1. Introduction
Fossil fuels are essential to modern society, as they are one of the most important contributors to world’s energy consumption. However, their excessive use is leading to a sharp increase in CO2 emissions, which creates many environmental concerns, such as the “greenhouse effect”. Statistical data show that carbon emissions of China increased by around 6 billion tons from 2001 to 2017. In 2016, China became the world’s largest emitter of greenhouse gases [1], so reducing carbon dioxide emissions is a challenging problem for China. A CaO-based carbon capture technology is considered promising for carbon emission reduction, and hence it is studied here in detail. The circulatory system contains a reactor to capture CO2 and a calcination furnace for regenerating CaO-based adsorbents [2, 3, 4]. However, calcination may also bring about deformation and sintering of particles of the adsorbent [5, 6]. After multiple carbonation–calcination cycles, the adsorption efficiency of calcium-based adsorbents decreased significantly [7, 8].
Calcium-based adsorbents have been modified using different methods: addition of other metal elements, changing the preparation methods, optimizing reaction conditions [9, 10, 11, 12]. Lee et al. [13] prepared an innovative, calcium-based adsorbent doped with Na2CO3 and found that its reaction with CO2 can take place at a lower adsorption temperature, thus reducing the energy demand. Pecharaumporn et al. [14] studied the performance of a synthetic Al–CaO-based system on CO2 capture and found that synthetic adsorbents with gluconic acid as precursor display a good adsorption capacity. Guo et al. [15] demonstrated the role of microstructure and oxygen vacancy (Zr–Ce) in CO2 adsorption and discovered that the synthetic adsorbent guaranteed good CO2 capture ability when the ratios Ca/Zr/Ce were 30:0.5:0.5 even after 35 carbonation–calcination cycles. Yoon et al. [16] added ZrO2 in CaO adsorbents using the sol–gel method to prepare a new adsorbent and obtained a CO2 adsorption capacity around 77.3 wt% (17.6 mol∕kg). Tong et al. [17] proposed a semi-industrial process for preparing calcium-based adsorbent pellets and demonstrated that the microsphere adsorbent containing 5 wt% uric acid had the best CO2 adsorption capacity. Wang et al. [18] manufactured stable Al–CaO-based adsorbents with different mass ratios using hydration, co-precipitation, and sol–gel spontaneous combustion and found that the preparation method had a significant impact on the structure of the synthesized adsorbents. Antzara et al. [19] investigated the behavior of CaO adsorbents containing ZrO2 using a fluidized-bed reactor and found that the deactivation rate of adsorbents was 16% lower after 20 carbonation–calcination cycles. Qin et al. [20] used a chemical combustion chain to provide the energy demand of regeneration of CaO particles and indicated that increasing water vapor pressure could significantly reduce the reaction deactivation rate of CaO/CuO sorbents in the Ca–Cu complex chemical circulation.
To further investigate the adsorption mechanism of CaO-based adsorbents, density functional theory (DFT) calculations have been widely used to study carbon capture [21, 22, 23, 24]. Ma et al. [25] investigated the reaction between CaO/Ca12Al14O33 adsorbent and CO2 in the presence of water vapor and proved that DFT calculations accurately predicted the adsorption results of CaO-based sorbents under different reaction atmospheres. Liu et al. [26] added different metal elements to calcium sorbents to study the adsorption performance and found that addition of Zr effectively improved the adsorption ability of CaO-based adsorbents. Fan et al. [27] analyzed the properties of a pressurized calcium-based adsorbent with steam in a spout-fluidized-bed reactor and reported that steam may have little catalytic effect on the carbonation of adsorbents. Guo et al. [28] studied the effect of Mg2+ in the CaO lattice for CO2 capture at high temperature. Further results showed that there was a strong adsorption effect between the CO2 molecule and O2−. Jensen et al. [29] studied the adsorption of CO2 on MgO and CaO by quantum chemical calculations and compared the data with the infrared spectra in the literature. Dai et al. [30] examined the behavior of H2O molecules on the surface of CaO (100). The results of the adsorption energy calculations proved that the interaction between H2O and CaO were due to chemical adsorption. Xu et al. [31] conducted experiments and DFT calculations on the adsorbent doped with different metal oxides. Ma et al. [32] synthesized an innovative calcium-based adsorbent with hollow core-shell structure and demonstrated that Al effectively delays the slagging and sintering of the synthetic adsorbent. However, there are only limited studies available on the combination of experiments and DFT calculations for different calcium-based adsorbents, and this has been investigated in the present study.
We have examined CaO-based sorbents containing different metal elements using a hydration method. The performance of the CaO-based adsorbent was tested in a fixed-bed reactor under different working conditions. DFT calculations were used to determine the key parameters of different adsorbents, which can help explain the experimental results.
