1 Introduction
Azlactones, or 2,4-substituted oxazolin-5-ones, are interesting intermediates for the synthesis of a variety of bioactive compounds, including aminoacids [1], peptides [2], heterocycles [3], N-substituted pyrroles [4], biosensors [5] and antitumor [6] or anticancer [7] compounds. The usual procedure for azlactone synthesis is the Erlenmeyer and Justus method [8], which involves the condensation reactions of different aryl aldehydes with hippuric acid in acetic anhydride as dehydrating agent and anhydrous sodium acetate as a homogeneous basic catalyst. A literature search revealed that different reagents have been used for the synthesis of azlactones involving perchloric acid [9], polyphosphoric acid [10], carbodiimides [11], POCl3 [12] and SO3 in dimethylformamide [13]. Other reported routes for the synthesis of azlactones by condensation reaction of aldehydes with hippuric acid in acetic anhydride as dehydrating agent are in the presence of various catalysts such as Al2O3 [14], Bi(OAc)3 [15], Bi(OTf)3 [16], silica-supported heteropolyacids [17], Yb(OTf)3 [18], Ca(OAc)2 [19], supported KF [20] and anhydrous zinc chloride[21]. Most of the methods are suitable; however, some of these procedures need high temperature (reflux temperature) and are difficult to handle. The presence of acetic anhydride may cause side reactions such as esterification or acylation. Therefore, replacement of acetic anhydride by other reagents is required.
Microwave irradiation is a well-known technique to promote the synthesis of a variety of compounds, where chemical reactions are accelerated because of the selective absorption of microwaves by polar molecules [22]. The use of this technique in organic synthesis [23–26] has attracted attention in recent years due to enhanced reaction rates, high yields, improved purity and ease of work up after the reaction.
2 Results and discussion
As a continuation of our recent studies, devoted to the development of practical, safe and environmentally friendly procedures for some important transformations [27–31], we wish to report an efficient, convenient and facile procedure for the condensation of aromatic aldehydes with hippuric acid to the corresponding azlactones, in the presence of inexpensively available tosyl chloride (TsCl) and dimethylformamide (DMF) system as condensing agent in the absence of solvents under microwave irradiation (Scheme 1).

Synthesis of azlactone derivatives.
First, to evaluate the synthetic potential of the proposed procedure and to optimize the reaction conditions, the reaction of benzaldehyde and hippuric acid was examined in the presence of TsCl and different bases such as sodium acetate, pyridine, triethylamine and dimethyl formamide under microwave irradiation. As can be seen from Table 1, the best result was obtained when the reaction was carried out in the presence of TsCl/DMF and in the absence of solvents under microwave irradiation.
Optimization of reaction conditions.
| Entry | Regent | Conditions | Time | Yield (%)a |
| 1 | TsCl | MW (300 W) | 2.5 | <10 |
| 2 | TsCl/NaOAc | MW (300 W) | 2.5 | 53 |
| 3 | TsCl/pyridine | MW (300 W) | 2.5 | 77 |
| 4 | TsCl/triethylamine | MW (300 W) | 2.5 | 70 |
| 5 | TsCl/DMF | MW (300 W) | 2.5 | 91 |
| 6 | TsCl/DMF | 80 °C | 2.5 | 75 |
| 7 | TsCl/DMF | MW (450 W) | 2.5 | 88 |
a Isolated yields.
To evaluate the generality of the process, several examples illustrating the present procedure for the synthesis of azlactones were studied (Table 2). The reaction of hippuric acid with various aromatic aldehydes bearing electron-withdrawing groups (such as nitro and halides), electron-donating groups (such as methyl and methoxy), was carried out under optimized conditions. In all cases, the corresponding products were obtained in good to high yields (Table 2).
