1. Introduction
Drug-induced nephrolithiasis is rarely discussed in medical literature except through isolated case reports. From an epidemiological point of view, only few data are available from laboratories that have analyzed urinary stones by physical methods such as infrared spectroscopy or X-ray powder diffraction [1, 2, 3]. In 1989, Jungers et al. have reported a 2% incidence of drug-induced urolithiasis [4]. Due to changes in drug availability with time, such incidence seems to be decreasing in the last decades [5]. Although it is uncommon, it is a seldom reported complication of the long-term and/or high-dose prescription of certain drugs with high renal excretion [5, 6]. Among these, the first drugs identified leading to nephrolithiasis and/or crystal nephropathy are sulfonamides [7]. Since the end of the 1930s, the first cases of kidney stones and renal failure related to the use of these products were reported, yet, widely prescribed for their antibiotic properties [7, 8, 9, 10]. Subsequently, the evolution of the pharmacopoeia with the development of more soluble sulfonamides gradually made the clinicians forget the renal risks associated with the crystallization of these products, even if some sporadic publications have described cases of urolithiasis or acute renal failure in patients treated with different sulfonamides antibiotics [11, 12, 13, 14, 15, 16]. For more than forty years, the presence of numerous drugs, namely foscarnet [17, 18, 19], atazanavir [20, 21], and cisplatin [22, 23] have been reported as causative of nephrolithiasis and/or crystalline nephropathy or have been identified in kidneys.
2. Main drugs involved in nephrolithiasis
2.1. Triamterene
At the end of the 1970s, nephrologists lost sight of sulfonamides, supplanted by a new molecule, triamterene, an antihypertensive of the pteridine family. In large studies conducted in the United States with laboratory analysis through physical methods such as X-ray diffraction or infrared spectrophotometry, it has been shown that 0.4% of all calculi analyzed by these laboratories were made, totally or partially, of triamterene metabolites [24, 25, 26, 27].
In Europe, and particularly in France, this drug has been identified with the same frequency as in the United States [28], which means that triamterene was the main cause of drug-induced stones in the world for nearly a decade, with also several reported cases of triamterene-induced crystal nephropathies [29, 30].
Studies conducted to identify risk factors for crystallization of triamterene [31] have shown less solubility in acidic urine vs alkaline urine and, above all, that low urine pH reduces its tubular reabsorption. Furthermore, the hepatic metabolism of triamterene led to the predominant formation of 4′-hydroxytriamterene sulfate with high urinary excretion (Figure 1). This metabolite was often abundant, sometimes predominant, and even almost pure in some stones [28, 32].
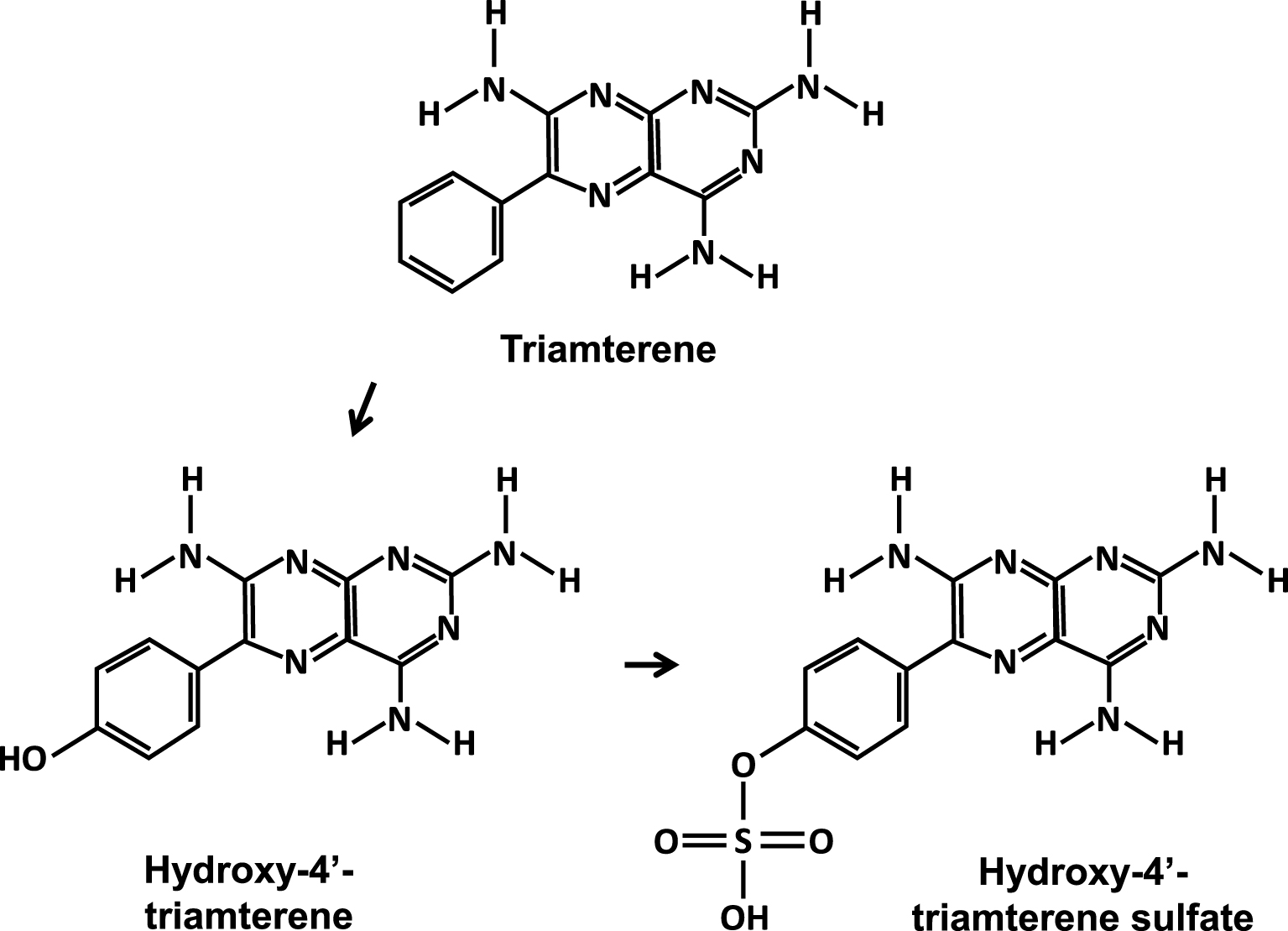
Triamterene and its major urinary metabolites.
