1 Introduction
Pyrochlore compounds are very interesting due to their physical and chemical properties, such as dielectric, piezoelectric, ferroelectric, magnetic and catalytic ones [1]. The general formula of a typical pyrochlore compound is A2B2O7, with a crystal structure belonging to a cubic system with lattice parameter a of about 10 Å and eight formula units per unit cell [2]. These materials have the high ionic conductivity required for a solid electrolyte and the mixed conductivity needed for an electrode material [3], raising the possibility to be incorporated in solid oxide fuel cell (SOFC) [4], which is currently one of the most promising candidate fuel cell systems and is an all-ceramic device operating at high temperature. The pyrochlore-like structures have recently attracted the attention of theoreticians [5] who predicted the existence of new pyrochlore-like structure materials. In this work we report the synthesis of a new pyrochlore-like-structure compound (Bi1.56Co0.44)(Sb1.48Co0.52)O7 and the study of the corresponding magnetic and electric properties.
2 Experimental
The compound were prepared by solid-state reaction of stoichiometric amounts of reagent-grade Bi2O3 (99.9%), Sb2O3 (99%), CoO (99%) (Aldrich Chemical Company Ltd). The mixture was ground for 1 h with an agate mortar and pestle. The resulting powder was calcined at 700 °C for 48 h in an alumina crucible. Then, the powder was again ground and calcined two more times successively at 800 and 900 °C for 72 h each. The resulting powder was re-milled for 1/2 h and uniaxially pressed into a pellet about 13 mm in diameter and 3 mm in thickness. It was then sintered at 1000 °C for 72 h. The sample was green in colour. The specimen was initially characterized by X-ray powder diffraction using an automated Philips θ–2θ Bragg–Brentano diffractometer. Data were collected using the Cu Kα radiation with 0.009° step size. Rietveld refinement [6] of the X-ray powder diffraction data was carried out using the FULLPROF program [7]. Polished specimens were examined using a conventional Philips SEM XL30 scanning electron microscope. Infrared spectroscopy was carried out with a Nexus Nicolet spectrometer between 400 and 4000 cm−1. The magnetic susceptibility of a powder sample was measured between 4 and 300 K with SQUID and from 100 to 800 K with a Faraday balance. The electrical conductivity measurements were carried out using an impedance Lock-in EG&G 7220-type with 10 mV AC signal amplitude at frequencies between 30 Hz and 120 kHz.
3 Results and discussion
3.1 Compound synthesis
The composition of this new compound was found starting from the pyrochlore-type structure compound Bi1.524Sb1.524Cu0.952O7+δ we synthesized recently [8] when we looked for a possibility to obtain a solid solution by partial substitution of bismuth [9], antimony or copper for other cations. Fig. 1 shows the existence of various phases when copper is totally substituted for cobalt. The stoichiometry Bi1.524Sb1.524Co0.952O7+δ resulted in a three-phase mixture of pyrochlore and small amounts of BiSbO4 [JCPDS/30-177] and CoSb2O6 [JCPDS/18-403]. As it was impossible to eliminate simultaneously both impurities, we managed to eliminate them successively. So, we eliminated CoSb2O6 by adding gradually a small amount of Bi2O3 and then we proceeded to the elimination of the BiSbO4 impurity following these steps:
| Bi1.524Sb1.524Co0.952O7+δ + q(Bi2O3) → Bi1.6Sb1.524Co0.952O7+δ − q′(CoSb2O6) |
| Bi1.6Sb1.524Co0.952O7+δ−x/2(Bi2O3 + Sb2O3) + y(CoO) → Bi1.56Sb1.48Co0.96O7 − x(BiSbO4) |
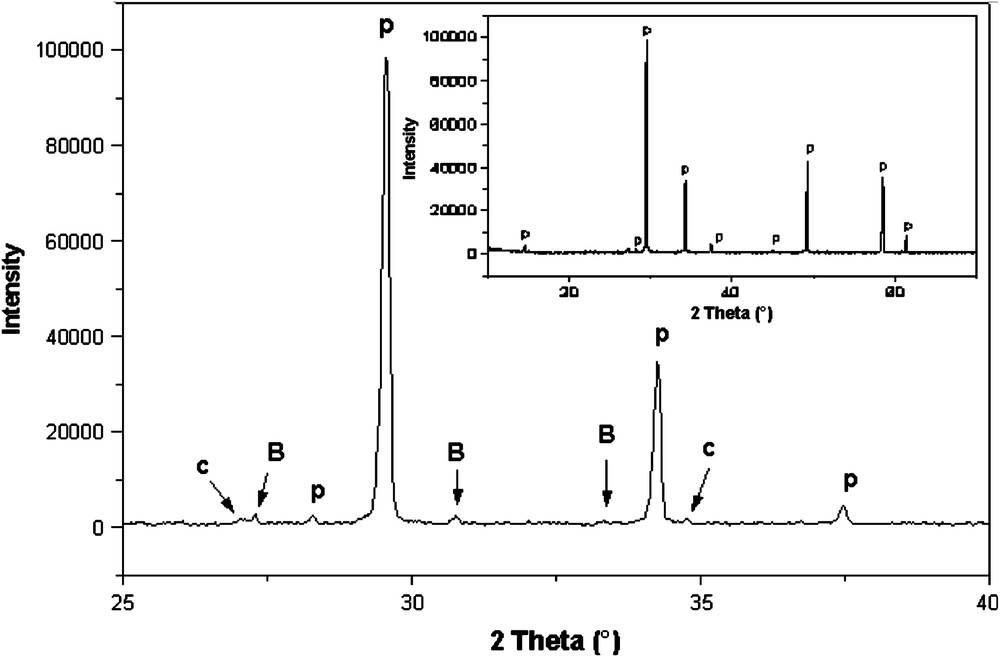
XRD pattern of Bi1.524Sb1.524Co0.952O7+δ (B: BiSbO4; C: CoSb2O6; P: pyrochlore).
The earlier Mössbauer study of the copper pyrochlore (Bi1.524−xMxCu0.476)(Sb1.524Cu0.476)O7+δ (M = Ca, Pb) compound [9] showed that antimony exhibits a +5 oxidation valence. Observed, indexed X-ray powder diffraction data for the pyrochlore with composition Bi1.56Sb1.48Co0.96O7 are given in Table 1. The 442 reflection (2θ = 52.504°), forbidden for an ideal pyrochlore structure [10], was easily observed in this pattern. Similar to that of Bi–Zn–Nb–O pyrochlores [10,11], the observation of this diagnostic reflection indicates the presence of displacements from ideal crystallographic sites. The consistent observation of this reflection indicates that the Bi–Co–Sb–O pyrochlore exhibits positional displacements. Furthermore, the stoichiometric location of the pyrochlore phase field, as also found for the analogous Bi–M–Nb–O systems (M = Zn, [10] Fe, [12] Mn [13]), occurs at substantially lower Bi concentrations than conventional formulations placing only Bi on the A sites and the smaller M/Nb cations on the B sites. The pyrochlore field includes compositions with “excess” B cations that require mixing of some Co on the A sites with Bi3+, as found for Zn in Bi–Zn–Nb–O pyrochlore [10].
Peak list of Bi1.56Sb1.48Co0.96O7 compound
| Pos. [°2Th.] | FWHM [°2Th.] | Rel. Int. [%] | h k l |
| 14.7610 | 0.0708 | 1.63 | 1 1 1 |
| 28.4044 | 0.0620 | 1.39 | 3 1 1 |
| 29.6950 | 0.0886 | 100.00 | 2 2 2 |
| 34.4042 | 0.0886 | 33.43 | 4 0 0 |
| 37.5928 | 0.0797 | 3.78 | 3 1 1 |
| 42.4443 | 0.2125 | 0.18 | 4 2 2 |
| 45.1533 | 0.0620 | 1.24 | 3 3 3 |
| 49.3942 | 0.0974 | 39.25 | 4 4 0 |
| 51.8211 | 0.1063 | 0.43 | 5 1 3 |
| 55.6884 | 0.1417 | 0.32 | 6 0 2 |
| 58.6476 | 0.0974 | 31.54 | 6 2 2 |
| 61.5206 | 0.0886 | 7.58 | 4 4 4 |
| 63.6435 | 0.1063 | 0.32 | 5 1 5 |
| 72.3652 | 0.0972 | 3.54 | 8 0 0 |
| 80.0638 | 0.0797 | 8.01 | 6 6 2 |
| 82.5833 | 0.0797 | 6.53 | 4 0 8 |
| 87.6114 | 0.2125 | 0.16 | 6 6 4 |
| 92.5655 | 0.1080 | 4.52 | 8 4 4 |
| 99.4585 | 0.1728 | 0.45 | 5 5 7 |
| 100.0765 | 0.1080 | 4.33 | 6 6 6 |
| 113.0903 | 0.1080 | 1.15 | 8 8 0 |
| 115.1373 | 0.1296 | 0.40 | 9 5 5 |
| 118.6532 | 0.2834 | 0.20 | 6 6 8 |
| 121.4987 | 0.1080 | 4.01 | 10 2 6 |
| 124.4627 | 0.1080 | 2.63 | 8 4 8 |
| 137.6713 | 0.1728 | 1.62 | 12 4 0 |
The scanning electron micrograph of the sample (Fig. 2) sintered at 1100 °C shows that the external area of the pellet is under spherical grain and porous shape. This compound remains stable until 1100 °C. But above this temperature, a new unidentified peak appears at 2θ = 26.7°.