Synthesis of azlactones with the use of TsCl/DMF system.
| Entry | Aldehyde (Ar) | Time (min) | Yield (%) a | Azlactone | M.P. (° C) | |
| Found | Reported [lit.] | |||||
| 1 | C6H5 | 2.5 | 91 | 3a | 166–167 | 167–168 [14] |
| 2 | 4-Me C6H4 | 2.5 | 90 | 3b | 141–143 | 143–144 [14] |
| 3 | 2,4-Cl2 C6H3 | 4 | 89 | 3c | 160–161 | 162–163 [18] |
| 4 | 4-MeO C6H4 | 4 | 90 | 3d | 156–157 | 155–157 [18] |
| 5 | 4-F C6H4 | 2.5 | 92 | 3e | 182–183 | 183–185 [14] |
| 6 | 3-NO2 C6H4 | 2.5 | 91 | 3f | 169–170 | 166–167 [18] |
| 7 | 2-Br C6H4 | 3 | 88 | 3g | 140–141 | 141–143 [32] |
| 8 | 3-MeO C6H4 | 3 | 87 | 3h | 100–102 | 99–102 [14] |
| 9 | 4-(Me2N) C6H4 | 3 | 93 | 3i | 210–211 | 212–214 [32] |
| 10 | 2-Cl C6H4 | 3 | 86 | 3j | 160–161 | 162–163[32] |
| 11 | 2-MeO C6H4 | 3.5 | 85 | 3k | 155–156 | 156–157 [14] |
| 12 | 4-NO2 C6H4 | 2 | 91 | 3l | 234–236 | 237–139 [32] |
| 13 | 2-Thiophene | 2 | 53 | 3m | 170–171 | 174–175 [14] |
a Isolated yields.
Aliphatic aldehydes such as acetaldehyde or propionaldehyde and ketones such as ethyl methyl ketone or cyclohexanone were also examined under the same conditions, but the corresponding products were isolated only in trace amounts.
A possible mechanism for this transformation is proposed in Scheme 2. The reaction of hippuric acid (III) with TsCl (I) and DMF (II) produced the Vilsmeier adduct as proposed by Higashi et al. for the activation of carboxylic acids [33]. This intermediate (IV), upon an intermolecular cyclization, yielded the azlactone (V) that supported by good evidence in Erlenmeyer synthesis [34]. Then, azlactone derivatives (VI) can be obtained from aldol condensation of azlactone (V) and carbonyl compounds.
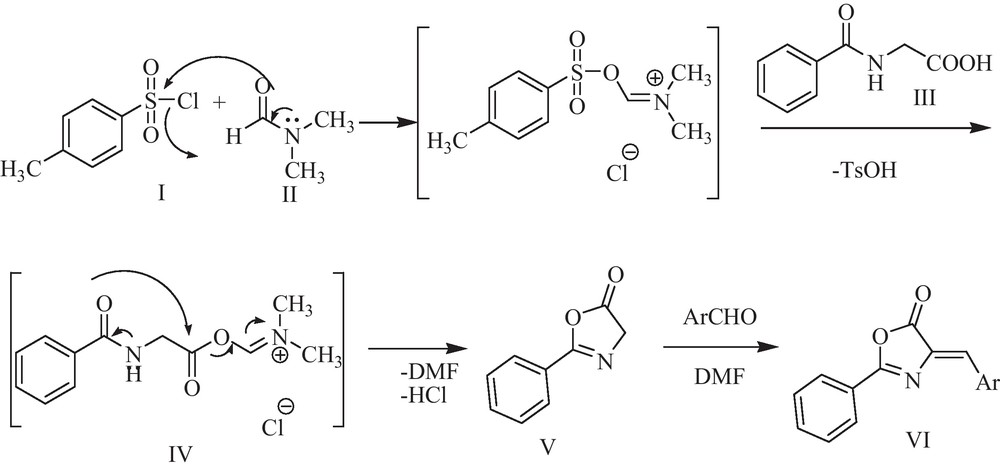
The most probable mechanism for the synthesis of azlactone derivatives.