2.2. Glafenin
Triamterene shared, at least in France, the first place among lithogenic drugs with another molecule, glafenin (not FDA approved, banned in early 1990s in Europe). It was a non-steroidal anti-inflammatory drug (NSAID) from the 4-amino-quinoline family which was widely prescribed by general practitioners, rheumatologists, and dentists because of its effective analgesic properties [33]. Several dozen cases of renal lithiasis (as well as biliary) were described between 1980 and 1993 [6, 34, 35, 36, 37, 38]. Glafenin-induced lithogenesis appeared to be related to the concomitant presence of bacteria in urine. Glafenin was metabolized by the liver and eliminated primarily by the kidney in the form of glucuronide glafenic acid, a soluble derivative without reason to crystallize. However, the stones were composed of free glafenic acid and, to a lesser extent, of free 4-hydroxy-glafenic acid, therefore unconjugated, and poorly soluble in urine (Figure 2).
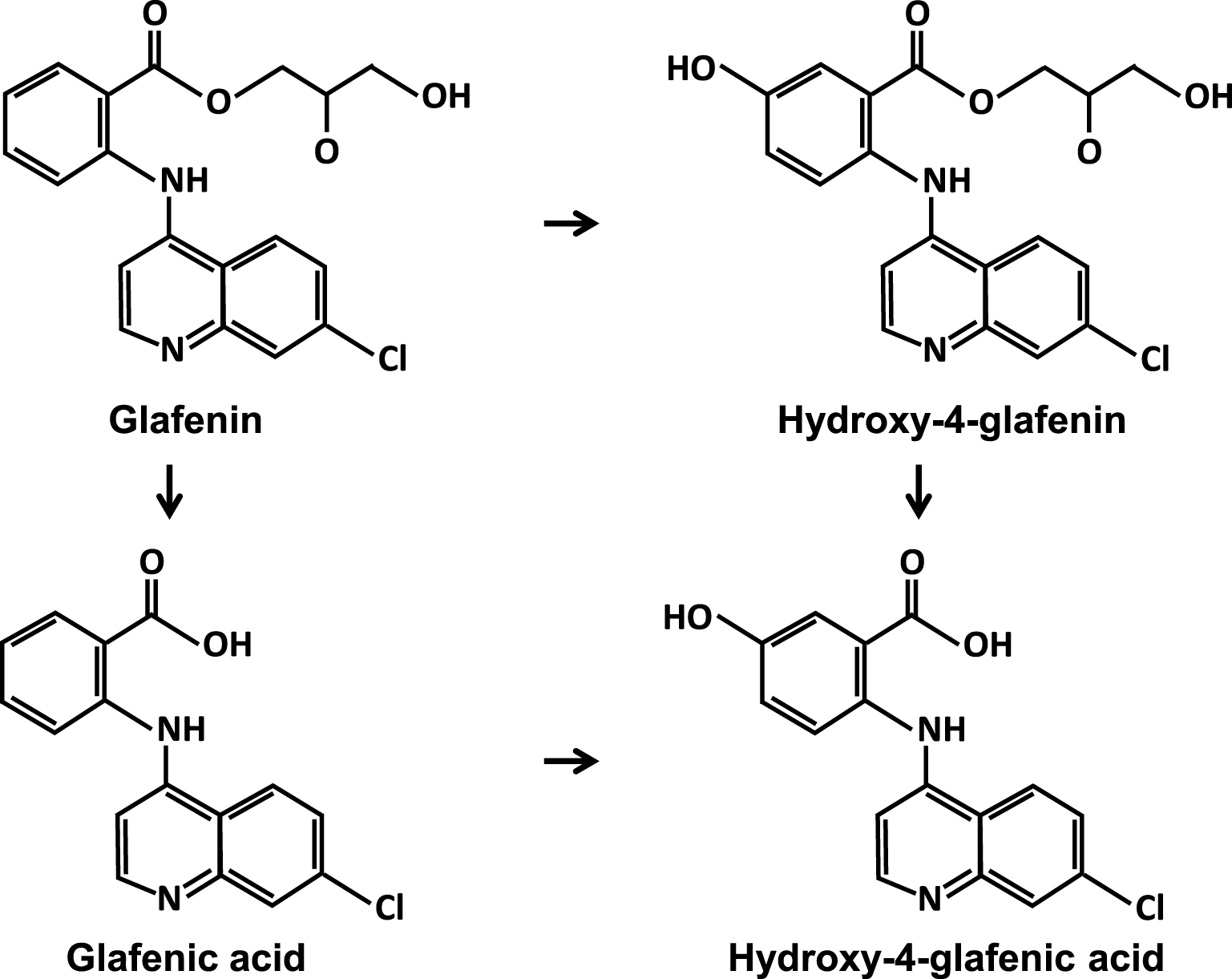
Glafenin and metabolites.
