1 Introduction
Lanthanides-based molecular catalysis has been steadily increasing in the last few years (e.g. see the 2002 special issue of Chemical Reviews, vol. 102, no. 6), and particularly in the field of polymerisation reactions [1]. Between the classical metallocenes and the more recent post-metallocenes developed for this specific area, the hemi-metallocene – also called “half-sandwich” – framework has attracted much less attention, despite spectacular catalytic abilities [2–4]. The major reason is that the hemi-lanthanidocenes have been until now rather difficult to prepare [5], undergoing comproportionation reactions, especially with the larger elements of the series [2,6], and even in the presence of a bulky cyclopentadienyl ligand [7,8].
Two general strategies have been employed until now to prepare half-sandwich lanthanide complexes: ionic or σ-bond metathesis [2]. Using the former method, we have shown earlier that half-sandwich lanthanide compounds of formula (C5Me4nPr)Ln(BH4)2(THF)n (Ln = Nd, n = 2; Ln = Sm, n = 1) could be isolated in the borohydride series, taking advantage of the bridging ability of the BH4 group. However, desolvation and clustering were observed, leading to the formation of hexamers [(C5Me4nPr)Ln(BH4)2]6 as the crystalline form [9]. On the other hand, the σ-bond metathesis method allows the straightforward formation of the expected product, but it requires highly sensitive homoleptic LnR3 precursors (R = alkyl [2], allyl [10], phenyl [11], or amido group [12]) [13]. Moreover, owing to the basicity of the R group, heating is often necessary to achieve the metathesis reaction, and ligand scrambling may be not completely excluded [5,11]. Ln(BH4)3(THF)3 are common lanthanide compounds, they are nowadays recognized as efficient precursors for organolanthanides syntheses [14], and we expected that they could be involved for σ-bond metathesis to produce C5Me5-supported compounds.
In this paper we describe the synthesis and characterisation, including X-ray structure, of a new ionic half-sandwich of neodymium, prepared according to a direct in situ σ-bond metathesis involving Nd(BH4)3(THF)3/BEM (butylethylmagnesium) and C5Me5H as starting materials. The isolated complex has the same efficiency as its neutral homologue – whose synthesis requires by contrast the previous preparation of KC5Me5 – for the controlled polymerisation of isoprene. Varying the nature of the cyclopentadiene allows us to evaluate very easily the impact of the Cp ligand upon the catalytic ability, without necessarily needing to isolate the related monocyclopentadienyl complex.
2 Results and discussion
2.1 Synthesis of complex 2
First attempts of a direct reaction between Nd(BH4)3(THF)3 (1) and C5Me5H were made, but failed, even after a prolonged reaction time (1H NMR monitoring). On the other hand, we recently established that Ln–alkyl bonds are readily obtained from the reaction of a [Ln]–(BH4) moiety with an alkylating agent, enabling the preparation of highly efficient catalytic systems for the polymerisation of non-polar monomers [3b,14–17]. Following this idea, a solution of BEM (nBuEtMg) was added to a 1:1 toluene mixture of C5Me5H and 1 at room temperature. A clean reaction took place within a few minutes, affording a compound consisting of one C5Me5 ligand for three BH4 groups whereas no precipitation of the expected Mg(BH4)2 occurred (Scheme 1).1
After slow concentration of the toluene solution, light blue single crystals could be isolated from gentle evaporation of the toluene solution. X-ray structure determination allowed us to establish [(C5Me5)Nd(BH4)3]2[Mg(THF)6] (2) as molecular formula for the isolated complex.2 It must be emphasized that this unprecedented synthetic method, that we call the “borohydride/alkyl route”, requires very mild experimental conditions and ordinary lanthanides precursors.