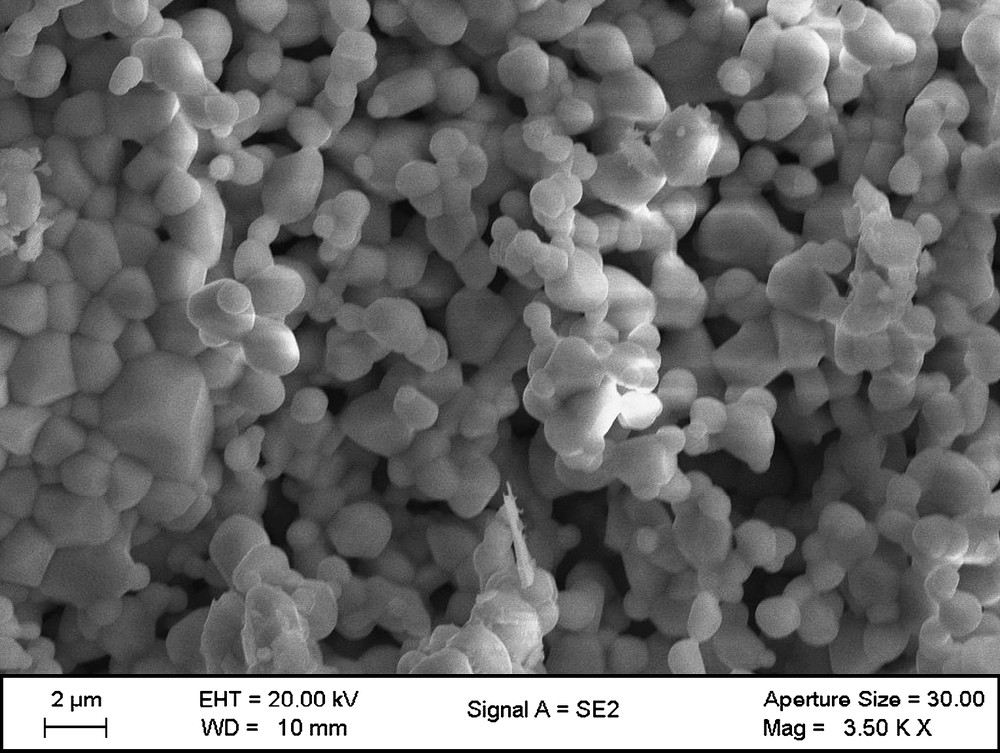
SEM micrograph of Bi1.56Sb1.48Co0.96O7 compound.
3.2 Structural refinement
The highly symmetrical ideal pyrochlore structure A2B2O7 crystallizes in space group Fd-3m with A and B cations on two special positions (16d, 16c) and oxygen atoms on two sites, 48f O and 8b O′, resulting in a single positional variable (x for the 48f-site oxygen atoms in the B2O6 octahedral network). As expected, this model resulted in anomalously large thermal parameters for the A-site cations and the O′ oxygen atoms in an initial refinement. The disordered structure was therefore modelled assuming static displacements of the A and O′ atoms, similar to that of analogous Bi–M–Nb–O pyrochlores (M = Zn [11], Fe [12], Mn [13]) whose structures were refined using neutron powder diffraction data. The refinement of the positional parameters for the disordered metal sites was carried out assuming displacements of the A2O′ network, as found for a number of other pyrochlores [14–17]. Refinement with only displacement of Bi gave bad residuals for the observed reflections; improved results were obtained when O′ was also displaced. The A-site Bi/Co metals were displaced to six partially filled 96g sites; however, the corresponding 96g position for O′ was not stable. Refinement of the O′ isotropic thermal parameter diverged, so a displaced position of 32e site was chosen. Other combinations were not stable. Theoretical, calculated and difference X-ray powder diffraction spectra are represented in Fig. 3. The final refinement results are given in Tables 2 and 3.
