1. Introduction
Urolithiasis prevalence is increasing in all industrialized countries, affecting more than 10% of the population [1, 2, 3, 4]. Among renal stone formers, uric acid stones affect 10% of patients, mainly men after the fourth decade [5, 6]. During the past years, several studies have shown evidence that metabolic syndrome and type 2 diabetes are associated with an increased risk of uric acid stone [7, 8, 9, 10]. Uric acid crystals and stone formation are specifically promoted by increased uric acid urinary concentration, due to purine and fructose consumption, and by low urinary pH [10, 11, 12]. Permanent acid urine over a 24-h period is a hallmark of metabolic syndrome and type 2 diabetes, due to impaired ammoniagenesis and to an increased daily net acid excretion in uric acid stone formers [10, 11, 12, 13]. Beyond these well recognized biological risk factors for uric acid stones, data from a large stone database provided evidence that uric acid stones originate mainly from the left kidney, whereas other stones are more homogenously distributed in both kidneys [4]. This original observation suggests that left kidney may conceal a specific risk factor for uric acid stone formation and we show herein that uric acid stone formers have an increased frequency of renal cysts in the left kidney.
2. Patients and methods
2.1. Morphoconstitutional analysis of renal stones
The Cristal laboratory has been collecting stones sent for identification and classification from >200 hospitals in France during several decades. Morphologic examination and classification of urinary stone surface and section were combined with Fourier transform infrared spectroscopy (FTIR) to classify stones and identify the different crystalline phases [14, 15]. Starting from a database of more than 78,000 urinary stones collected in adults between 1990 and 2020, stones whose origin side (left or right kidney) was certain have been selected, limiting the study to 38,349 stones. Other clinical data including the notion of diabetes were recorded in the database when available. The proportions of the main stone components were assessed, as well as the internal structure of the stone according to FTIR and to the morphoconstitutional stone classification [14, 15]. All stones have been analyzed by a single investigator (MD).
2.2. Lateralization of uric acid renal excretion and urine pH in kidney stone formers
To assess whether kidney side would influence uric acid excretion or urine pH in kidney stone formers, we measured uric acid and pH in urine from left or right kidney collected during reno-ureteroscopic procedures. Urine samples were collected in 38 renal stone formers at the beginning of reno-ureteroscopic procedures in the left (22 patients) or the right kidney (16 patients) in a single center (Tenon Hospital, Paris). In parallel, bladder urine (i.e. mixed urine from the left and the right kidney) was collected. We deliberately did not collect urine from both kidneys during the same procedure to stay in the routine practice procedure and avoid any risk of ureteral or kidney damage on the other side. Serum samples were collected during preoperative check-up. All patients gave a written consent and the procedure was in accordance with routine practice. Serum and urine parameters including uric acid, creatinine, and pH were assessed as previously described [16].
We assessed uric acid/creatinine ratio in urine and uric acid fractional excretion defined by the formula: (urine uric acid ∗ serum creatinine)/(serum uric acid ∗ urine creatinine). The lateralization ratio was defined by the (left or right kidney uric acid/creatinine)/(bladder uric acid/creatinine) ratio. Urinary pH was measured in all samples.
2.3. Uric acid fractional excretion and pH in renal transplant kidneys
To assess whether kidney side would influence uric acid excretion or urine pH, we also measured urinary uric acid and pH in a cohort of renal graft recipients who received either a left or a right kidney. Uric acid fractional excretion and fasting urine pH were analyzed retrospectively in 181 renal transplant recipients whose data had been recorded in the Bichat Hospital (Paris) transplant recipient cohort [17]. Renal transplant recipients had a systematic measure of GFR 3 months or one year after kidney graft and uric acid fractional excretion was calculated as stated above.