As the glucuronidation properties are important in the liver, ensuring detoxification of many substances, the hepatic origin of free glafenic acid was unlikely. Moreover, it is known that certain bacteria have very active beta-glucuronidases which have been shown to be implicated in bilirubin deconjugation, at the origin of certain pigment gallstones [39, 40]. More than 80% of patients who developed glafenic acid stones were found to have a clinically symptomatic urinary tract infection, which is quite unusual in drug-induced stones [5].
Identified species were mainly Escherichia coli, a species known to possess glucuronidases, and, more rarely, Proteus mirabilis which by its capacity to strongly increase the urinary pH, can saponify the glucuronide function.
Apart from renal lithiasis, perhaps linked to a particular environment, glafenin has been shown to be a nonexceptional cause of degradation of renal function, sometimes of crystalline origin, but above all for reasons of renal toxicity linked to immuno-allergic reactions [41, 42], which led to the publication of a special issue of the Presse Médicale journal in 1972 [43] and to the withdrawal of this molecule in 1992 after multiple cases, sometimes fatal, of anaphylactic shock and liver toxicity.
2.3. Protease inhibitors
In the 1990s came the protease inhibitors used in tri-therapies against HIV, first indinavir sulfate [44, 45, 46, 47, 48] then barely ten years later, atazanavir [49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60]. In both cases, nephrolithiasis was explained by the short drug half life and its high urinary excretion, leading within 90–120 min after intake, to a peak elimination of poorly soluble unchanged form. As for sulfonamides and other drugs able to crystallize in the urinary tract, nephrolithiasis [49, 50, 51] and/or crystalline nephropathy [52, 53, 54, 55, 56, 57, 58, 59, 60] cases were reported, new anti-retroviral drug protocols with the appearance of new molecules have led to a significant reduction in stones and drug-induced renal complications over the past five years.
2.4. Antibiotics
Certain molecules from antiseptics or antibiotics class, such as amoxicillin, ceftriaxone, ciprofloxacin or vancomycin, are still occasionally reported as drug nephrolithiasis [61, 62, 63, 64, 65, 66, 67] and more often responsible for crystalline-induced nephropathies [68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83]. It should be noted that ceftriaxone and ciprofloxacin crystallize in the form of salts, calcium for ceftriaxone, magnesium for ciprofloxacin, which suggests that the individual metabolic status of the patient may be involved in the crystallogenic risk of these drugs [84, 85].
2.5. Sulfadiazine
In the context of the opportunistic infections in AIDS patients [86], cases of lithiasis and acute renal failure reappeared in the early 1990s, induced by a first-generation sulfonylurea, sulfadiazine, the only one still currently used in clinical practice (Figure 3). It is still effective against severe forms of toxoplasmosis as the small size of the molecule allows it to cross the blood–brain barrier, but clinicians had forgotten how to avoid renal complications related to the high-dose prescription of this molecule, often several grams per day [87, 88, 89, 90, 91].
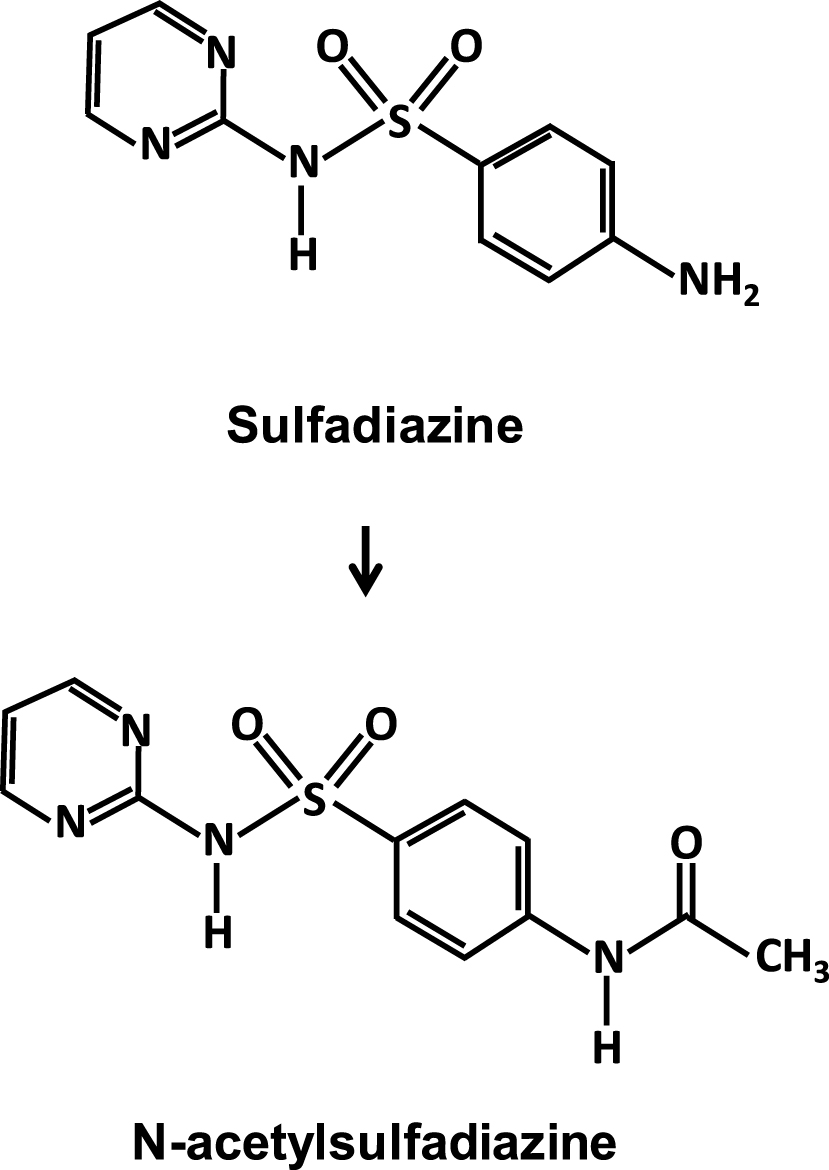
Molecular structure of sulfadiazine and N-acetylsulfadiazine identified in nephrolithiasis and crystal nephropathies.