XRD spectrum of Al2O3-doped sample. Lower tracks show spectra for Ca(OH)2, CaCO3 and Ca(Al2O4) for comparison.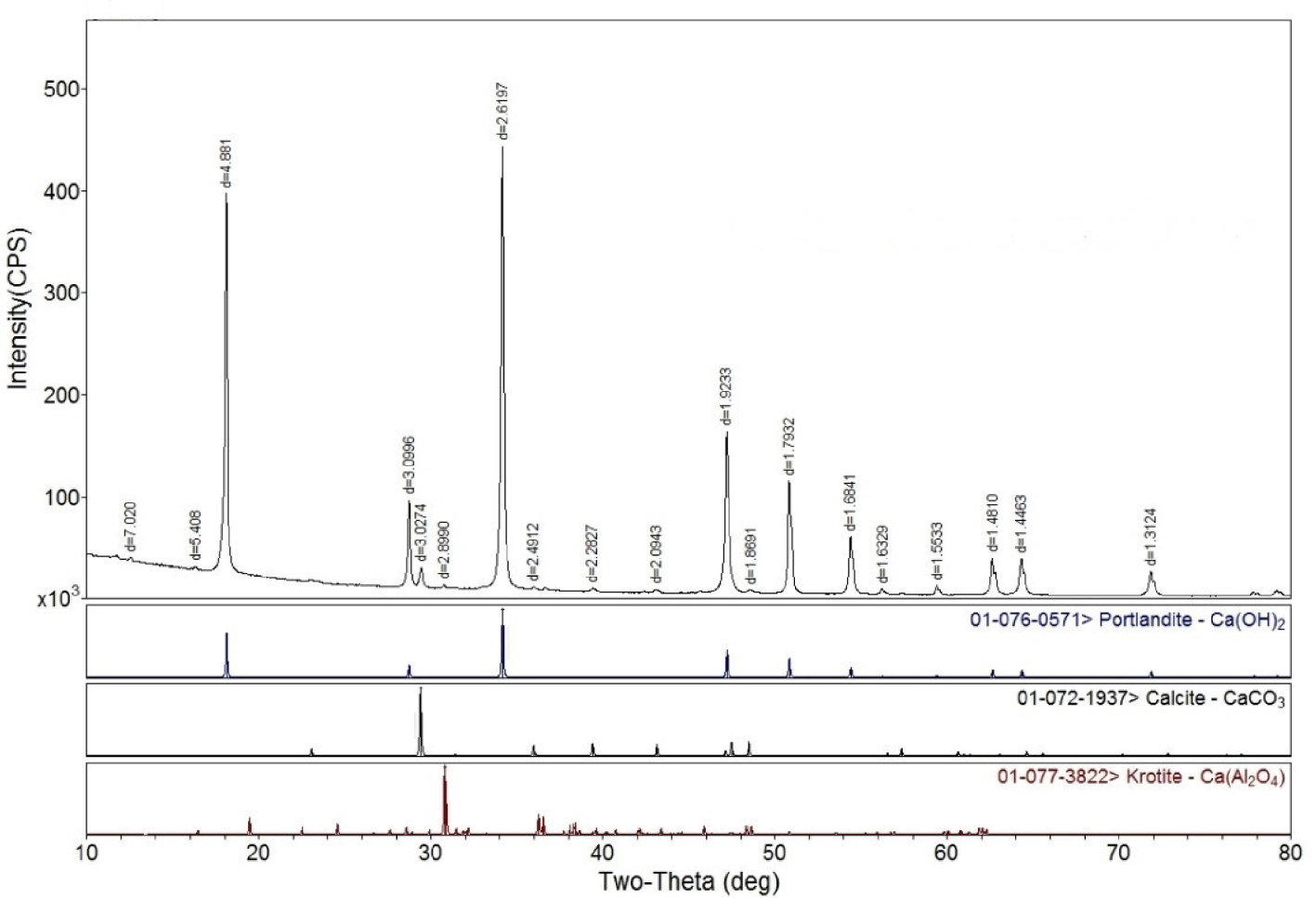
2. Experimental part
2.1. Materials
Modified CaO-based adsorbents were prepared using the hydration method. Analytical reagent (AR)-grade CaO particles were used for the experiment. Other materials included ZrO2 (99%), 200–300-mesh Al2O3, and CuO (99%). Five adsorbent samples doped with metal oxides were prepared: (1) 95 wt% CaO + 5 wt% ZrO2; (2) 95 wt% CaO + 5 wt% Al2O3; (3) 95 wt% CaO + 2.5 wt% ZrO2 + 2.5 wt% Al2O3; (4) 95 wt% CaO + 5 wt% CuO; (5) 100 wt% CaO. All samples were evenly stirred in deionized water. After allowing samples to stand for 30 min, a specimen was put into an oven and dried for 12 h at 100 °C, and particles with diameter of 200 μm were chosen for experiment. X-ray diffraction (XRD) shows essentially the same main Ca(OH)2 and secondary CaCO3 peaks for all 5 samples (Figure 1 shows a typical spectrum obtained with the Al2O3-doped sample). Due to moisture remaining after drying, CaO particles reacted with H2O to form Ca(OH)2; then a small amount of Ca(OH)2 and CO2 reacted to form CaCO3. Therefore, samples were subsequently calcined for 3 h at 900 °C to remove Ca(OH)2 and CaCO3.
2.2. Experimental apparatus
We used a fixed-bed reactor in our experiments. The outer and inner diameters of the quartz tube were 125 and 120 mm, respectively, and its length was 1400 mm. The samples were placed in a quartz boat and gently pushed to the center of the quartz tube. The heating and cooling rates were set at 15 and 20 °C/min, respectively. The carbonation reaction temperature varied from 600 to 750 °C, depending on the working condition, and the calcination temperature was set at 850 °C. The reaction time for carbonation and calcination was set at 25 and 15 min, respectively. Here, 500 mg of experimental samples was investigated for 20 carbonation–calcination cycles under various working conditions.
The CO2 and N2 gas valves were connected to the fixed-bed reactor. The total gas flow was fixed at 400 ml/min. The purge and furnace gases composed of N2 were used to effectively preserve the fixed-bed reactor. The carbonation conversion rate of the synthetic adsorbent was defined as
| (1) |
The XRD analysis was carried out to study the specific distribution of crystal phase in the sample. To determine the crystal structure, atomic coordinates, and absolute configuration, we used a Gemini XRD analyzer, with a CCD detector and a cryogenic system.
2.3. Preparation of DFT calculation
We established the crystal structure model using Materials Studio software to perform the calculation. The CaO crystal has a cubic structure. The lattice constant after optimization is 4.862 Å, which is in agreement with the experimental results [33]. The C–O bond length and O–C–O bond angle of the CO2 cell are 1.173 Å and 179.895°, respectively, which is consistent with the experimental results [34]. The CaO crystal cell is defined by adopting crystal indices (100). A vacuum layer with a thickness of 15 Å is chosen. Simultaneously, the CaO (110) surface is modified by adding Zr, Al, and Cu to achieve the doping of metal elements and structure optimization.
Calculations were performed using the general gradient approximation method and the Cambridge Serial Total Energy Package (CASTEP) module. The cut-off energy value of 570 eV was selected for truncation. The energy difference between adjacent ion steps was less than 1.0 × 10−5 eV/atom. The maximum atomic interaction force, maximum atomic stress, and atomic displacement were 0.3 eV/nm, 0.05 GPa, and 0.0001 nm, respectively. Here, the energy convergence standard of the electron step in the self-consistent field operation was 10−5 eV/atom. The K point was set as 3 × 4 × 1 to ensure the accuracy of calculation. The adsorption energy Ead is defined as
| (2) |
Adsorption efficiency of different modified CaO-based adsorbents at 650 °C.