2.2 X-ray structure of [(C5Me5)Nd(BH4)3]2[Mg(THF)6] (2)
Complex 2 is a trinuclear ionic compound comprising two anionic half-neodymocene trisborohydride [(C5Me5)Nd(BH4)3]− moieties and one cationic hexa-THF magnesium [Mg(THF)6]2+ adduct (Fig. 1) that alternate in the unit cell without direct cation–anion interaction. The asymmetric unit contains two slightly different trimetallic Nd/Mg/Nd entities. The Nd anions have a pseudotetrahedral tri-legged piano-stool geometry. It is noteworthy that the Nd–B distances (six coordination) fall in a narrow range of 2.582(5)–2.586(5) Å, typical of monomeric borohydrido complexes bearing a tridentate Nd–(η3-H)3B–H terminal group [14,18]. All hydrogens belonging to borohydride groups could be located; geometric parameters (B–H, Nd–H distances and B–H–Nd angles) confirm the η3-mode, likely distorted, however [16,19]. The Mg cation exhibits the octahedral geometry with oxygen atoms of THF molecules. The absence of coordinated THF to the neodymium atom points out the higher affinity of the lanthanide toward ionic ligands.
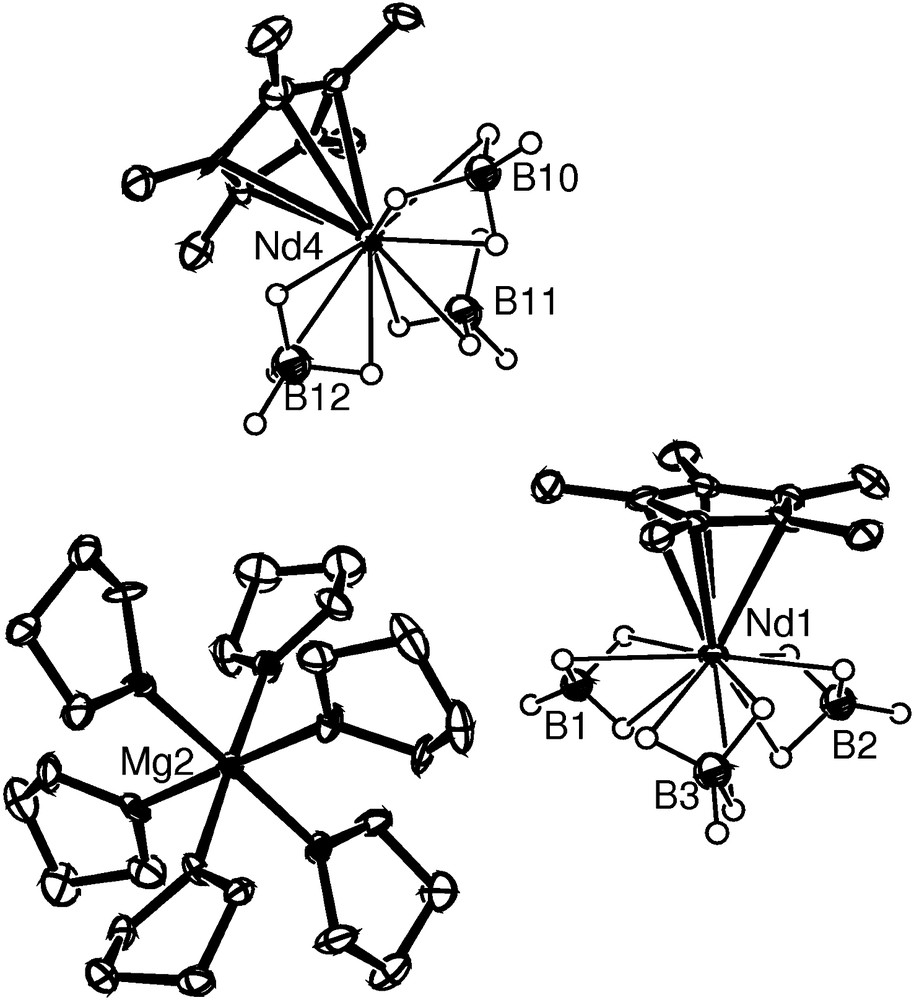
ORTEP structure of one of the two entities of the asymmetric unit showing the molecular structure of 2 (thermal ellipsoids at the 30% level; non-BH4 hydrogen atoms omitted for clarity).
Though structurally characterized molecular CpRLnX3− (CpR is a substituted cyclopentadienyl ligand) species have been observed occasionally [10,20], the borohydride anionic [(C5Me5)Nd(BH4)3]− is singular. Moreover, we checked (1H NMR, C6D6) that a solution of 2 prepared in our one-pot procedure remained unchanged after 20 h at 75 °C1. Thus, (C5Me5)LnX3− appears as a stable molecular entity in the borohydride series, with respect to comproportionation or clustering. The ionic trinuclear structure of compound 2 is comparable to that of [Mg(THF)6][Nd(allyl)4]2(2THF), obtained by ionic metathesis, with discrete [Mg(THF)6]2+cation and allyl neodymate anions [21].