Sulfadiazine leads to stone formation and crystalline-induced acute kidney injury due to the low solubility in acidic urine of the molecule and its main metabolite, N-acetylsulfadiazine, mainly excreted by the kidney. Not all sulfonamides used have this propensity to easily crystallize in urine, either because of a lower dosage, a better solubility, or finally because of their own metabolism limiting their urinary excretion.
3. Sulfasalazine and mesalazine
In this work we addressed the specific case of sulfasalazine. It is a sulfonylurea that has been used for several decades for the treatment of inflammatory bowel diseases, mainly in ulcerative colitis and Crohn’s disease [92]. It is also used to a lesser extent in the treatment of rheumatoid arthritis [93]. To our knowledge, this is the only drug which has led over the last thirty years to two different forms of nephrolithiasis and urinary crystallization, one concerning sulfapyridine, the other 5-aminosalicylic acid, two molecules resulting from the catabolism of sulfasalazine.
Sulfasalazine, also called salazosulfapyridine (Figure 4), is used per os at high doses as starting treatment (4–6 g/d) and at lower doses (2–3 g/d) for the long-term maintenance treatment.
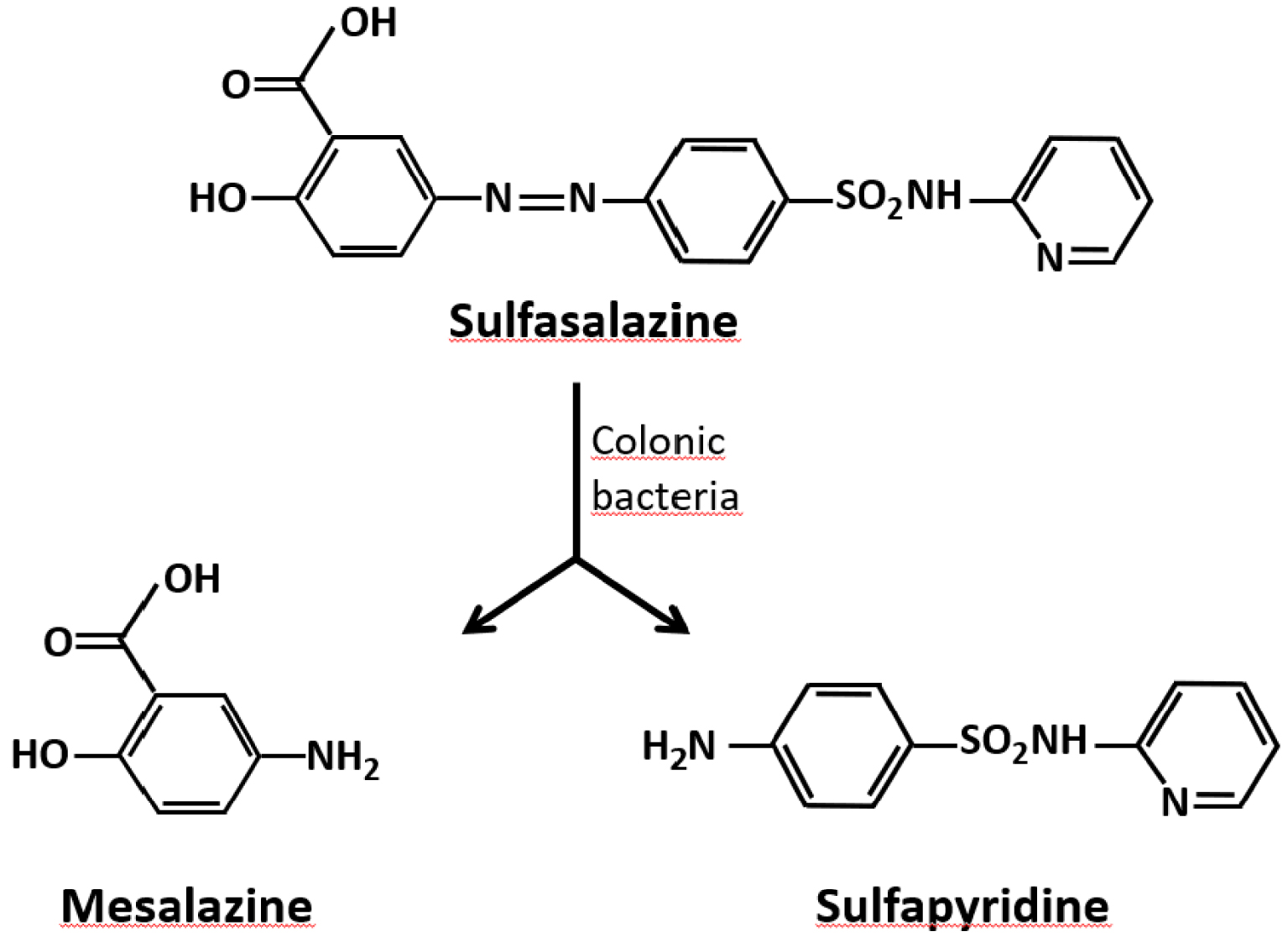
Simplified metabolism of sulfasalazine in the digestive tract.