Adsorption efficiency of different modified CaO-based adsorbents at 700 °C.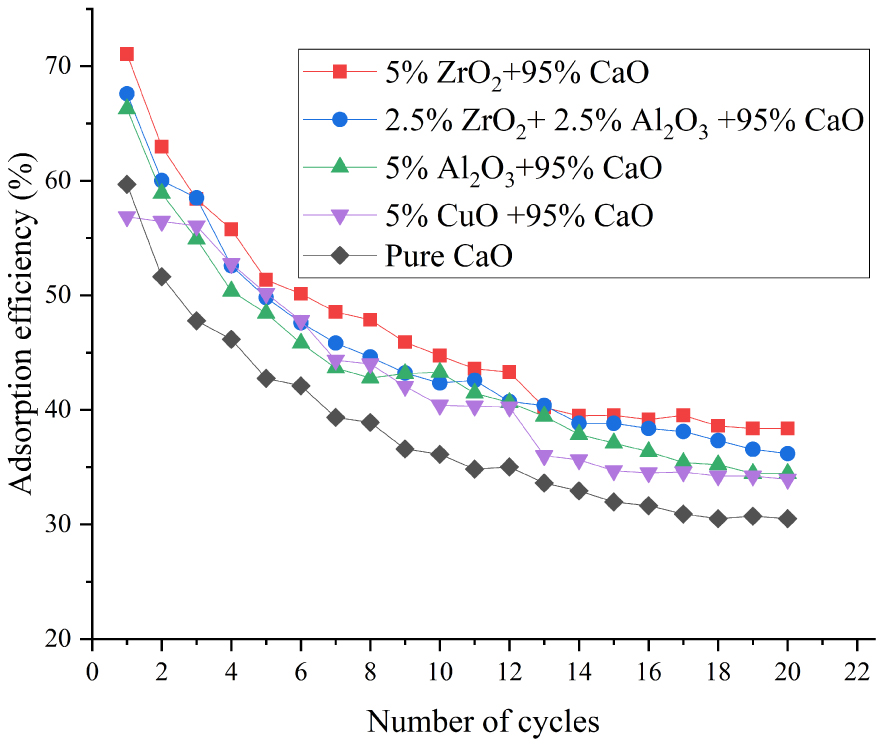
Effect of temperature on CaO-based adsorbents doped with 5% ZrO2.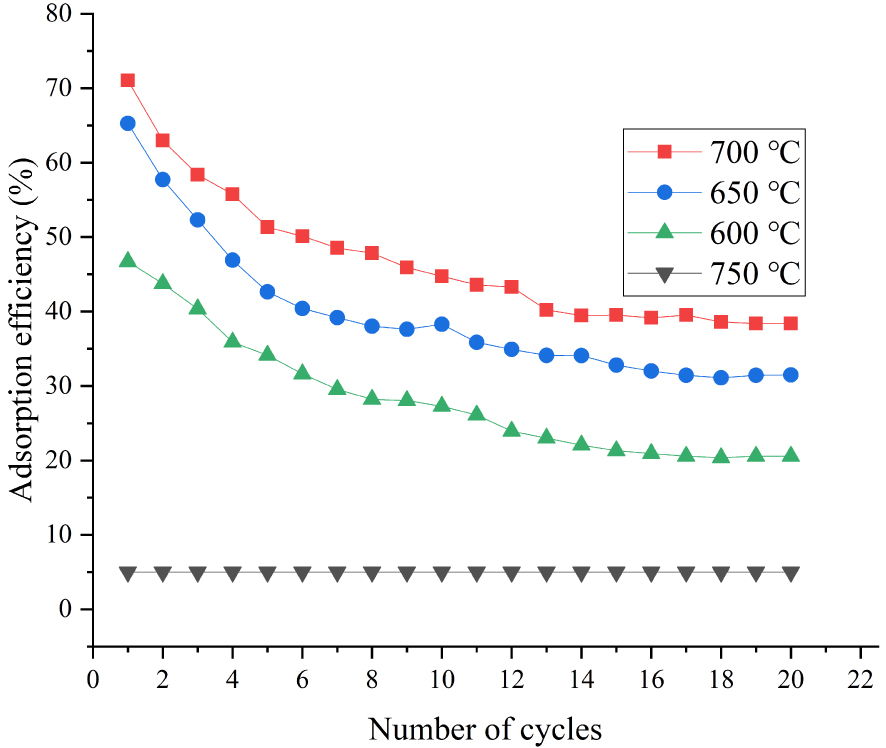
Effect of temperature on CaO-based adsorbents doped with 2.5% ZrO2 + 2.5% Al2O3.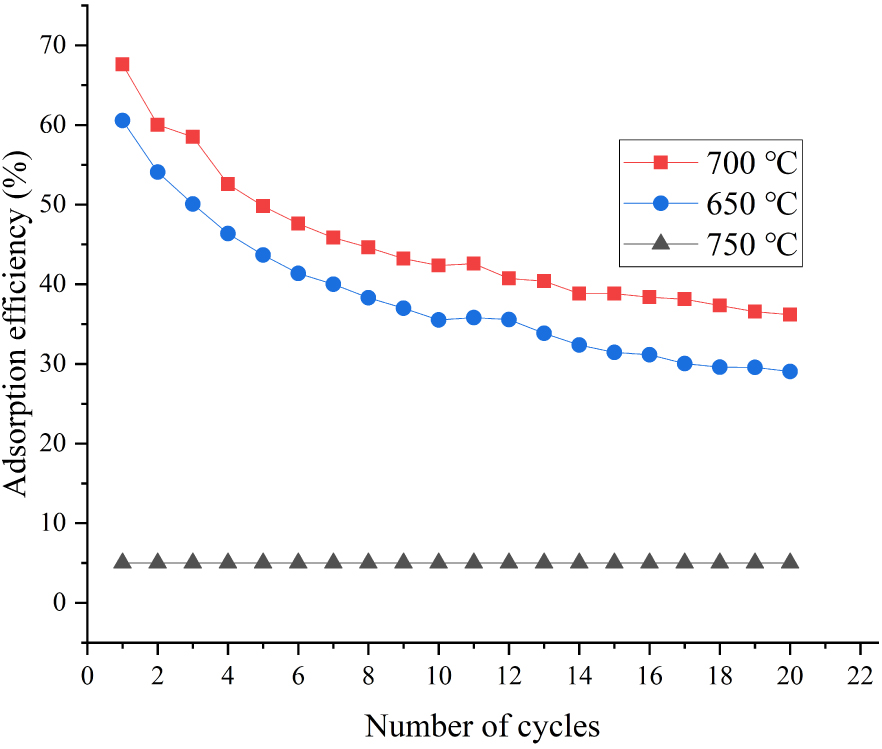
3. Results and discussion
3.1. Performance test of modified adsorbents
3.1.1. Adsorption properties of different modified CaO-based adsorbents
We evaluated the performance of modified adsorbents after multiple carbonation–calcination cycles. During the experiment, N2/CO2 and N2, respectively, were the carbonation and calcination atmospheres. Figures 2 and 3 show the experimental results of the adsorption efficiency of adsorbents after 20 carbonation–calcination cycles at 700 and 650 °C, respectively, at 15 vol% CO2 concentration. The adsorption efficiency of all adsorbents at 700 °C was higher than that at 650 °C, which indicated that the increase in temperature was beneficial to the adsorption reaction. When the carbonation temperature was 700 °C, the initial efficiency of the adsorbent with 5 wt% ZrO2 was 72%. After 16 carbonation–calcination cycles, the adsorption efficiency appeared to be stable at 38.4%, which represented an increase of approximately 9.4% compared with a pure CaO adsorbent. The adsorption efficiency of adsorbents doped with Al2O3 and CuO after 20 cycles was 34.5% and 34%, respectively. It should be pointed out that the adsorption capacity of CaO-based adsorbent doped with Cu decreased significantly during the 12–13 carbonation–calcination cycles. The adsorbent was inactivated due to fusion at high calcination temperature. However, lower calcination temperature also guaranteed that the adsorbent with Cu showed a good carbon capture capability. Overall, the adsorbent doping with 5 wt% ZrO2 demonstrated the strongest anti-sintering ability and the best adsorption ability.