2.4. CT-scan analysis of kidneys in uric acid and calcium oxalate stone formers
To assess whether uric acid stone formation would be influenced by morphological changes in the left kidney, we assessed kidney and urinary tract morphology by CT-scan in uric acid stone formers. We analyzed CT-scans of uric acid stone formers who fulfilled the following criteria: (1) uric acid stones analyzed in the Cristal laboratory and (2) CT-scan performed in the radiology unit of Tenon Hospital at ±1 year from stone removal or expulsion. Overall, 25 patients fulfilled the criteria (23 males/2 females, mean age 62 years). A control group of 25 patients matched by age and sex and affected by calcium oxalate stones who performed a CT-scan in the same conditions was designed (23 males/2 females, mean age 60 years). In the view of the high number of cysts observed in left kidneys of uric acid stone formers, a trained radiologist (CM) reviewed all CT-scans retrospectively (unblinded procedure as uric acid density is lower than calcium oxalate). Renal or ureteral malformations including the presence of cysts and the number, size and type of renal cysts were recorded.
2.5. Statistical analyses
χ2 test was used for epidemiological data. Fisher’s exact test and Mann–Whitney test were used to compare other categorical or quantitative variables, respectively. Reported values represent mean ± SEM and median ± interquartile range-IQR. A p value < 0.05 was considered significant. Statistics were performed independently by two authors using both NCSS 6.0 and Statview softwares.
3. Results
3.1. Uric acid renal stones lateralization
Among more than 78,000 renal stones analyzed between 1990 and 2020, 38,349 stones from adult patients, whose origin (left or right kidney) was certain, were identified. Among these stones 3195 had uric acid as main component and 61% of these stones originated from the left kidney (p < 0.001), a specific feature of uric acid stones since stones made principally of calcium oxalate (monohydrate/whewellite and dihydrate/weddellite), carbapatite, brushite, struvite, or cystine were more homogeneously distributed (Figure 1). The notion of diabetes was recorded for 416 patients whose uric acid stone was analyzed. Among these diabetic patients, the lateralization ratio (left/right kidney stones) was lower than in all other patients affected by uric acid stones (1.26 versus 1.6; p = 0.02, Figure 1).

Lateralization of uric acid stones on the left side. Lateralization ratio (number of left kidney stones/number of right kidney stones) is shown for 38,349 stones whose main component is calcium oxalate monohydrate (COM), calcium oxalate dihydrate (COD), apatite (CA), brushite (BR) uric acid (UA, global and with/without notion of diabetes), struvite (PAM) or cystine (CYS). ∗: p < 0.001. Lateralization ratio was significantly lower when patients affected by uric acid stones were known to be affected by diabetes (#: p = 0.02). Masquer
