1 The hydroxymercuration–hydrodemercuration sequences
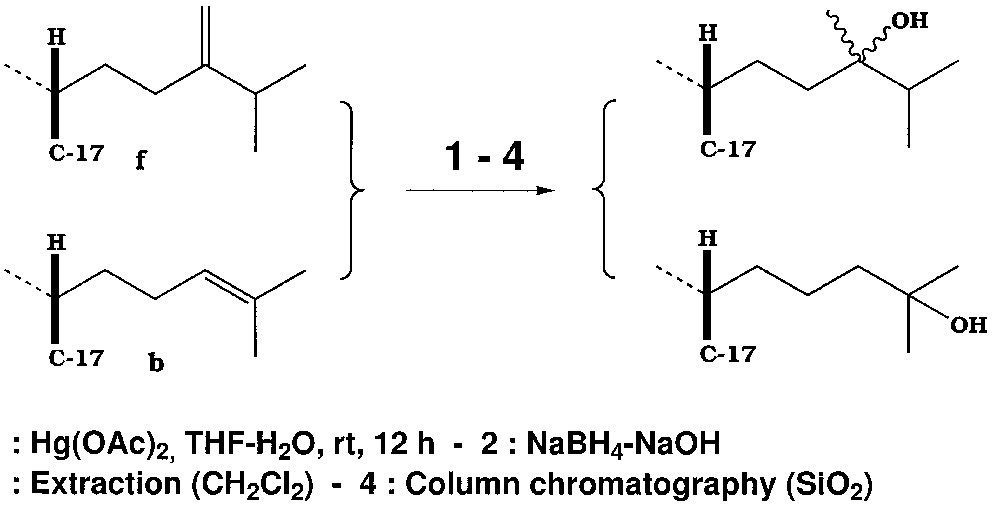
The reaction of mercuric acetate with unsaturated substrates [1, 2] leads to the addition on the double bond of the groups –OH on one side, and –HgOAc on the other. It can be followed by an acid hydrolysis or by the easy reduction of the C–Hg bond with sodium borohydride in sodium hydroxide/water [1], to yield the alcohol corresponding to a Markovnikov addition of water on the double bond; by working in methanol, one obtains the corresponding methoxy derivative. These sequences have been studied on a wide variety of sterically hindered simple olefins (increased substitution on the double bond). The only case of complete inertness so far found was that of trans-di-t-butylethylene [3–5]; the reaction has been made even more selective by the use of mercuric pivalate [6]. The action of ultrasound has also been studied [6].
On more complex substrates, already in 1971, we had observed that desmosterol (24-dehydrocholesterol) 7b, both trisubstituted double bonds of which react with other electrophilic reagents, gives quantitatively, after treatment with mercuric acetate followed by NaBH4, 25-hydroxy-cholesterol [7, 8]; the nuclear Δ5 double bond, which is quite reactive towards most electrophilic reagents, was left untouched. This remarkable selectivity has been confirmed in 1992, and made use of to prepare a 25-hydroxysterol [9], but this study has not been extended. (A reason for the small number of studies involving mercuric acetate is obviously the poisonous nature of the reagent and of the products of the reaction. Care must be taken, even when working with small amounts, during the reaction and for the disposal of the residues. One of the authors can bear witness of the spectacular – but temporary and not personal – consequences of insufficient care.)
2 The reactivity of sterols towards oxymercuration
We have now investigated the reactivity of a wide variety of substances, all closely related to cholesterol, with double bonds differently located and substituted (Fig. 1), and treated them according to the general scheme summarised in Fig. 2.
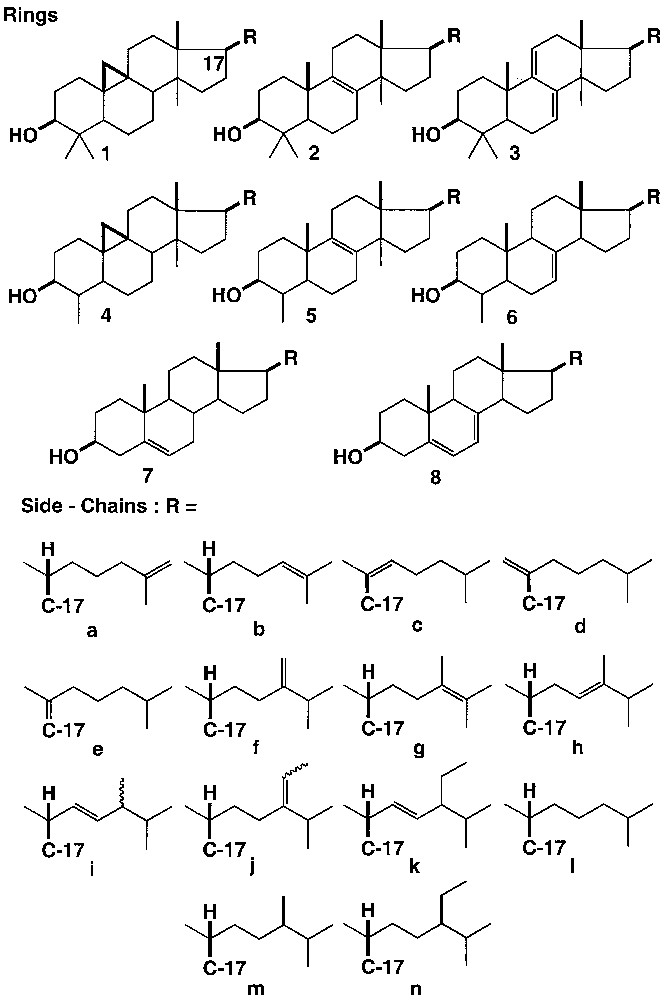
Any of the tetracyclic ring moieties 1–8 can in principle be associated with any of the side-chains a–n. We have tested the following substrates:
– 4,4-dimethyl sterols: 1b, cycloartenol; 1l, cycloartanol; 1f: 24-methylene cycloartanol; 2b: lanosterol; 2f, 24-methylene lanostenol; 2l, lanostenol; 3b, agnosterol; 3l, 24-dihydroagnosterol;
– 4-methylsterols: 4f, cycloeucalenol; 4j (E), cyclofuntumienol, 5b, 31-norlanosterol; 6b, 24-dehydrolophenol;
– sterols:7a, 25-dehydrocholesterol; 7b, desmosterol; 7c, 20(22)dehydrocholesterol (E) + (Z); 7c, 20(22)dehydrocholesterol (E) + (Z); 7d, 20(21)dehydrocholesterol; 7e, 17(20)dehydrocholesterol; 7f, 24-methylene cholesterol; 7g, ergosta-5, 23-dienol; 7h, ergosta-5, 24-dienol; 7i, brassicasterol; 7j (E), fucosterol; 7j (Z), isofucosterol; 7k, stigmasterol; 7l, cholesterol; 7m, campesterol; 7n, sitosterol; 8l, 7-dehydrocholesterol; 8i, ergosterol.