About 20% of the ingested dose is absorbed through the digestive mucosa and metabolized in the liver. The peak serum concentration is reached between 3 to 12 h after taking the drug, depending on the individual. Sulfasalazine is essentially cleaved by the gut microbiota into sulfapyridine and 5-amino-salicylic acid (5-ASA, also named mesalamine or mesalazine), which constitute two active forms of the drug in the digestive mucosa (Figure 4).
The absorption of these metabolites is considered to be low, but variable depending on the individual and the condition of the intestinal mucosa. Sulfapyridine is absorbed much faster and importantly than mesalazine. Both of them can undergo hepatic acetylation, the speed and extent of which depend on the acetylation functions of the liver (with genetic and other drug intake influence) [94].
The N-acetylated derivatives (Figure 5) essentially are eliminated in the urine [95]. Due to their low solubility, they can lead to the formation of crystals and stones in the urinary tract. The use of sulfapyridine for its antiseptic properties in the 1940s had already led to cases of crystal nephropathies, including several fatal cases, observed in particular among certain Indian soldiers of the British army, after administration of doses of ⩾4 g/d, while most patients were hydrated and had alkaline urine [96, 97, 98, 99, 100]. Hypersensitivity related to the ethnicity of the subjects had been mentioned [101].
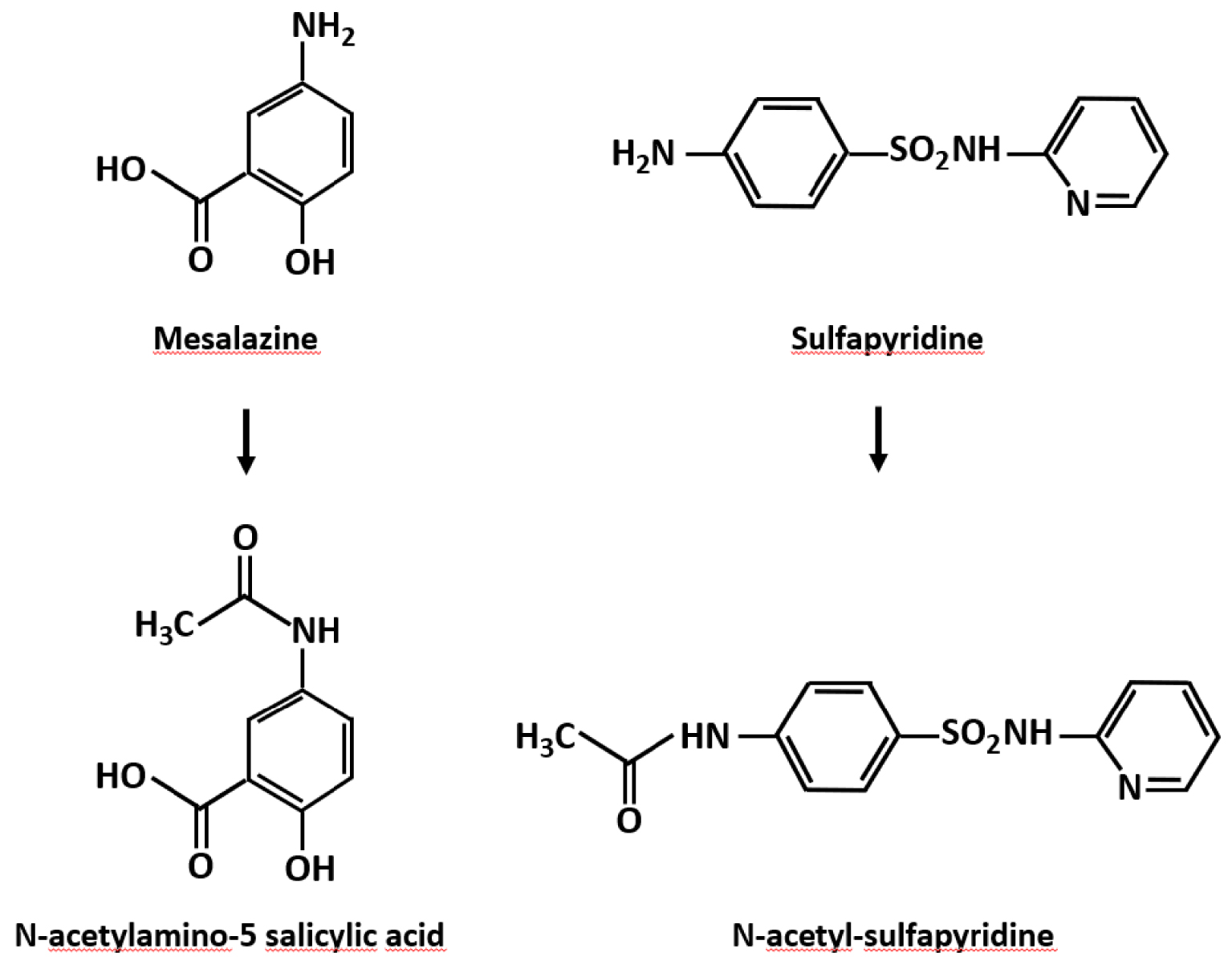
N-acetyl derivatives of sulfasalazine metabolites.
Forty years later, the use of sulfasalazine, transformed by the gut microbiota into sulfapyridine, corresponds to a slightly different context, but which does not exclude the complications initially reported for sulfapyridine. The cleavage of sulfasalazine by intestinal bacteria releases quantities of sulfapyridine, a part of which may be more or less rapidly absorbed and transported to the liver where it is transformed into an N-acetyl derivative which will then be mainly excreted in the urine. In fact, as early as 1993, a first case of N-acetylsulfapyridine lithiasis was described by Sillar and Kleinig [102] in a patient treated with sulfasalazine and the following year a new case of bilateral lithiasis composed of N-acetylsulfapyridine was reported by Erturk et al. [103]. Several other cases of urolithiasis were then reported [104, 105, 106, 107, 108] and also several cases of kidney injury resulting from crystal deposition in the parenchyma [105, 106, 109].