2.3 Isoprene polymerisation
Precatalyst 2 combined with 1 equiv of BEM afforded a very efficient initiator toward isoprene polymerisation (Table 1), whose catalytic behaviour was fully comparable to the one obtained from neutral (C5Me5)Nd(BH4)2(THF)2 (3) with 1 equiv of BEM using a procedure that we previously described [3b]. As can be seen from the Mn values, such polymerisation process was found to be “living” with one growing chain per Nd.
Isoprene polymerisation with (borohydrido half-neodymocene/BEM) catalysts
| Catalytic systema | Yield [%] | Rate of trans-PI [%]c | Mnd | Mw/Mn | Mn(calc.)e |
| 2/1 BEM | 84 | 98.2 | 58 200 | 1.16 | 56 000 |
| 2′b/1 BEM | 68 | 98.0 | 46 500 | 1.16 | 48 800 |
| 3/1 BEM | 80 | 97.4 | 52 300 | 1.18 | 53 700 |
| 4′b/1 BEM | 57 | 96.7 | 31 200 | 1.41 | 37 300 |
| 5′b/1 BEM | 60f | 91.4 | 25 300 | 1.90 | 40 100 |
| 1/1 BEMg | 87 | 95.5 | 58 200 | 1.35 | 52 100 |
a Conditions: 10 μmol Nd, 1 mL toluene, [monomer]/[Nd] = 1000, T = 50 °C, t = 2 h.
b In situ prepared precatalyst Nd(BH4)3(THF)3/HCpR/0.5 BEM, 2′: CpR = C5Me5, 4′: CpR = 1,2,4-Ph3C5H2, 5′: CpR = C5H5.
c Determined by both 1H and 13C NMR integrations.
d Determined by Steric Exclusion Chromatography calibrated with PS standards.
e ([monomer]/[Nd]) × 68 × (yield%).
f t = 24 h.
g See Ref. [25].
The “living” character of the latter catalyst is confirmed by a double-feed monomer experiment, showing the typical GPC profiles (Fig. 2) [22].
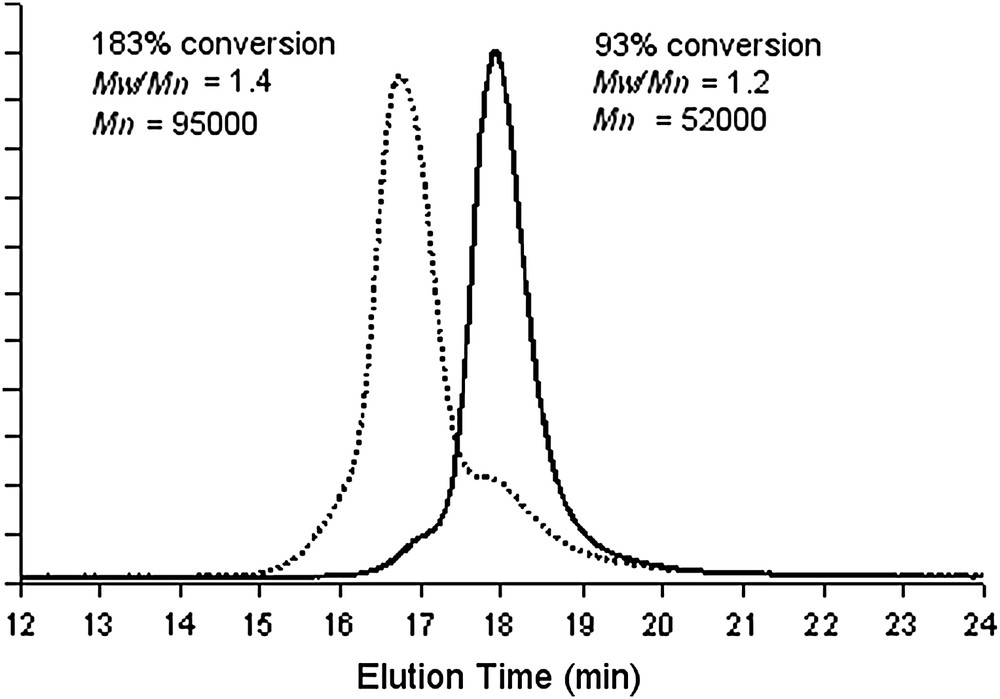
GPC profiles before (plain line) and after (dot line) the second feed of isoprene obtained with 3/1 BEM catalytic system.