Lateralization of uric acid stones on the left side. Lateralization ratio (number of left kidney stones/number of right kidney stones) is shown for 38,349 stones whose main component is calcium oxalate monohydrate (COM), calcium oxalate dihydrate (COD), apatite (CA), brushite ... Lire la suite
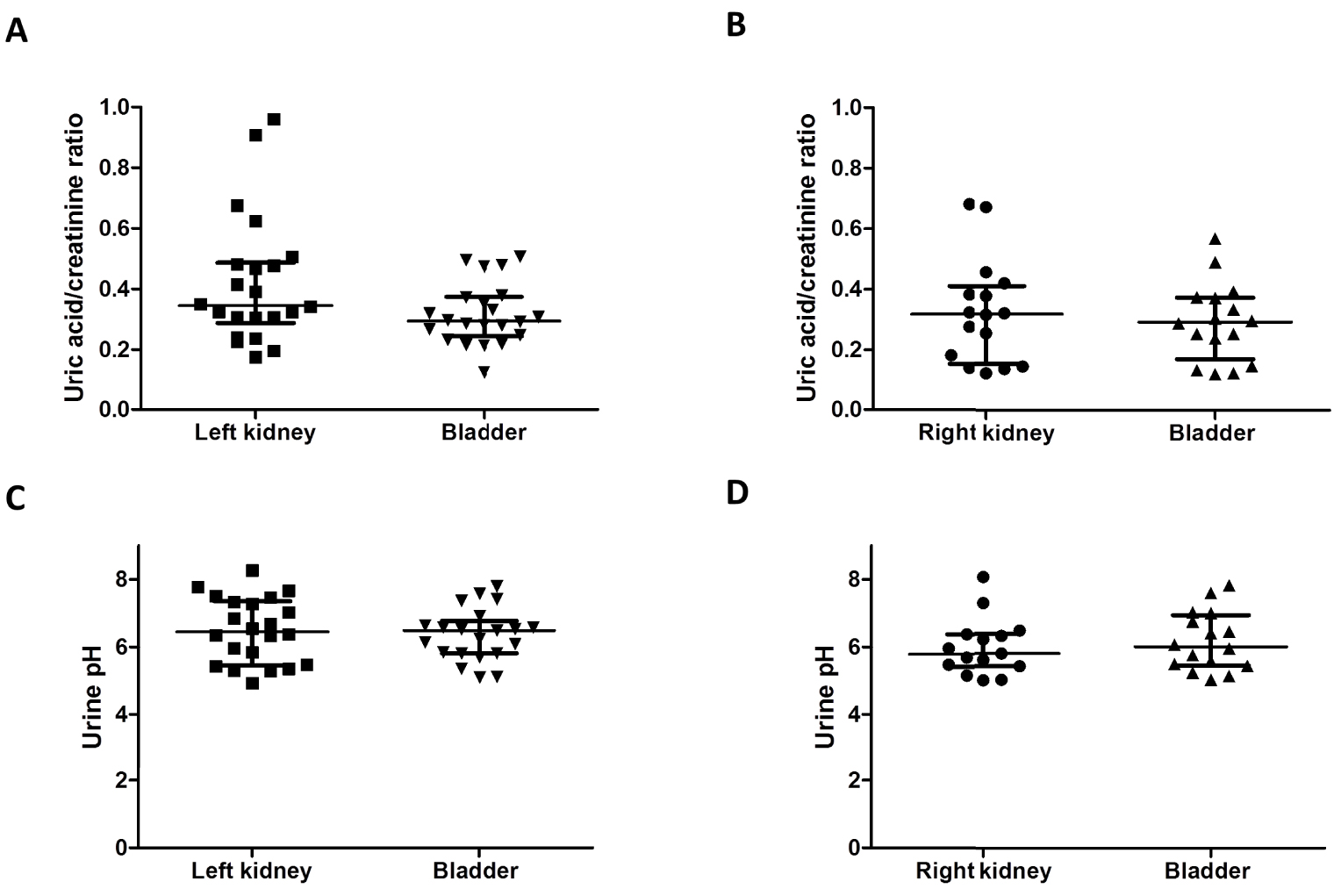
(A,B) Absence of lateralization of uric acid excretion in kidney stone formers. Uric acid/creatinine ratio was measured at the same time in the urine from the left or the right kidney at the beginning of reno-ureteroscopic procedures and in the bladder of 38 patients (mixed urine from both kidneys). We did not see a significant lateralization of uric acid excretion on the left side. Results are shown as median ± IQR. (C,D) Absence of lateralization of urine pH in kidney stone formers. pH was measured at the same time in the urine from the left or the right kidney at the beginning of reno-ureteroscopic procedures and in the bladder of 38 patients (mixed urine from both kidneys). We did not observe a lower pH on the left side. Results are shown as median ± IQR (brackets show IQR 25–75). Masquer
(A,B) Absence of lateralization of uric acid excretion in kidney stone formers. Uric acid/creatinine ratio was measured at the same time in the urine from the left or the right kidney at the beginning of reno-ureteroscopic procedures and in the ... Lire la suite
3.2. Similar composition of urine from left and right kidneys
Uric acid supersaturation in urine is driven by (i) low pH and (ii) high uric acid concentration: both parameters were analyzed in urine from left and right kidneys during renoureteroscopy and in kidney allograft recipients. Uric acid/creatinine median ratio was similar in urine collected in both left and right kidney at the beginning of ureteroscopic interventions performed in routine practice (0.35 [0.30, 0.48] mmol/mmol and 0.32 [0.17, 0.39] mmol/mmol respectively (p = 0.15, Figures 2(A) and (B)). When compared to the bladder urine (integrating left and right kidney uric acid secretion), there was no significantly increased uric acid excretion in the left kidney (left and right lateralization ratio respectively at 1.19 [0.97, 1.78] and 1.05 [1.00, 1.20], p = 0.31). In the same vein, urinary pH did not differ significantly in lateralized or mixed urines. Urinary median pH from the left and right kidney was at 6.45 [5.55, 7.31] and 5.78 [5.44, 6.34] respectively (p = 0.11, Figures 2(C) and (D)). Among the 38 patients whose urine has been analyzed, most had calcium oxalate stones and uric acid stones have been reported in 3 patients only. Of notice, these three patients had a left lateralization ratio of uric acid at 1.26, 1.81 and 2.41 respectively.