Following various studies on the mode of action of sulfasalazine, it quickly became clear that 5-ASA released by bacterial hydrolysis of sulfasalazine represented an interesting compound due to its lower digestive absorption and its longer action in situ. For this reason, 5-ASA, also called mesalazine, replaced sulfasalazine just over two decades ago. Considered to be the main active anti-inflammatory metabolite, mesalazine is effective in the treatment of ulcerative colitis and moderate forms of Crohn’s disease [110]. Its efficacy is considered to be proportional to its intraluminal concentration [111, 112]. It is used orally (tablets or granules) or rectally (suppositories or suspension) at doses of 2–4 g/d.
The absorption of mesalazine from the intestinal mucosa appears to be variable among individuals and slower than that of sulfapyridine. Overall, its absorption is estimated to be around 60% of the ingested dose, around 35% in the small intestine and 25% in the colon. The absorbed mesalazine is then metabolized by the liver and other tissues to the N-acetyl derivative which is mainly excreted in the urine [113]. In fact, several dozens of cases of tubulointerstitial nephritis during mesalazine treatment have been reported, but no case of lithiasis [114, 115]. The retained mechanism was cellular nephrotoxicity by analogy with aspirin and phenacetin [114]. The first cases of 5-ASA nephrolithiasis were described in 2013, one by Hasan and Tiselius [116], the other by Jacobsson et al. [117]. The first observation concerned a 32-year-old woman treated with mesalazine at a dose of 6 g/d for ulcerative colitis. The stones were spontaneously expelled. They were analyzed by the Herring laboratory in the USA that revealed they were composed of free mesalazine. Reducing the dose to 2 g/d with increased diuresis prevented recurrence. In the second case, it was also a 32-year-old woman treated during only six months with mesalazine at an unknown dosage. The patient expelled several stones identified as derivatives of mesalazine, without further indication. In 2018, a third case was reported in a 23-year-old woman treated for four years for ulcerative colitis with mesalazine at a dose of 4 g/d, plus local administration of 500 mg [118]. She presented with a left renal colic rapidly resolving with medical management. A computerized tomography without injection did not show any stone. No stones were recovered, but the following year, during a new renal colic, the patient expelled small orange-colored stones of which infrared analysis revealed to be composed of mesalazine. The following year, Simsek and de Boer described the case of a 33-year-old woman receiving 4 g/d of mesalazine for ulcerative colitis, who began to spontaneously expel stones after six months of treatment while she had no history of nephrolithiasis [119]. The calculi were composed of 85% mesalazine without specifying the other possible compounds. On the occasion of this case report, the authors recall that 48 cases of urolithiasis under treatment with mesalazine were the subject of reports to the European Pharmacovigilance Commission (Eudra Vigilance), but that only the four cases reported above have demonstrated the presence of the drug in the stones. Two other cases were reported in 2020 by Vilchez et al. in subjects treated over several years with 4 g/d of mesalazine [120]. Finally, data from FDA reports updated in March 2021 implied 64 cases of kidney stones while patients were treated with mesalazine [121] with no detailed information regarding stone composition.
4. Case presentation
A series of cases is presented here, of ten patients with mesalazine kidney stones, collected between 2014 to 2020 (8 women and 2 men), being the biggest case-series to date. They were treated either for ulcerative colitis or for Crohn’s disease with daily doses of mesalazine between 2 g (n = 1) and 4 g (n = 9). The data for each patient are summarized in Table 1.
The average age was 34.8 years, the disease had been progressing for 4.5 years without flare-up while on treatment. All patients had normal kidney function. Patients had been taking the treatment for four years on average before the first stone event. None of them had previous history of nephrolithiasis, most of the calculi were spontaneously passed, and six of the ten patients stopped the treatment due to this side effect.
All stones and crystals were identified as pure mesalazine by Fourier transform infrared (FTIR) spectroscopy or X-ray diffraction. The infrared spectrum is presented in Figure 6.