3.1.2. Effect of carbonation temperature on the performance of modified adsorbents
We also investigated the performance of modified adsorbents at different carbonation temperatures. The adsorption efficiency of adsorbents doped with (a) 5 wt% ZrO2, (b) 2.5 wt% ZrO2 + 2.5 wt% Al2O3, and (c) 5 wt% Al2O3 increased by (a) 6.9%, (b) 7.15%, and (c) 7.43% when the carbonation temperature increased from 650 to 700 °C (20 carbonation–calcination cycles). The effect of temperature on the performance of adsorbents can also be clearly seen in Figures 4–6. However, adsorption efficiency decreased significantly at 750 °C (Figure 7). Figure 8 further confirmed 700 °C as the optimal temperature.
Effect of temperature on CaO-based adsorbents doped with 5% Al2O3.
Effect of temperature on modified CaO-based adsorbents after 20 cycles.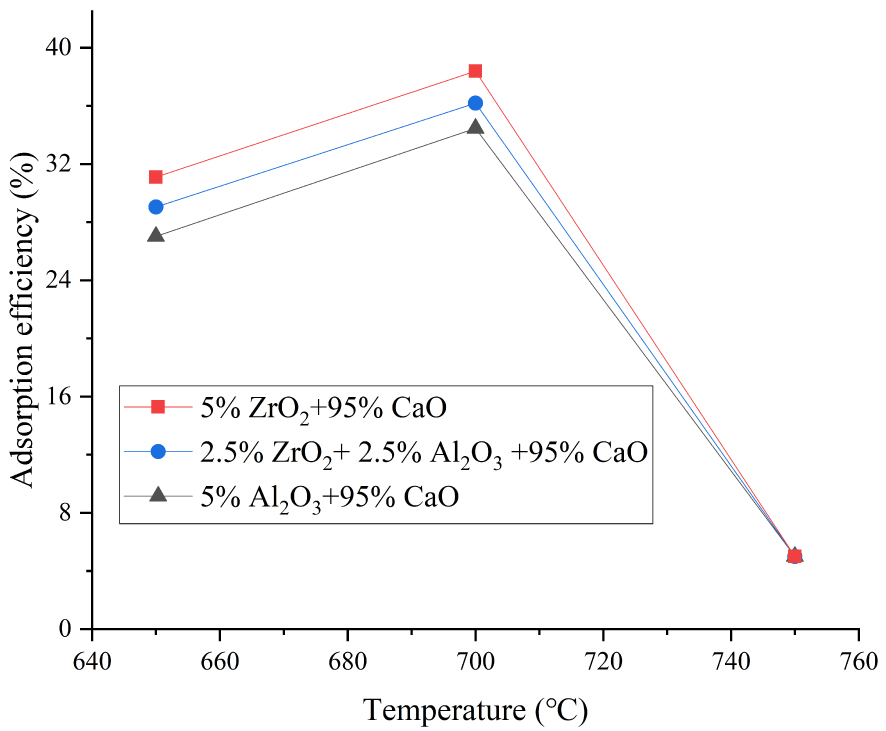
Optimal adsorption temperature of CaO-based adsorbents doped with 2.5% ZrO2 + 2.5% Al2O3.
3.1.3. Effect of carbonation time on the performance of modified adsorbents
The effect of carbonation time on the adsorption efficiency of adsorbents was also investigated. The 2.5 wt% Al2O3 + 2.5 wt% ZrO2 adsorbent was our main object of study. The carbonation temperature was 700 °C, and the atmosphere was N2/CO2 with 15% CO2 concentration. As shown in Figures 9 and 10, the adsorption efficiency increased by 23.2%, 8.2%, and 4.7% for 0–5 min, 5–15 min, 15–25 min, respectively. The initial steepness of the curve in Figure 10 shows that the carbonation reaction was very fast between 0–5 min. Between 5 and 15 min (the transition period), the adsorption efficiency of adsorbents increased relatively rapidly. However, the adsorption reaction became slow between 15 and 25 min (reaction diffusion stage). In particular, the 0–5 min rapid stage of carbonation ensured good CO2 capture capacity.