In addition, uric acid fractional excretion and urinary pH were analyzed in 181 renal transplant recipients who received either a right (n = 71) or a left kidney (n = 110). Kidney side (in the donor) did not affect significantly uric acid fractional excretion or fasting urinary pH in the recipient (Figures 3(A), (B)).
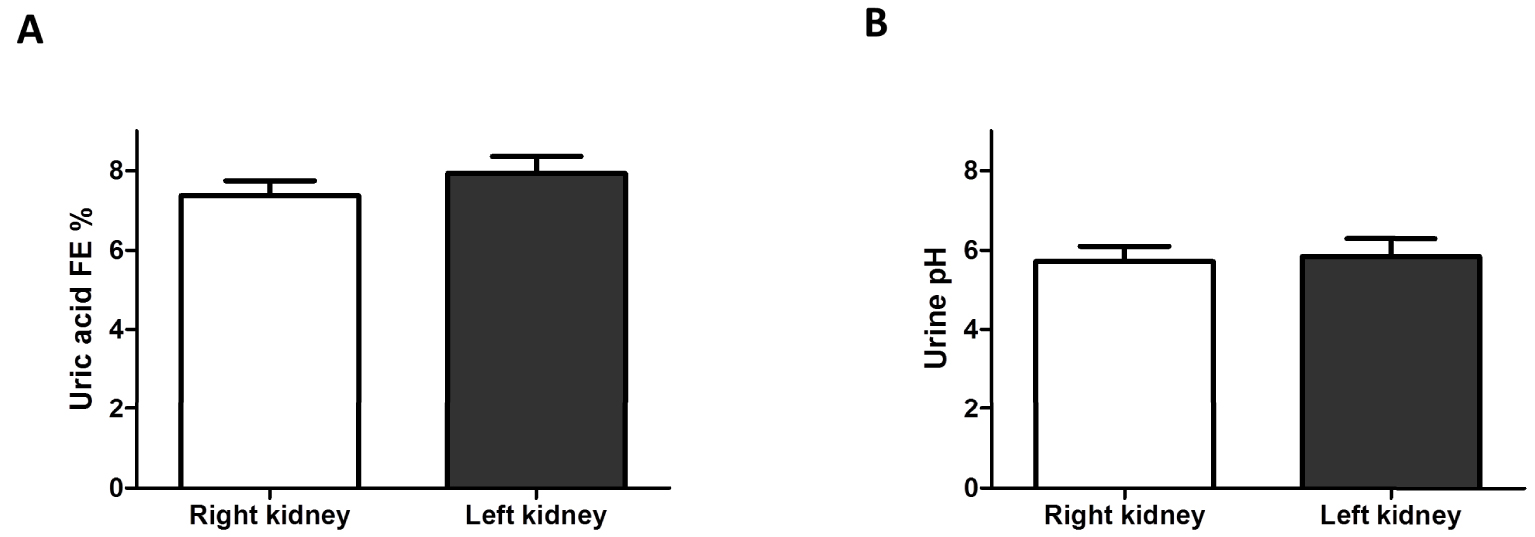
(A) Uric acid excretion in renal transplant recipients grafted with left or right kidney from the donor. Uric acid fractional excretion (FE) was similar in renal transplant recipients who received either a right or a left kidney. Results are shown as mean ± SEM. (B) Fasting urine pH in renal transplant recipients grafted with left or right kidney from the donor. Fasting urine pH was similar in renal transplant recipients who received either a right or a left kidney. Results are shown as mean ± SEM. Masquer
(A) Uric acid excretion in renal transplant recipients grafted with left or right kidney from the donor. Uric acid fractional excretion (FE) was similar in renal transplant recipients who received either a right or a left kidney. Results are shown ... Lire la suite
3.3. CT-scan analysis of kidneys in uric acid and calcium oxalate stone formers
To assess whether kidney structure would be related to uric acid stone formation, CT-scan were retrospectively analysed in 25 kidney stone formers affected by uric acid urolithiasis and 25 patients affected by calcium oxalate urolithiasis. The frequency of renal cysts was higher in patients affected by uric acid stones (68%) than in calcium oxalate stone formers (16%, p < 0.001). In uric acid stone formers, 9 had left renal cysts, 7 had bilateral cysts and 1 had right renal cysts (Figures 4(A)–(C)). Cysts