Ultimately, we found that a mixture made of 1, C5Me5H, and BEM in the 1/1/1.5 ratio shows a very similar reactivity toward isoprene. In that latter case, it is likely that (i) a half-sandwich compound forms in solution (precatalyst 2′, Table 1) from the reaction between 1, C5Me5H, and 0.5 equiv of BEM, (ii) the subsequent combination of this precatalyst with the residual equivalent of BEM affords the catalytic species (Scheme 2). The somewhat lower activity of this catalytic system is tentatively ascribed to the number of THF molecules initially coordinated to the neodymium atom.
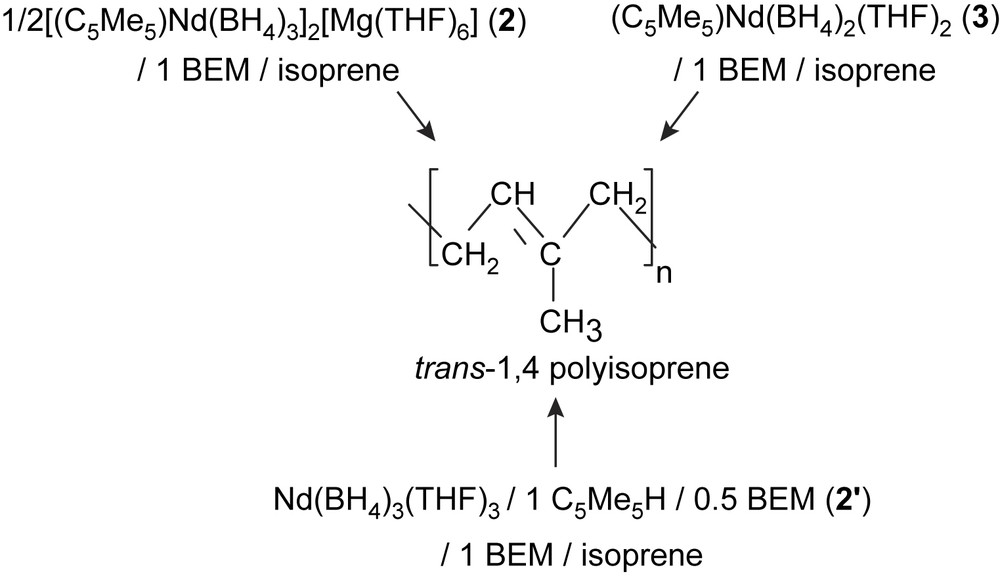
Synthesis of trans-polyisoprene from 2, 2′ and 3, in combination with BEM.