Effect of carbonation time on CaO-based adsorbents doped with 2.5% ZrO2 + 2.5% Al2O3.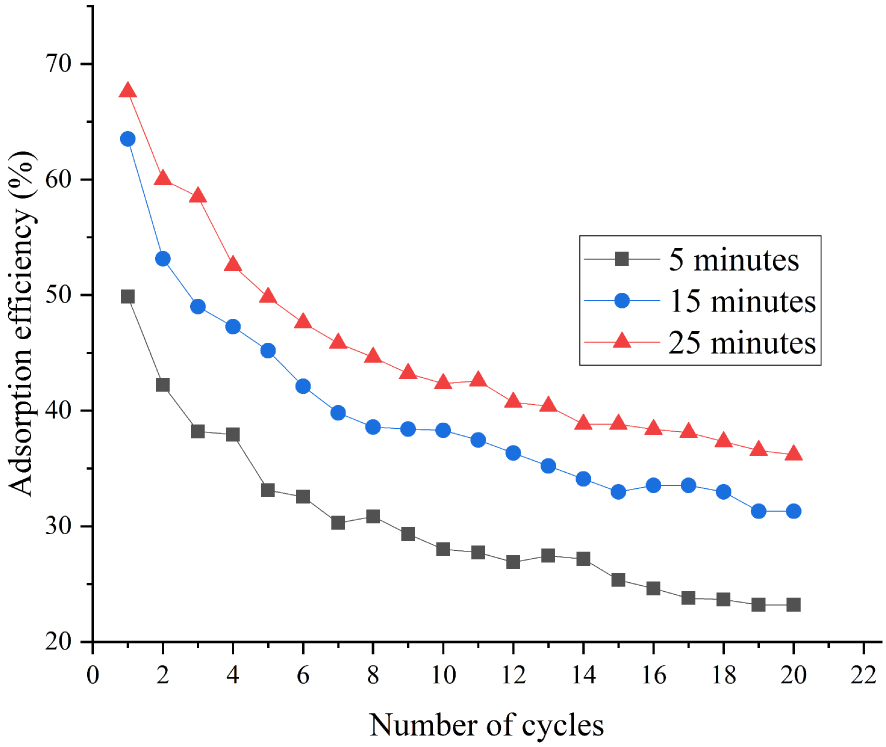
Adsorption reaction analysis of CaO-based adsorbents doped with 2.5% ZrO2 + 2.5% Al2O3 after 20 carbonation–calcination cycles.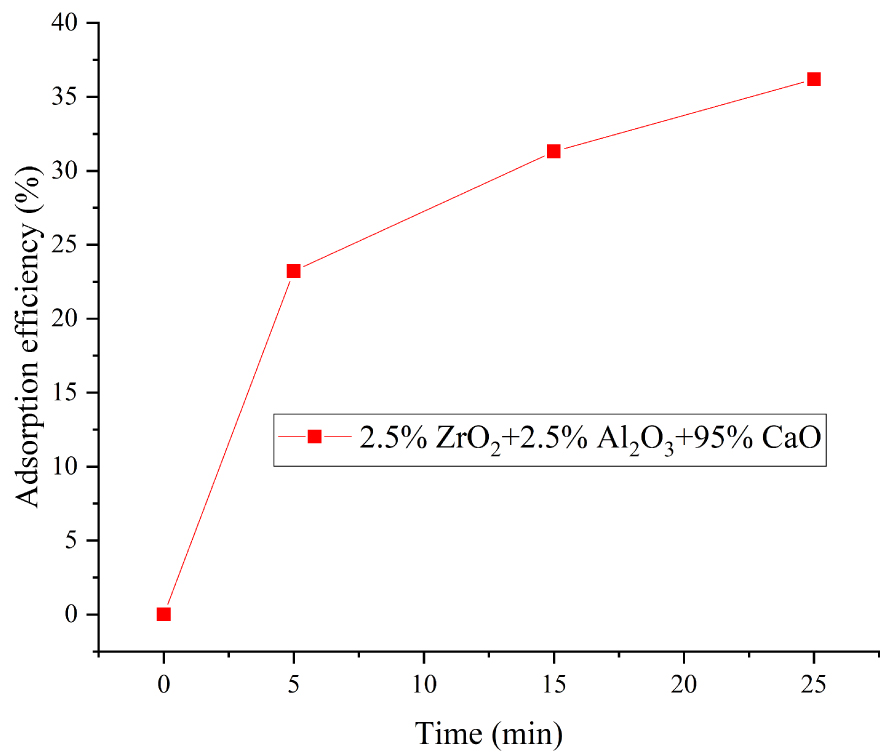
3.1.4. Effect of CO2 concentration on the performance of modified adsorbents
We also studied the effect of CO2 concentration on the adsorption performance of modified adsorbents. A CaO-based adsorbent doped with 5 wt% ZrO2 was set as the main object of study. Although the volume fraction of CO2 in coal-fired flue gas was about 15%, it was imperative to study the CO2 concentration higher than 15% in order to determine the performance of adsorbents more clearly. The adsorption efficiency of adsorbents was investigated under increasing carbonation–calcination cycles with 40, 15, and 8 vol% CO2. The working conditions in the calcination stage were the same in all experiments. Figure 11 shows that the adsorption efficiency of adsorbents was nearly uniform under 40% and 15% CO2 but decreased sharply with 8% CO2. Figure 12 demonstrates that the efficiency was consistent during the first cycle. However, after 20 carbonation–calcination cycles, the adsorption efficiency of adsorbents with 40 vol% CO2 concentration was higher by approximately 9.5% than with 15 vol% CO2.
This phenomenon was explained as follows. First, the whole carbonation reaction process was divided into two stages: the rapid reaction stage and the reaction diffusion stage. Thereafter, the CO2 diffusion rate in the carbonation stage was determined by temperature and CO2 concentration. In addition, the sintering degree was based on the same calcination temperature and time for all adsorbents. Figures 11 and 12 suggest that the CO2 diffusion rate is mainly depended on CO2 concentration at 650 °C, but determined by temperature and CO2 concentration together at 700 °C. The diffusion rates with 40 and 15 vol% CO2 are similar at 650 °C (both curves essentially overlap). The diffusion rate with 8 vol% CO2 was far slower than that under high CO2 concentration, which led to the sharp decrease in adsorption efficiency.
Figure 12 shows that the diffusion rate of the CO2 molecule was rapid with 40 vol% CO2 concentration at 700 °C, so the adsorption efficiency of adsorbents in rapid reaction stage was also higher. However, the CO2 molecular diffusion rate with 15 vol% CO2 was relatively slow, and the CaCO3 produced in the rapid reaction stage might result in pore blockage, which would ultimately reduce the adsorption efficiency.