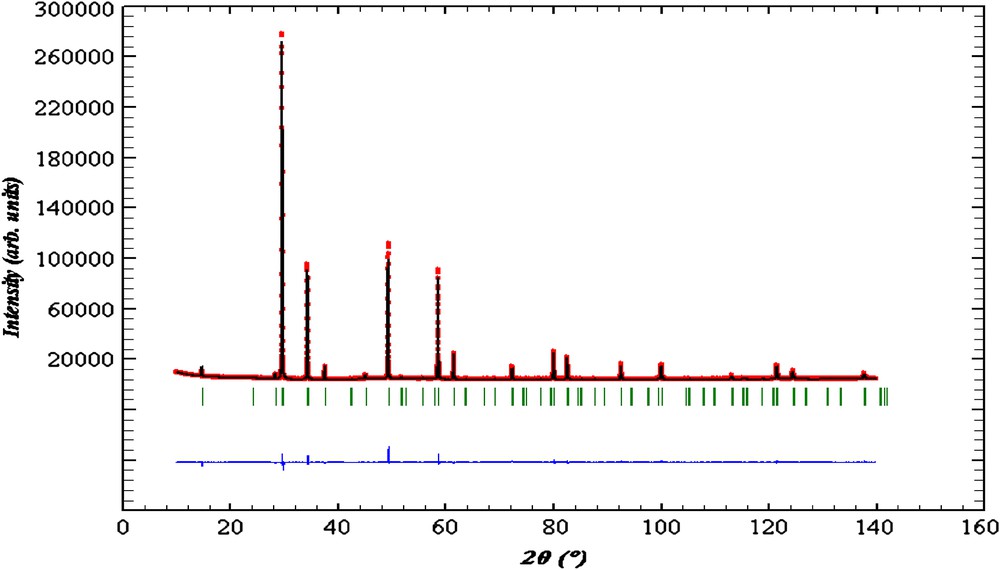
Theoretical, calculated and difference X-ray powder diffraction of Bi1.56Sb1.48Co0.96O7 compound.
Rietveld refinement results
| Global formula | Bi12.48Co7.68Sb11.84O56 |
| Formula mass | 5398.17 g |
| Crystalline system | Cubic |
| Space group | Fd-3m (No. 227) |
| Cell parameter | 10.44896(2) Å |
| Cell volume | 1140.825(4) Å3 |
| Wyckoff sequence | Fdcb |
| Reflexion number | 70 |
| Refine parameters number | 35 |
| Zero point (°2θ) | 0.00000 |
| Sycos | 0.11859 |
| Function profile | Pseudo-Voigt |
| Half width parameters | U = 0.016031(0) |
| V = −0.010584(0) | |
| W = 0.012087(0) | |
| Asymmetrical parameters | 0.243040(0) |
| 0.028510(0) | |
| 0.000000 | |
| 0.000000 | |
| Rwp | 3.09 |
| Rp | 2 |
| Rexp | 1.39 |
| RBragg | 4.38 |
| Rf | 4.65 |
Atomic position and site occupation
| Atom | Position | Occupation | x | y | z | Biso [Å2] |
| Bi(1) | 96g | 0.1300 | 0.51618(31) | 0.51618(31) | 0.48031(25) | 0.487(49) |
| Co(1) | 96g | 0.0367 | 0.51618(31) | 0.51618(31) | 0.48031(25) | 0.487(49) |
| Sb(2) | 16c | 0.74 | 0.00000(0) | 0.00000(0) | 0.00000(0) | 0.050(12) |
| Co(2) | 16c | 0.26 | 0.00000(0) | 0.00000(0) | 0.00000(0) | 0.050(12) |
| O | 48f | 1 | 0.31668(23) | 0.12500(0) | 0.12500(0) | 0.957(7) |
| O′ | 32e | 0.25 | 0.35551(137) | 0.35551(137) | 0.35551(137) | 0.050(6) |
The results suggest to write the initial composition as (Bi1.56Co0.44)(Sb1.48Co0.52)O7, where the cobalt is distributed between A and B sites. Polyhedral representation of this structure is given in Fig. 4. Every octahedron forms the B-site and the arrangement of the octahedral, sharing vertices, generates hexagonal cavities which are the A sites. The value of the isotropic thermal agitation factor 0.487(49) Å2 in the A-site can be interpreted by the good model used for the resolution following the presence of two kinds of cations with different radius: bismuth (RVIII = 1.17 Å) and cobalt (RVIII = 0.90 Å) [18]. This result is in agreement with works made by Withers et al. [19]. They showed in their neutron diffraction studies of (Bi1.5Zn 0.5)(Nb1.5Zn0.5)O7 pyrochlore-like compound that for bismuth and zinc, 96g-site (with O′ in 96g) as well as 96h-site (with O′ in 32e) is more stable than 16d-site. Lately Vanderah et al. [20] synthesized an isotype phase of formula (Bi1.56Co0.44)(Nb1.48Co0.52)O7 which confirms the accuracy of the chemical formula of our compound.