Example of treatment of a sterol mixture.
The substrates used vary in their ring structures (Fig. 1, Rings) and/or in the nature of their side-chain (Fig. 1, Side chains).
They have been isolated over the years from various plant sources: leaves, barks, and mostly fats from seeds of African plants, collected during the activity of one of us (G.C.) in the University of Yaoundé (Cameroon). Each unsaponifiable fraction was first easily separated by chromatography on alumina or silica into three fractions of different polarities: 4,4-dimethylsterols, 4-methylsterols, and sterols proper, by order of elution. Each one of these fractions was further analysed by the usual analytical methods (TLC, GC, column chromatography on AgNO3-impregnated silica gel, HPLC), and characterised by NMR and MS. 24-Methylenecycloartanol 1f and cycloeucalenol 4f were isolated from the heartwood of ‘tali’ (Erythrophlœum guinense, Cæsalpiniæ); agnosterol 3b was obtained from diepoxy-8,24-lanostanol, and other sterols were obtained from Funtumia elastica [10] and Holarrhena sp. (Apocynaceæ). To widen the range of substrates studied, we have furthermore prepared some sterol mixtures by dehydration of 20-hydroxycholesterol [11] and of (24R,S)24-hydroxy-24-methyl-cholesterol [12]. Finally, we have also used commercially available sterols: cholesterol, 7-dehydrocholesterol, ergosterol, stigmasterol, ‘lanosterol’ (containing 24-dihydrolanosterol, see below), and reference products isolated during our own earlier research [13, 15–20]. The disubstituted double bond of cholest-2-ene reacts to give a mixture of 2β- and 3β-hydroxy cholestanes [14]. Some 30 closely related sterols were thus available for testing (Fig. 1).
The oxymercuration–hydrodemercuration sequence can also be run on complex mixtures, which leads to a considerable simplification in the isolation of pure components. For instance, an extract of Holarrhena sp. leaves (1 g) contained at least seven different sterols (GC: 7b, 7f, 7j, 7k, 7l, 7m, 7n); after oxymercuration–hydrodemercuration, the mixture produced was chromatographed over silica gel, with hexane-ethyl acetate, and gave first five unreactive sterols (450 mg, GC, 7j, 7k, 7l, 7m, 7n), then 24R,S 24-hydroxy-24-methylcholesterol (150 mg) coming from 24-methylenecholesterol (7f), and finally 25-hydroxycholesterol (205 mg) coming from desmosterol 7b. Similarly, from 10 g of a mixture of sterols isolated from seeds of Funtumia elastica, we obtained 4.5 g of 25-hydroxycholesterol [7].
3 Conclusions
It is rather surprising that the double bonds located on the ring system of naturally occurring mixtures of sterols or tetracyclic triterpenes, while they are quite reactive towards other electrophilic reagents like bromine or peracids, are inert towards mercuric acetate. This shows the higher steric requirements of the last reagent. One can also remark that the same selectivity accrues for the enzymatic systems involved in the reduction or the alkylation of the side-chain of the tetracyclic triterpene precursors of sterols, which leave intact the ring double bonds.
Appendix Procedure for the preparation of pure lanostenol and 25-hydroxylanosterol
A commercial sample of ‘lanosterol’ (Sigma, 1 g) containing about 40% of 24-dihydrolanosterol (lanostenol) (GC) was dissolved in THF (10 ml). A solution of mercuric acetate (1.658 g) in water (10 ml) was added and the mixture was stirred at room temperature during 10 h. A 0.5-M NaBH4 solution in 3-M sodium hydroxide (10 ml) was added. After extraction with chloroform and chromatography on silica gel, the unreacted lanostenyl acetate was isolated (0.36 g). Further elution with hexane (95)-Et2O (5) eluted the pure 25-hydroxylanosterol (0.54 g, 1H NMR).