Effect of CO2 concentration on CaO-based adsorbents doped with 5% ZrO2 at 650 °C.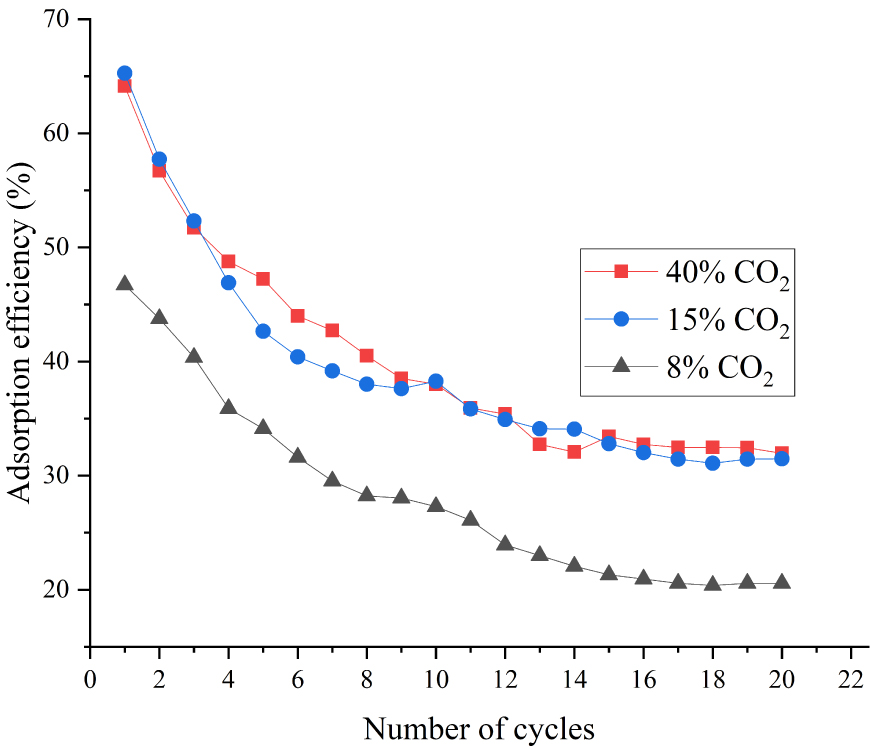
Effect of CO2 concentration on CaO-based adsorbents doped with 5% ZrO2 at 700 °C.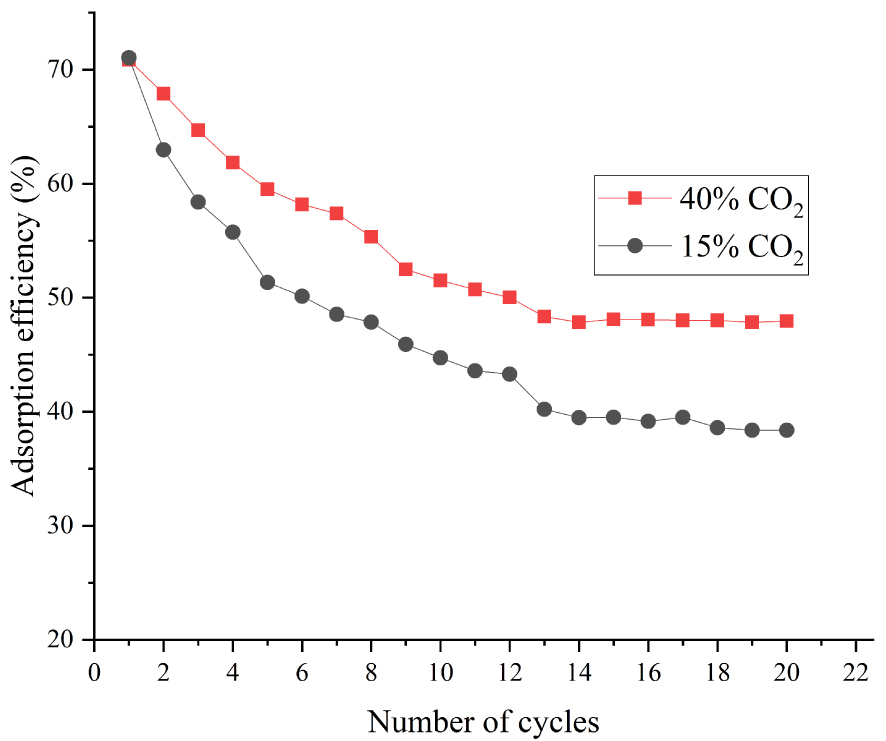
Adsorption model of different modified CaO-based adsorbents.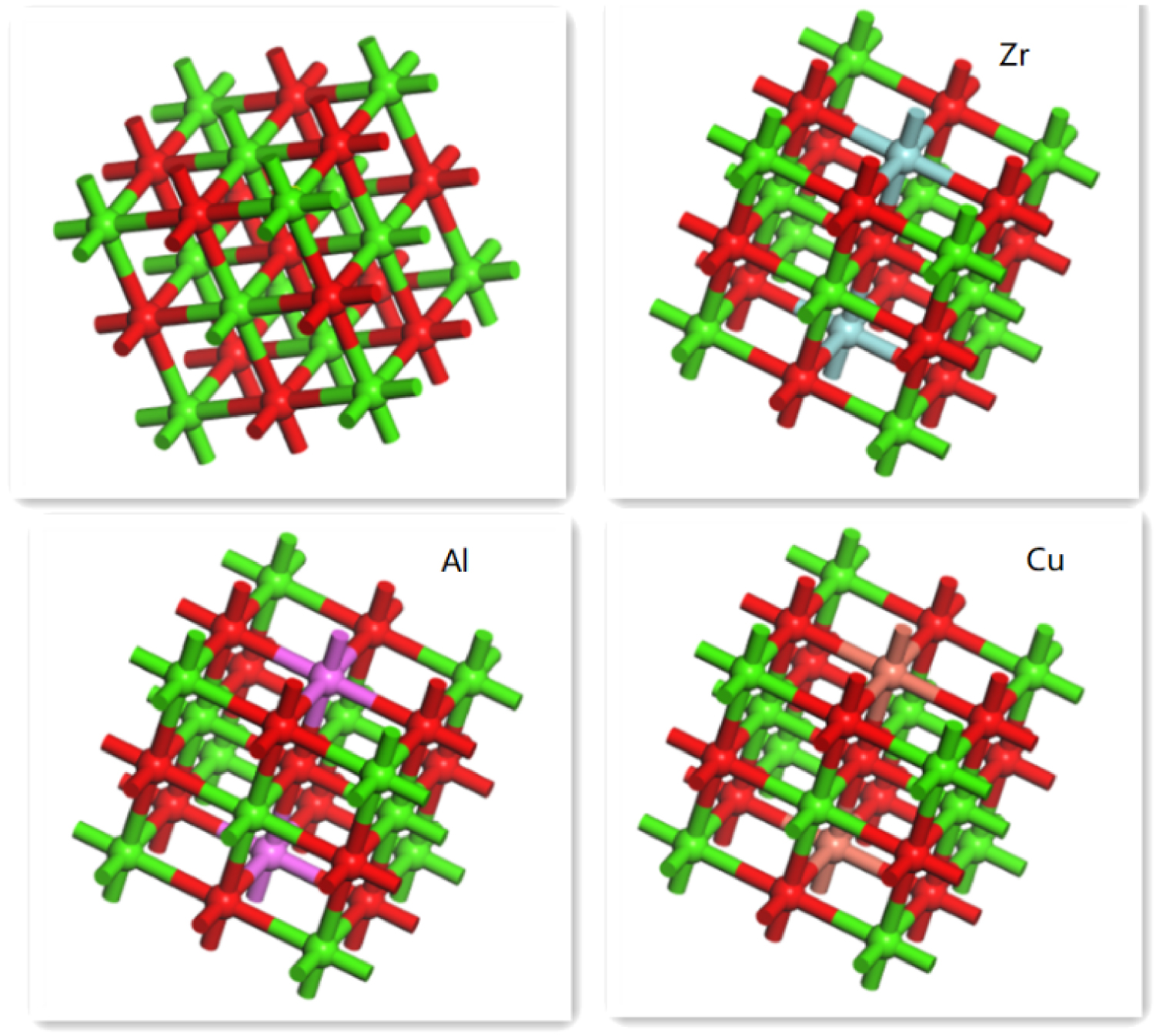
Main results of DFT calculation
| CaO (100) | Al–CaO (110) | Zr–CaO (110) | Cu–CaO (110) | |
|---|---|---|---|---|
| Ca–O bond angle/° | 90 | 82.74 | 73.4 | 83.587 |
| C–O bond length/Å | 2.9 | 2.796 | 2.329 | 1.506 |
| O–C–O bond angle/° | 141.1 | 142.6 | 119.05 | 132.76 |
| O–C–O bond length/Å | 1.208 | 1.176 | 1.3 | 1.24 |
| Ead/eV | 1.715 | 1.98 | 2.37 | 1.81 |
PDOS of CaO adsorbent.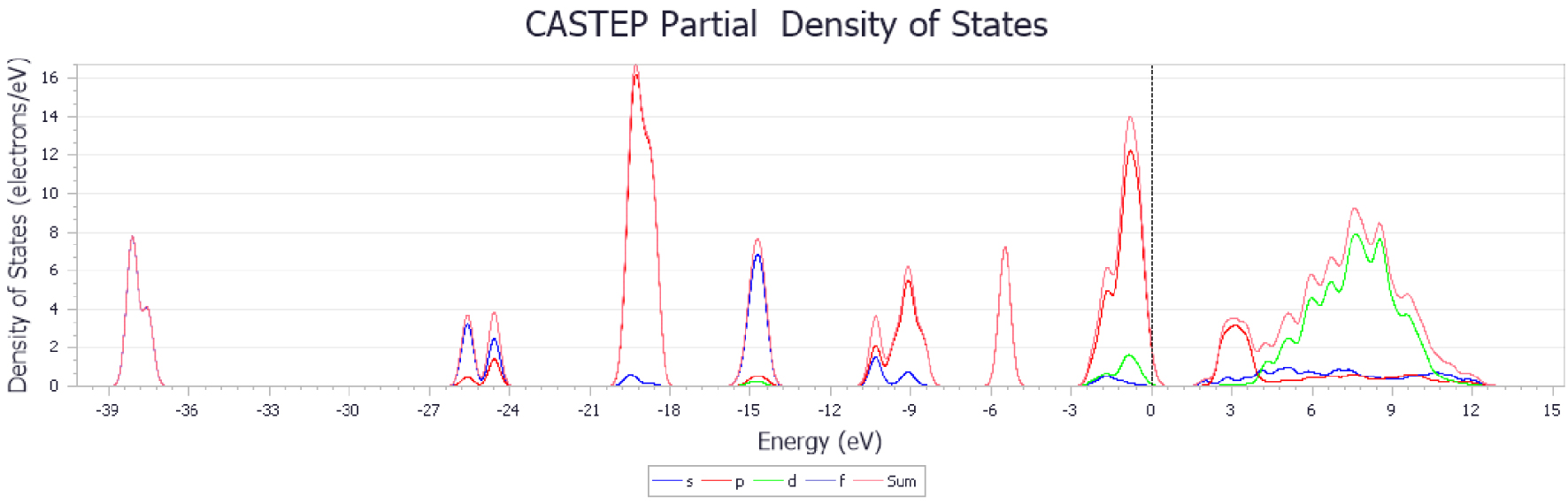
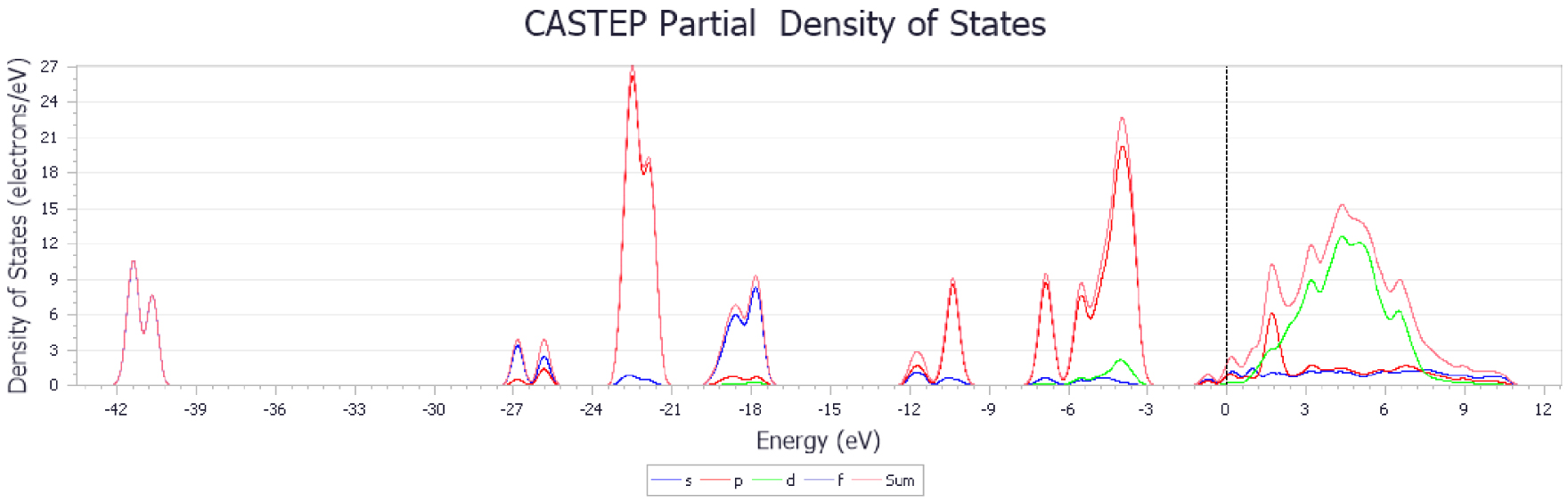
PDOS of CaO-based adsorbent doped with ZrO2.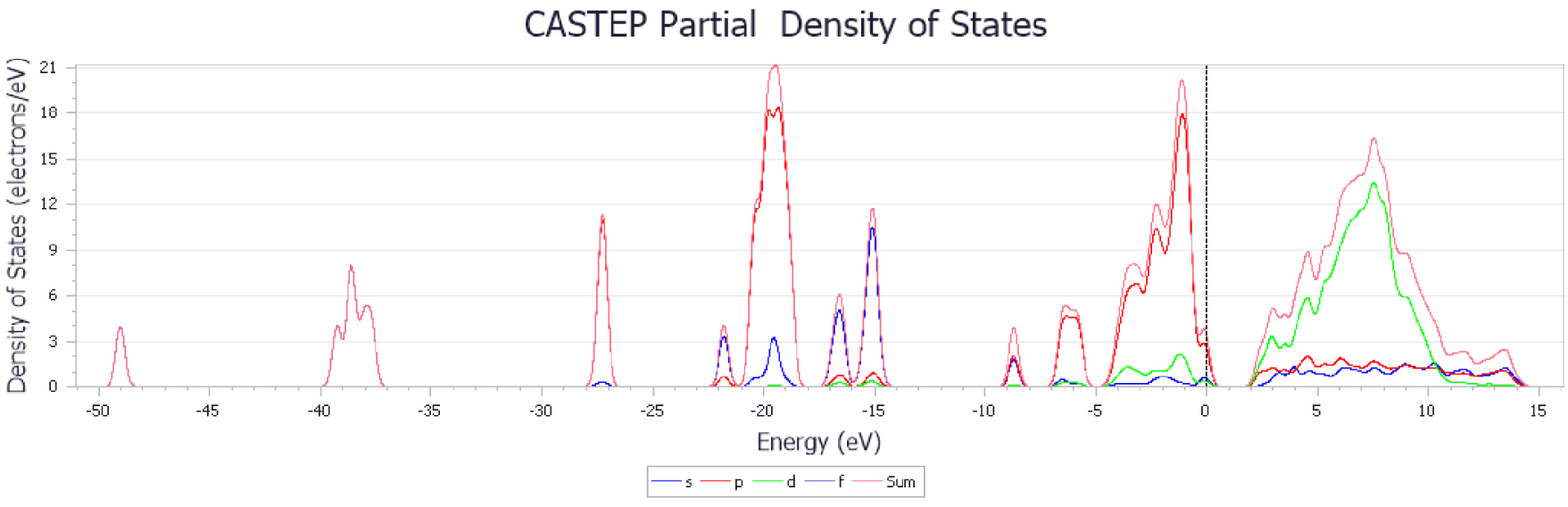
3.2. Results of DFT calculation
To verify the experimental results for adsorbents under various working conditions, DFT calculations were performed using MS software. The carbonation reaction between CaO-based adsorbents doped with different metals and CO2 molecules was studied in detail. The structure of CO2 molecules and CaO cells has been calculated before [25, 35]. However, in this study, the adsorption reaction between CaO-based adsorbents and CO2 was emphasized. Figure 13 shows the adsorption models of CO2 molecules on the CaO-based adsorbents doped with different metal elements. It could be seen that CaO (110) crystal cells had multiple adsorption sites, including O-top, Ca-top, bridge, and hollow sites. We found that the possibility of forming an anionic carbonate-like structure was high for CO2 molecules on the O-top site compared with other adsorption sites. Other studies showed that CO2 molecules were also inclined to be