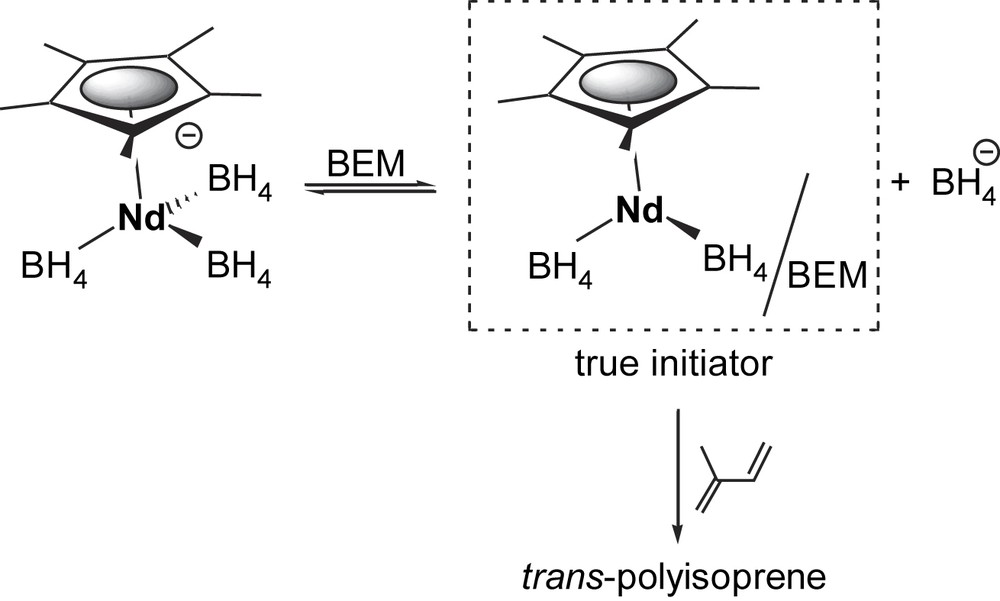
According to the high and very close levels of trans-selectivity, along with the controlled macromolecular data – fitting well with one growing chain – the three different pathways most probably involve the same half-sandwich catalytic species. One can thus propose that 2 dissociates in solution in the presence of 1 equiv of BEM [23] (Scheme 3) to afford a Nd/Mg bimetallic compound bearing one Nd–alkyl active bond, as already observed from 3.3
To generalize the above procedure, additional in situ experiments were carried out with 1,2,4-Ph3C5H2 and C5H5 as ligands (precatalysts 4′ and 5′, respectively) [24]: in both cases, the process was much less controlled and the activity was lower (Table 1), showing the specific role played by the presence of a C5Me5 ligand in the coordination sphere of the neodymium atom for such catalysis.
3 Conclusion
To conclude, we report herein that the “borohydride/alkyl route” is an elegant alternative for the preparation of half-sandwiches of early lanthanides, enabling a one-pot and high yield synthesis and starting with simple precursors. By using this method, it is possible to prepare in situ and very easily sophisticated lanthanide-based catalysts, but also to simply evaluate the impact of a CpR ligand upon a catalytic process in a high throughput screening context.
Further results related to the extension of this “borohydride/alkyl route” to the preparation of lanthanidocenes are in progress and will be published in a forthcoming paper.
Acknowledgments
The authors wish to thank Anne-Marie Cazé for GPC analyses and the Conseil régional Nord-Pas-de-Calais for financial support (ARCIR Nanocat No. AF/CBC/MLM/No. 2005-13).
1 Bulk synthesis: [(C5Me5)Nd(BH4)3]2[Mg(THF)6] (2): 291 mg (0.53 mmol) of a solution of BEM diluted in 5 mL of toluene were added dropwise, at room temperature, to a solution of 1 (430.0 mg, 1.06 mmol) and C5Me5H (195 mg, 1.43 mmol) in toluene (30 mL). The mixture turned from light purple to blue immediately. After 12 h stirring at room temperature, the resulting blue solution was filtrated to eliminate the insoluble residues present in very small quantities (ca. 20 mg). The solution was concentrated of one third and after 12 h, a crop of well-formed crystals (60 mg) could be collected. The mother liquor was then concentrated to ca. 2 mL, providing a blue crystalline powder which was rinsed twice with pentane and finally dried under vacuum (m = 402 mg). Complex 2 was found quite soluble in aromatic solvents. Total yield: 83.3%. Anal. Calc. C44H102B6O6Nd2Mg: C, 47.80; H, 9.20. Found: C, 46.84; H, 9.88. 1H NMR (C6D6) δ: 63.8 (ν br, 12H, BH4, ν1/2 = 600 Hz), 8.28 (s, MeCp, 15H), 1.57 (s, THF, 12H), 0.54 (s, THF, 12H).
2 Compound 2 (C44H102B6MgNd2O6) crystallizes in the monoclinic space group P21 with a = 11.064(2), b = 30.027(4), c = 17.525(3) Å, β = 106.586(2)°, V = 5580(2) Å3, and ρ = 1.315 g cm−3 for Z = 4. CCDC-606630 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK; fax: +44 1223 336 033 or deposit@ccdc.cam.ac.uk).
3 According to an NMR study, the active species involved in the catalytic process from 3 is probably a [(C5Me5)Nd]–(μ-BH4)–Mg bimetallic one, see Ref. [3b].