were often multiple in affected kidneys. Two patients had left parapyelic cysts and 2 had bilateral parapyelic cysts. Among the 25 patients affected by calcium oxalate stones, only 2 had left renal cysts and 2 had right renal cysts. Kidney stones were evidenced in CT-scans but there was no evidence for close relationships between cysts and stones or chronic obstruction (Figures 4(A), (B)). None of these patients was affected by autosomal dominant polycystic kidney disease (ADPKD).
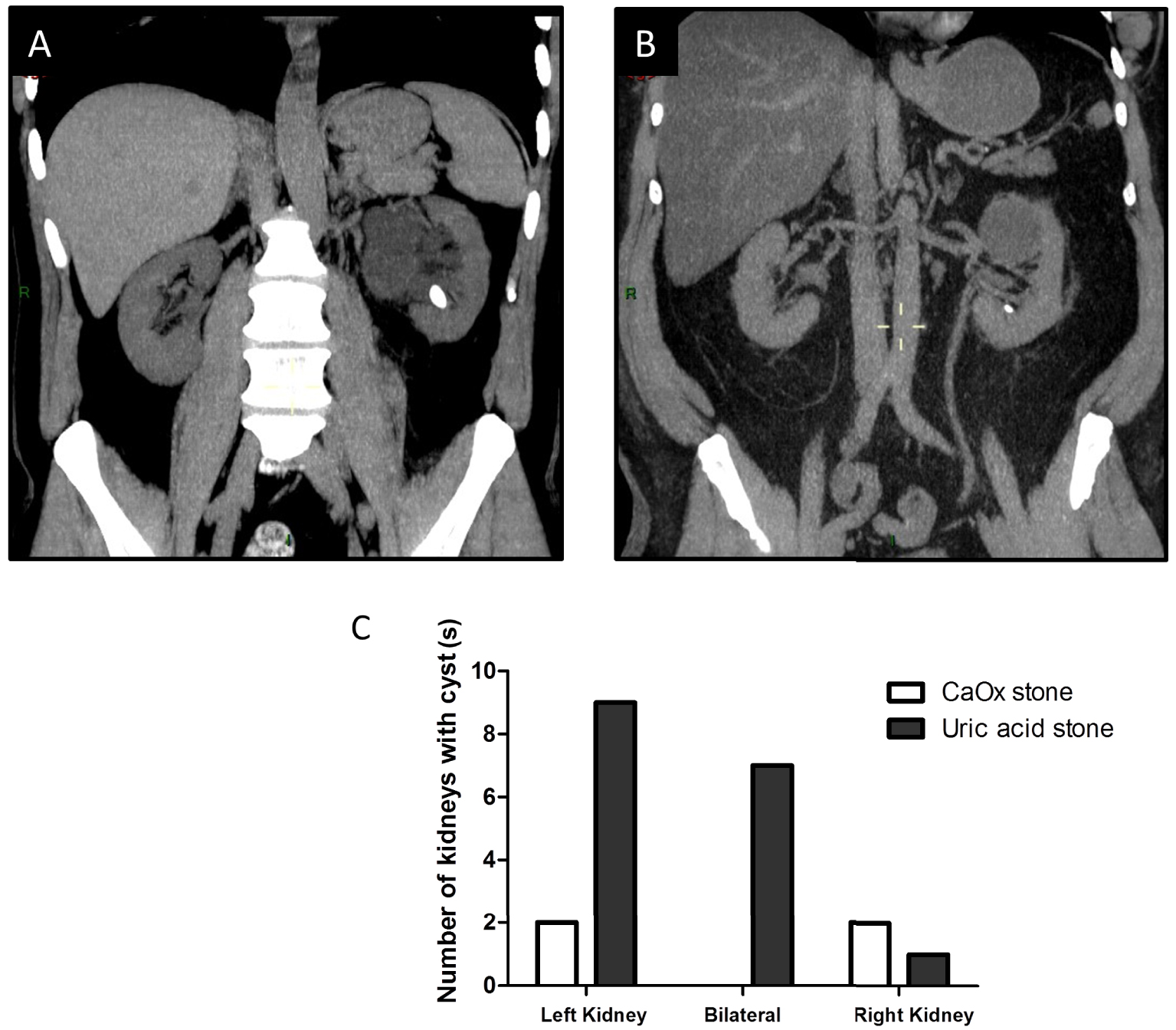
(A,B) Representative CT-scans from patients affected by renal cysts and uric acid stones. Patients were sometimes affected by (multiple) parapyelic cysts or isolated cysts without clear evidence of spatial relationships between stones and cysts. (C) Number of left, right or bilateral cysts in 25 uric acid stone formers (SF) and 25 calcium oxalate (CaOx) stone formers. We observed a dramatic number of left renal cysts in uric acid stone formers when compared to control CT-scans performed in 25 CaOx stone formers (p < 0.001). Masquer
(A,B) Representative CT-scans from patients affected by renal cysts and uric acid stones. Patients were sometimes affected by (multiple) parapyelic cysts or isolated cysts without clear evidence of spatial relationships between stones and cysts. (C) Number of left, right or ... Lire la suite
4. Discussion
Based upon a collection of 38,349 stones from adult stone formers, 3195 were made of uric acid (main component) and predominated in the left kidney. Although a moderate left/right lateralization was observed in calcium oxalate (1.09 and 1.11 for whewellite and weddellite as main component, respectively), brushite (1.17) and cystine (1.11) stone formers, the lateralization ratio was dramatic in uric acid stone formers (1.55, more than 61% in the left kidney). In addition, CT-scan analysis evidenced an association between uric acid stones and left renal cysts.