adsorbed at the O-top position [26]. Therefore, we only investigated the adsorption conditions at the O-top site. The adsorption results are given in Table 1, including adsorption energy and bond length angle. The adsorption energy of pure CaO was 1.715 eV. The structure of the CaO crystal cell changed slightly upon CO2 adsorption, the Ca–O bond angle remained 90°. The C–O bond length was 2.9 Å, and the bond angle of the CO2 molecule was 141.1°.
The results show that the adsorption energy of CO2 molecules is higher with metal-doped CaO-based adsorbents, especially for Zr. The higher adsorption energy ensured that the C–O bond was more stable, and the adsorbents had much stronger ability to resist sintering. Figures 14–17 show the changes in energy band structure of different modified CaO-based adsorbents after adsorption reaction. In summary, the partial density of state of CaO-based adsorbents doped with different metal elements was shifted from right to left. In particular, when the peak height of the waveform in figures is large, then the degree of hybridization between different atoms is higher.
Figure 15 shows the best adsorption performance compared with other results. It was found that the structure in CO2 molecules and CaO crystal cells had also changed and the O–C–O bond angle got smaller, which was caused by the deformation in electrons from the O2− transferred to the C atom. It should be noted that there was no obvious change in the energy band structure of Cu-doped adsorbent, although some researchers have observed benefits for carbon reduction [20, 36, 37, 38]. It might be explained that the method was applied for the CO2 catalytic reduction rather than adsorption reaction. The Cu-doped adsorbent may have melted during the experiment at high calcination temperature. The experimental results are consistent with the DFT calculations for all the carbonation temperatures examined here. It was clear that the Zr-doped adsorbent had the best adsorption efficiency after 20 carbonation–calcination cycles.