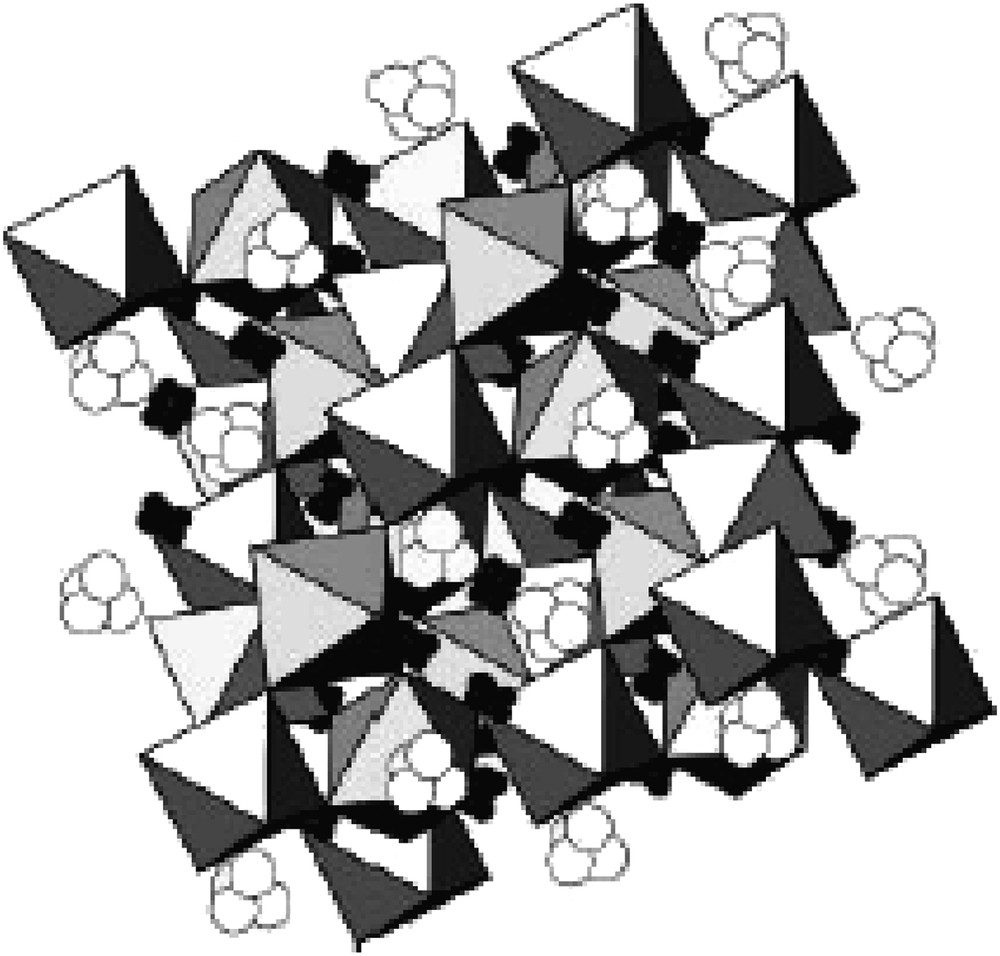
(Bi1.56Co0.44)(Sb1.48Co0.52)O7 cubic pyrochlore structure.