Previous studies evidenced a trend toward lateralization of kidney stones in the left kidney. For instance, Buck et al. have observed in 127 normocalciuric stone formers that 50.4% had left kidney stones and 41.7% right kidney stones, and in 148 hypercalciuric patients that 46.6% had left kidney stones and 40.6% right kidney stones [18]. Nevertheless, stone composition was not assessed and left lateralization was not statistically significant.
Uric acid crystals and kidney stones are promoted by (i) urinary uric acid concentration, due to low diuresis and/or increased uric acid excretion and (ii) low urine pH. Acid urine pH results mainly from decreased renal ammoniagenesis, a common setting in diabetes and metabolic syndrome, and from an increased acid load [7, 8, 9, 10, 11, 12, 13]. The increasing prevalence of obesity, metabolic syndrome and diabetes may explain why uric acid urolithiasis is relatively frequent in industrialized countries, resulting in about 10% of renal stones [4, 5, 6]. Our studies provide evidence for the first time to our knowledge that more than 60% of uric acid stones originate from the left kidney, a specific feature since other stones are equally distributed in both kidneys. Interestingly, the lateralization ratio was significantly lower in patients declared to be diabetic (1.26). Uric acid stones may form in both kidneys in patients affected by diabetes with low urine pH, whereas local factors may increase the risk of uric acid stone formation in the left kidney.