4. Conclusions
In this study, CaO-based adsorbents doped with different metal oxides were prepared using the hydration method. The performance of the modified adsorbents under various working conditions was studied and the results show that the modified calcium-based adsorbent doped with 5 wt% ZrO2 has excellent CO2 adsorption capacity. The adsorption efficiency is around 38.4% after 20 carbonation–calcination cycles for a carbonation temperature of 700 °C and 15 vol% CO2, which is 9.4% higher than that of the pure CaO adsorbent. The adsorbents doped with other metal oxides demonstrate higher anti-sintering ability under high calcination temperature, which suggests a promising research direction for industrial applications. The effect of carbonation time on adsorption efficiency shows that extending carbonation time enhances CO2 capture. In addition, the reaction model for modified adsorbents was established by DFT calculation to further explain the experimental results. The results indicate that the adsorption energy of adsorbents doped with metal oxides is higher than that of pure CaO, and the values are 2.37 eV for ZrO2–CaO, 1.98 eV for Al2O3–CaO, 1.81 eV for CuO–CaO, and 1.715 eV for pure CaO, respectively. The maximum adsorption energy of the adsorbent doped with 5 wt% ZrO2 is 2.37 eV. The greater adsorption energy is favorable to strengthen the anti-sintering ability of adsorbents, so the theoretical results of DFT calculation are consistent with the experimental results.
Acknowledgment
This work was supported by The National Key Research and Development Program of China (No. 2019YFE0100100-05).



 CC-BY 4.0
CC-BY 4.0