3.3 Infrared spectroscopy
The IR absorption bands of solids in the range of 100–1000 cm−1 are usually assigned to vibrations of ions in the crystal lattice [21]. The infrared lattice vibration frequencies of some pyrochlore compounds with a formula A2B2O7 have been studied. It is known that there are seven IR-active optic modes in the infrared spectra of a pyrochlore compound, and the highest frequency and the most intense band (ν1) at about 600 cm−1 is from the B–O stretching vibration in the BO6 octahedron; the weak band (ν2) at about 500 cm−1 is from the A–O′ stretching vibration and the weak band (γ3) at about 400 cm−1 is from the A–O stretching vibration in the AO6O′2 polyhedron in A2B2O7, respectively [1]. As Fig. 5 shows, there are five absorption bands in the range 400–1000 cm−1 of the infrared spectra of the (Bi1.56Co0.44)(Sb1.48Co0.52)O7 compound. These bands are at 845, 677, 600, 582 and 412 cm−1, respectively. The weak and strong bands are at 412, 582, 600 cm−1, respectively. These bands are just in the region of the A–O and the A–O′ stretching vibrational frequency in the AO6O′2 polyhedron of the pyrochlore compound, respectively. Hence, it may be reasonable to assign the band (ν1 = 412 cm−1) to the (Bi/Co)–O stretching vibrations and the band (ν2 = 582 cm−1) and (ν′2 = 600 cm−1) to the Bi–O′ and the Co–O′ stretching vibrations in the (Bi/Co)O6O′2 polyhedron, respectively. This is because the stretching vibration frequency (ν) of the bond relates to the mass of the bonding atoms [22]. The more the mass of the bonding atoms, the lower the stretching vibration frequency of the bond. The band (ν3 = 677 cm−1) may be from the B–O stretching vibration in the BO6 octahedron. McCauley [23] measured the infrared absorption spectra of several pyrochlore-structure materials. The data showed a number of very weak absorption bands in the 800–1100 cm−1 region, leading to the suggestion that this lattice mode is an indication of an additional structural complexity. As stated before, Withers et al. [19] indicated the bond length difference for the A–O′ bond in the A2O′ substructure. Their data show the longer bond length is 2.351 Å and the shorter bond length is 1.961 Å, about a 20% difference. Hector and Wiggin [24] showed that the displacements of both the O′ anion and the A-site cation must be cooperative within domains and this may lead to one A–O′ bond being shortened and the other being lengthened. According to this picture, the vibration of the shorter A–O′ bond may correspond to the phonon mode around 845 cm−1 and the vibration of the longer bond may lead to a phonon mode around 582 and 600 cm−1. It has been recently proposed that the disorder of A and O′ ions is due to static displacements in all pyrochlores in which the A cation has active lone pairs [15]. And it has been noted [25] that in some pyrochlores the lone pair character of Bi3+ is reduced by mixing of the Bi 6s electron pairs and the d orbitals (of Bi-site cations).
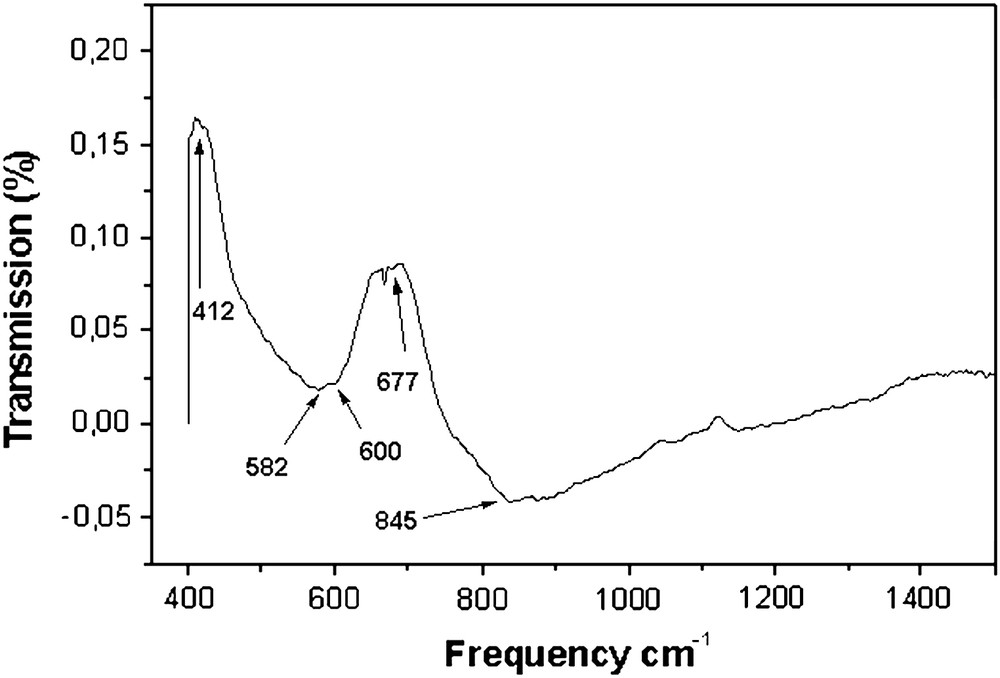
Infrared spectra of (Bi1.56Co0.44)(Sb1.48Co0.52)O7 compound.