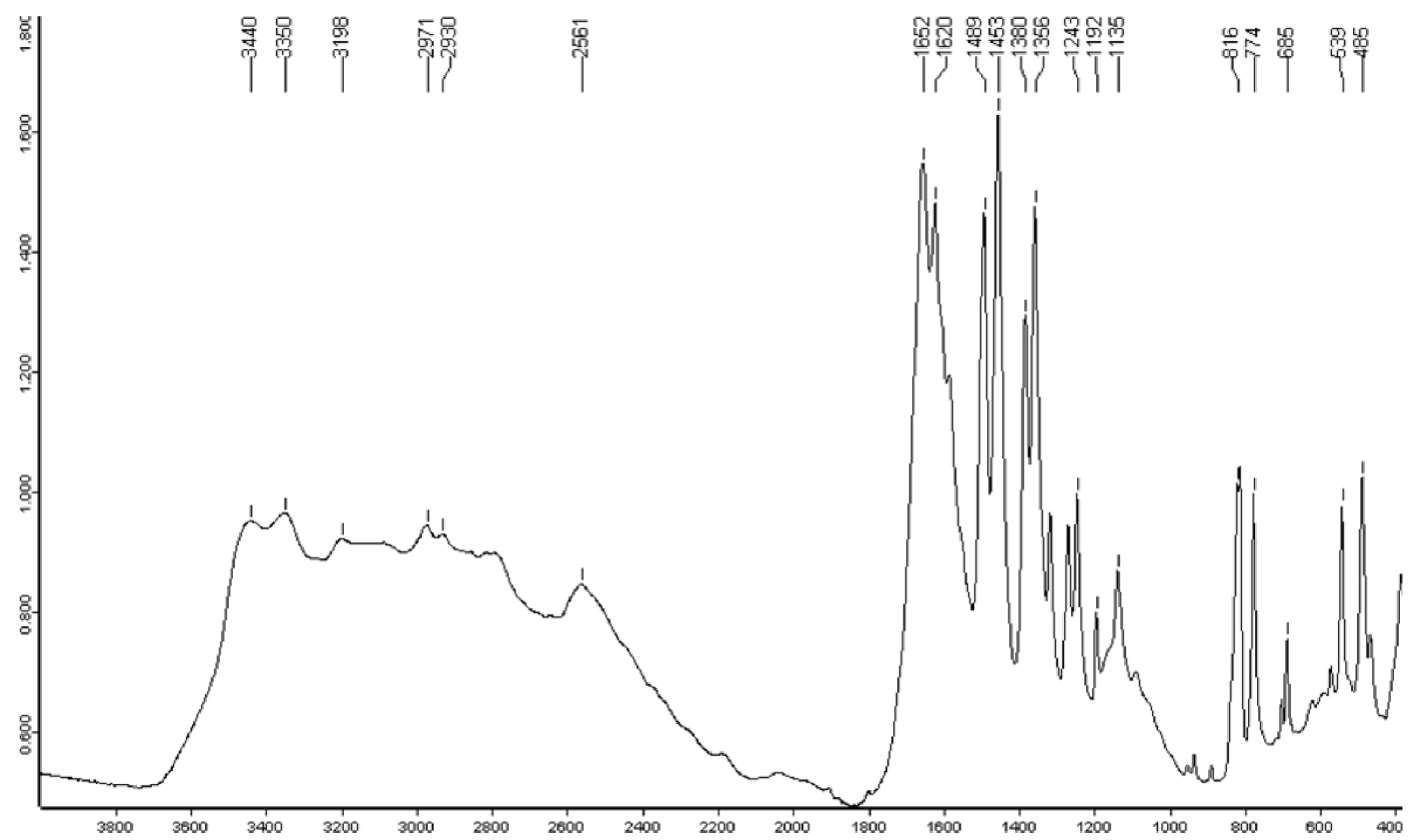
Infrared spectrum of mesalazine (5-aminosalicylic acid) stone. Y -axis: infrared absorption intensity; X-axis: wavelength (cm−1).
Patient characteristics
| Patient characteristics | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex category | Female | Female | Female | Female | Male | Female | Female | Female | Female | Male |
| Age (years old) | 29 | 50 | 29 | 44 | 30 | 24 | 38 | 33 | 60 | 48 |
| Pathology | UC1 | CD2 | UC | CD | UC | UC & CD | CD | CD | UC | UC |
| Number of year of disease progression (years) | 1 | 15 | 6 | 7 Mo3 | 4 | 3 | 2 | 15 | 12 | 4 Mo3 |
| Nature of material identified | Stone | Stone | Stone | Stone | Stone | Stone∗+ Crystalluria | Stone | Stone | Stone | Stone |
| History of previous nephrolithiasis under 5-ASA | No | No | No | No | No | No | No | Yes | Yes | No |
| Posology of medication (g/d) | 4 | 4 | 4 | 4 | 2 | 4 | 4 | 4 | 4 | 4 |
| Route of admission | po4 | po | po | po | po | po | po | po | po | po |
| Duration of medication intake before stone (years) | 1 | 2 | 6 | 0.5 | 1 Mo3 | 3 | 0.5 | 15 | 12 | 4 Mo3 |
| Stone treatment | Spont5 | JJ stent | Spont | Spont | Spont | Spont | Spont | Spont | Spont | Spont |
| Treatment discontinuation | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | No |
| Stone recurrence | No | No | Yes | No | No | No | No | No | No | No |
| Other lithogenic drugs | No | No | No | No | No | No | No | No | No | No |
Legend: Ulcerative colitis1, Crohn’s disease2, Months3, Per OS4, Spontaneous emission5, Stone not available for analysis∗.
The stones had a particular morphology, with a rough orange- or pink-colored surface. The section was identical to the surface without detectable organization. For one of the patients, the stone could not be recovered, but the examination of the urine revealed the presence of needle-shaped or rod-shaped crystals, more or less aggregated, whose infrared spectrum after centrifugation and drying of the sediment revealed that they were made from pure mesalazine (Figure 7).