Based upon the analysis of urine biochemistry from the left or the right kidney collected in kidney stone formers, we did not find a lateralization of uric acid excretion or a lower pH in urine from the left kidney. Of notice, most of patients were affected by calcium oxalate stones, consistent with kidney stones epidemiology. In addition, left or right grafted kidneys do not differ statistically regarding uric acid excretion or urinary pH. Overall, these results rule out the hypothesis that kidney side would influence kidney physiology, at least in calcium oxalate stone formers and kidney donors.
Nevertheless, patients affected by uric acid stones are more frequently affected by renal cysts than renal calcium oxalate stone formers, particularly in the left kidney.
On the one hand, it has been reported that both hyperuricemia and high uric acid fractional excretion are independent “risk factors” for the presence of simple renal cysts [19]. The presence of renal cysts might be a consequence of hyperuricemia and/or increased uric acid excretion, potentially through the formation of urate crystals in renal tissue or uric acid crystals in renal tubules.
On the other hand, the presence of cysts could be responsible for uric acid crystal formation or aggregation. Kidney cysts are the ending product of tubular alterations resulting in tubule dilation and inverse cellular polarity associated with aberrant ionic transports [20]. The content of renal cysts was analyzed, revealing an increased uric acid and urea concentration in cystic fluid compared to plasmatic levels whereas electrolyte concentrations were similar [21]. We may therefore hypothesize that tubular cells undergoing cystic formation would be responsible for locally increased uric acid and/or increased proton excretion. Interestingly, the proportion of uric acid stones has been previously described to be high in patients affected by ADPKD, due to PKD1 or PKD2 genes mutation [22, 23]. A majority of ADPKD patients has a low urinary pH (<5.5), probably due to a defect in ammoniagenesis but the underlying mechanisms remain elusive [23, 24, 25]. It seems likely that low urinary pH in polycystic patients, in addition to the distorted renal structure, may enhance uric acid stone risk. It has been shown that Pkd1 ± mice on 129/Sv genetic background (which are not affected by renal cysts) develop increased uric acid excretion when compared to controls. Surprisingly, the nestin-Cre Pkd1-targeted cystic mice, on a c57bl/6 genetic background, do not have increased uric acid excretion [26]. The mechanisms underlying the role of Pkd1 in uric acid transport remain unknown. Another hypothesis is that renal distortion due to cysts may promote urine stasis and crystal formation, growth or aggregation. However, these hypotheses do not address why uric acid stones are lateralized on the left side.
Previous studies have shown that renal malformations are more often found on the left side, including renal agenesis/aplasia, renal ectopia, pelviureteral junction obstruction and non-obstructive non-refluxing megaureter [27]. Of notice, unilateral multicystic dysplastic kidneys are more frequently located on the left side [28]. It has been hypothesized that genes that are differentially expressed at both sides of the embryo during development would be involved in unilateral kidney malformations. Among these genes, those involved in the cilium function are particularly of interest since they are involved both in abnormal left–right body axis and cystic diseases. For instance, mutations of INVS causing nephronophtisis type 2 or mutations of BBS genes responsible for Bardet–Biedl Syndrome are related to renal cyst formation and, in some cases, random left–right axis specification resulting in situs inversus [29, 30]. Mice affected by nek8 or pkd2 loss develop cystic kidney disease and left–right asymetric defect [31]. We may hypothesize that the predominance of renal cysts in the left kidney would explain the lateralization of uric acid stones.
Our study suffers from limitations. It would be of interest to assess whether uric acid stone formers affected by isolated left cysts have an increased uric acid fractional excretion or lower urine pH in the left kidney. Although we analyzed lateralized uric acid excretion in a large number of patients, only 3 were affected by uric acid stones. Interestingly, left lateralization ratio of uric acid seemed increased in 2 of them but we did not collect data relative to kidney morphology in these uric acid stone formers and the number of patients was too low to draw any conclusion. To date, we have no proof that isolated cysts or nephrons undergoing cystic formation modify urine composition.
In conclusion, we describe that uric acid stones predominate on the left side and are frequently associated with left renal cysts. These observations deserve further studies to identify genes involved in left renal cysts and uric acid stone formation.
Conflicts of interest
The authors declare no conflict of interest.




 CC-BY 4.0
CC-BY 4.0