The results obtained from the condensation reaction of benzaldehyde, 4-methoxybenzaldehyde and 4-nitrobenzaldehyde with hippuric acid under optimized conditions were compared to the best ones published so far for this reaction using inorganic or organic catalysts, the data listed in Table 3. It showed that the TsCl/DMF system was a fairly good reagent for this reaction in terms of short reaction time and simplified conditions.
Preparation of azlactone 3a, 3d and 3l under different conditions.
| Entry | Regent | Conditions | Time (min)/Yield (%)a | Ref. | ||
| 3a | 3d | 3l | ||||
| 1 | TsCl/DMF | MW (300 W) | 2.5/91 | 4/90 | 2/91 | – |
| 2 | Polyphosphoric acid | 80–95 °C | 90/90 | 90/90 | – | [10] |
| 3 | Ac2O/Yb(OTf)3 | 40 °C | 30/82 | 30/84 | 60/83 | [18] |
| 4 | Ac2O/Silica-supported heteropolyacids | Toluene/reflux | 60/95, 96 | 60/93, 94 | – | [17] |
| 5 | Ac2O/KF-Al2O3 | Reflux | 60/81.2 | 60/74.4 | 60/72.6 | [20] |
| 6 | POCl3 | Reflux | 20/86 | 10/73 | 15/90 | [12] |
| 7 | Ac2O/Bi(OAc)3 | Reflux | 60/88 | 60/56 | – | [15] |
a Isolated yields.
3 Conclusion
In summary, we have developed a new, microwave assisted protocol for the synthesis of azlactone derivatives from condensation of aromatic aldehydes with hippuric acid using TsCl/DMF system as a condensing agent. In addition to the efficiency and simplicity, high yields, short reaction time and the ease of workup make the method advantageous.
4 Experimental
4.1 General
Products are known compounds and were characterized by comparison of their spectral data (1H NMR, IR) and melting points with those reported in the literature. IR spectra were recorded as KBr disc on a galaxy series FT-IR 5000 spectrometer. NMR spectra were recorded on a brucker spectrometer in CDCl3 with TMS as an internal standard. Microwave irradiation was carried out in a National Microwave Oven, Model No. NN-K571MF (2450 MHz).
4.2 General procedure
To a mixture of aromatic aldehydes (1 mmol), hippuric acid (1.1 mmol) and tosyl chloride (1 mmol), DMF (1 mmol) was added and the reaction mixture was irradiated using the microwave oven for the appropriate time according to Table 2. The progress of the reaction was monitored by TLC. Upon completion of the reactions, a mixture of EtOH–H2O (2:1.5 mL) was added to it, the suspension was stirred for 5 min and the precipitate filtered. The crude products were purified by recrystallization from EtOH.
4.3 Representative spectral data
3a: IR (KBr) (νmax): 3070, 1786, 1649, 1450, 1301, 1158, 760, 657 cm−1. 1H NMR (300 MHz, CDCl3) δ = 2.27 (s, 1 H), 7.42–7.63 (m, 6 H), 8.18–8.21 (m, 4H).
3b: IR (KBr) (νmax): 3065, 1793, 1649, 1602, 1490, 1301, 1160, 981, 858, 764 cm−1. 1H NMR (300 MHz, CDCl3) δ = 2.41 (s, 3 H), 7.21–7.34 (m, 3 H), 7.50–7.62 (m, 3H), 8.11–8.19 (m, 4H).
3g: IR (KBr) (νmax): 3058, 1793, 1650, 1550, 1323, 11650, 860, 761 cm−1. 1H NMR (300 MHz, CDCl3) δ = 7.35–7.65,(m, 6 H), 7.77 (s, 1H), 8.21 (d, J = 7.4, 2H), 8.95 (d, J = 7.7, 1H).
Acknowledgment
We gratefully acknowledge the financial support from the Research Council of Islamic Azad University, Dezful Branch.