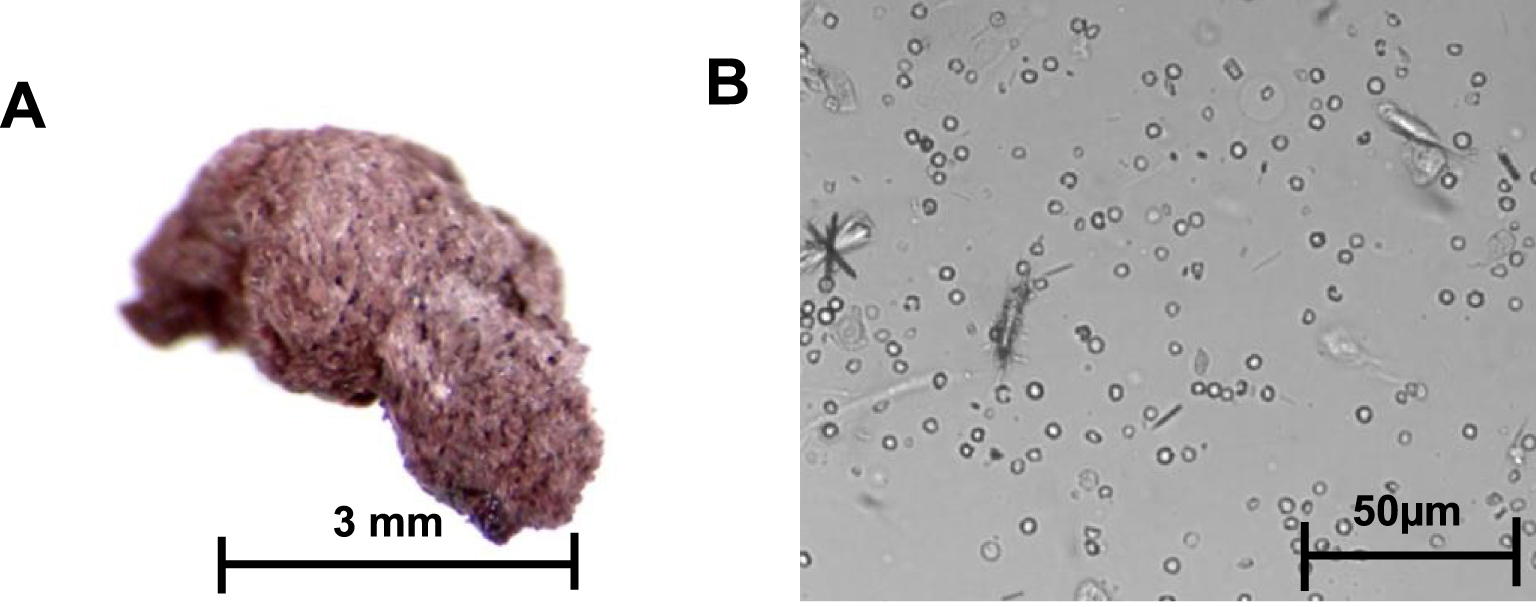
A. Mesalazine stone; B. Mesalazine crystalluria, original magnification: 400×.
5. Discussion
From a general point of view, drugs may be divided into two different categories according to the mechanism involved in calculi formation [5, 122]. The first one includes poorly soluble drugs that favor crystallization and calculi formation. The second category includes drugs that enhance calculi formation through their metabolic effects (loop diuretics, carbonic anhydrase inhibitors, laxatives).
In the case of mesalazine, only the unmetabolized drug was clearly identified in urinary stones and crystals. Although no study has been carried out to date to verify it and understand its mechanisms, it can be thought that the formation of urinary stones composed of mesalazine is preceded by a digestive absorption of this compound greater than in other subjects, may be due to a peculiar digestive environment or metabolism.
Furthermore, patients with inflammatory bowel disease suffer from chronic hydro electrolytic losses, increasing therefore the urinary concentration of the drug above its solubility product, thus favoring its crystallization. Moreover, due to the more rapid urinary excretion (approximately 1 h) [123] of nonmetabolized mesalazine by comparison to that of N-acetyl derivative, one would expect, as it had been observed for protease inhibitors [5] at a peak excretion within two hours of absorption. In addition, in low acetylators for genetic reasons or because of multiple drugs therapy, it is reasonable to expect higher mesalazine concentrations in plasma and urine, with an increased risk of crystallization in urine.
In patients with normal renal function, the plasma concentration of mesalazine can reach 2–3 μg/mL [108], which suggests an urinary excretion of 20–30 mg within 60–120 min after absorption of the product. In patients with low urine output (1 L/d, or 40 mL/h), a urine concentration of 600–650 mg/L can be predicted and it can probably be higher in subjects with very low urine output. Knowing that the urinary solubility of mesalazine is approximately 800 mg/L [112], it is quite possible that, if the circumstances are favorable (higher dosage, increased digestive absorption, less hepatic N-acetylation, low diuresis, urine pH < 6), mesalazine supersaturation can lead to crystallization in the kidneys and urinary tract.
Renal toxicity of 5-ASA has been well documented, yet, none of our patients experienced renal impairment. Perhaps, as recently reported for vancomycin, mesalazine-related kidney injury could be, at least in part, related to crystallization of the drug [71]. To prevent such consequences, it could be relevant to increase water intake associated to the drug intake and within 2 h after, as has been shown to be efficacious for preventing stones with indinavir [6].
Of note, among our 9 cases of mesalazine stones, all were made of pure mesalazine. No metabolic compound neither acetylated metabolites were detected in the stones by FTIR spectroscopy. Another remarkable point of our small cohort of patients is the very high gender ratio F/M, which was equal to 4.0 while the sex ratio F/M for common stone disease is about 0.5 or less in western countries. However, it seems that women are slightly more exposed than men to inflammatory bowel diseases, especially for Crohn’s disease. A sex ratio F/M was reported ranging from 1.1 to 1.35 for ulcerative colitis and from 1.18 to 1.65 for Crohn’s disease [124, 125, 126, 127], i.e., a female to male ratio significantly lower than in our series of stone cases. Such a finding suggests a special sensitivity of women to the risk of forming mesalazine stones. However, it was not reported that women have a mesalazine metabolism significantly different from that observed in men. Further studies are required to explain such difference between genders.
6. Conclusion
Drug-containing stones are infrequent and result in most cases from specific characteristics of the drug, i.e., high dosage, high urine excretion, low solubility of the drug or its metabolites. Regarding mesalazine, the occurrence of urine crystallization could be underestimated. Several factors may be involved in stone formation: higher absorption of mesalazine by the gut, lower acetylation of the drug by the liver, lower urine volume than in patients free of stones, all of which deserve further investigations.




 CC-BY 4.0
CC-BY 4.0
Vous devez vous connecter pour continuer.
S'authentifier