3.4 Magnetic susceptibility
Magnetic data obtained for the compound indicate that the field dependence of the magnetization confirmed that the sample was overall paramagnetic in nature with an effective moment of 5.04μβ for the cobalt. The observed effective magnetic moments (μeff/(mol) Co), calculated from the slopes of the Curie–Weiss fits, are similar of those observed for other Co-containing pyrochlores [26] giving the range of values reported for high-spin Co2+ (4.6μβ/Co–5.2μβ/Co)[27]. The temperature dependence of the magnetic susceptibility and its inverse are shown in Fig. 6. Above 100 K the inverse magnetic susceptibility was linear and that range was fitted to the Curie–Weiss law. The extrapolation of the linear fits indicated small negative temperature intercepts −49.48 (θ (K)), suggesting the presence of weak antiferromagnetic cooperative interactions.
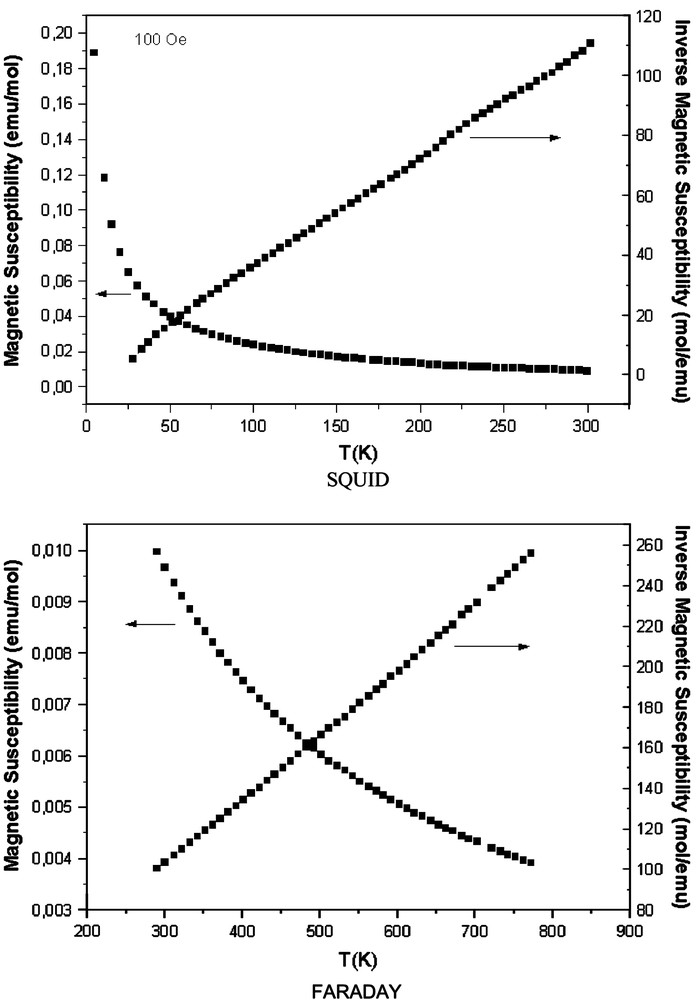
Magnetic susceptibility and inverse magnetic susceptibility versus temperature of Bi1.56Sb1.48Co0.96O7 compound.
3.5 Electrical characterization
The typical complex impedance diagram Z″ = f(Z′) of the compound obtained at 420 K is presented in Fig. 7. It shows a non-deformed unique circle arc passing by the origin and having a centre displaced under the abscissa axis. The resistance R of the sample at each temperature was determined from the intersection of abscissa axis (zero phase-shift angle) with the circle arc at low frequencies [28]. Fig. 7 shows the variation log(σ) versus the reciprocal temperature (1/T). The electrical conductivity obeys the Arrhenius relation: σ = σ0 exp(−Ea/KBT) where σ0 represents a pre-exponential factor, KB the Boltzmann constant, Ea was estimated from the slope of log(σ) versus T−1. The activation energy (Ea) of the compound is 0.41 ± 0.02 eV. These results reveal a semiconducting behaviour of the material.
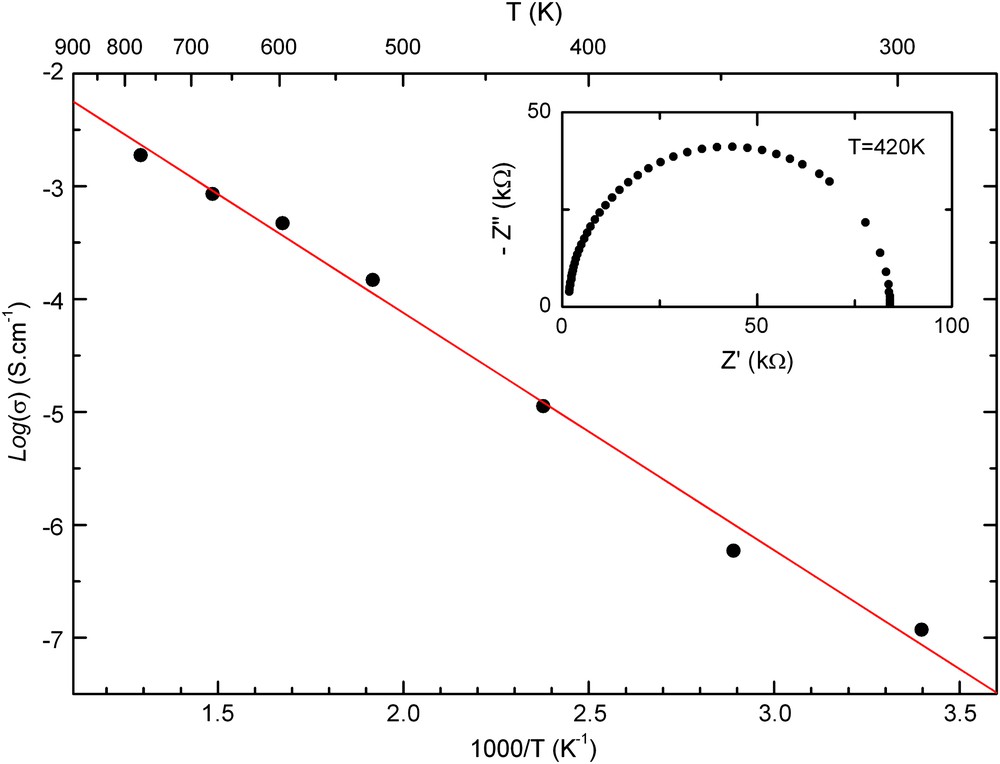
Logarithmic conductivity variation versus inverse temperature (1/T) with the impedance complex diagram at 420 K.
4 Conclusion
A new pyrochlore compound with formula (Bi1.56Co0.44)(Sb1.48Co0.52)O7 was synthesized by solid-state reaction at 1000 °C. X-ray powder diffraction pattern for this composition is consistent with both the cubic pyrochlore unit cell with a = 10.44896(2) Å and Fd-3m symmetry. Rietveld refinements confirmed an overall B2O6·A2O′ cubic pyrochlore structure with Co cations present on both A and B sites. The IR-active bands characteristic of A and B sites of the pyrochlore compound have been detected by infrared spectroscopy. Magnetic characterization confirmed a “+2” oxidation state of the cobalt cation. Electric measurements at high temperature revealed an increasing of the electrical conductivity, which reached 1.88 × 10−3 (ohm cm)−1 at 775 K and which lets us think about a semiconducting behaviour of the material. The substitution of the A sites cations for transition metals is under investigation and will be published later.


