1 Introduction
Protection and deprotection of organic functions are important processes during multi-step organic synthesis [1,2]. The choice of a method for the functional group's transformations depends on its simplicity, high yields of the desired products, short reaction times, low cost of the process and ease of the work-up procedures.
Trimethylsilylation of labile hydroxyl groups is one of the most important strategies for the protection of alcohols [3]. These functional organosilyl ethers are stable under various conditions, soluble in non-polar solvents, have high thermal stability and are easy to remove under acid or base hydrolytic conditions [4]. They also help to increase the volatility for analysis in gas-chromatography and mass spectrometry (GC–MS) [5]. A variety of reagents have been employed for the introduction of the trimethylsilyl group [6–13], among which hexamethyldisilazane (HMDS), as a cheap and commercially available reagent [14,15], is the most widely used. Its handling does not need special precautions, and the work-up is not time consuming, as the by-product of the reaction is ammonia, which is simple to remove from the reaction medium. However, the main drawback is the poor silylating capacity of HMDS which is a deterrent in its application.
Between the several methods available for the protection of amines, N-tert-butylcarbonylation has attracted the attention of many organic chemists. This considerable attention can be attributed to the stability of N-tert-butylcarbamates againt bases, nucleophiles, catalytic hydrogenation and racemization [16,17], as well as the easy removal of the Boc moiety by reagents, such as CF3CO2H, formic acid, (3 N) HCl in ethyl acetate or 10% H2SO4 in dioxane. Among the different reagents which are available for the N-Boc protection of amines [18], di-tert-butoxypyrocarbonate [(Boc)2O] is the most popular one because of its commercial availability, low cost, stability and efficiency. However, there are several drawbacks behind the classical N-Boc protection techniques using this reagent. The tedious work-up takes time and side products are generated. Moreover, the catalysts used to promote the reaction are most of the time very costly and non-recoverable [19].
Among the many protecting groups for alcohols, phenols, amines and thiols, acetate is used with high frequency. The acetylation is typically performed using acetic anhydride in the presence of either base [20–23] or acid catalysts [24–43]. Although various acetylation methods are available, most have one or more drawbacks, including long reaction times, harsh conditions, harmful organic solvents, and tedious work-up procedures. One of the most promising solutions to these problems seems to be the use of green and insoluble catalysts or of eco-friendly solvent-free conditions. When an insoluble catalyst is used, it can be separated easily by filtration and recycled. Furthermore, the reported examples have demonstrated that heterogeneous catalysts typically require easier work-up procedures. Also, solvent-free synthetic methods are valuable for environmental and economical reasons [44–46].
In recent years, the use of green reagents in organic reactions has attracted the attention of many organic chemists. This attention can be attributed to the reduction of environmental pollution and the cost of the applied methods.
Rice husk, as a thin but abrasive skin in nature, which covers the edible rice kernel, contains cellulose, hemicellulose, lignin, silica, solubles, and moisture [47,48]. The worldwide annual rice husk output is about 80 million tons and over 97% of the husk is generated in developing countries [49]. In the course of decades, rice husk has found different applications in chemistry and industry. For example, unmodified rice husk has been evaluated for its ability to bind zinc(II) and other metal ions [50,51]. On the other hand, various modifications have been done on rice husk in order to enhance its sorption capacities for metal ions and other pollutants [52,53]. In addition, both rice husk and rice husk ash are used as potential raw materials in ceramics, cements and silica-based industries [54].
2 Experimental
2.1 General
Chemicals were purchased from Fluka, Merck, and Aldrich chemical companies. All yields refer to the isolated products. Determination of the purity of the substrate and monitoring of the reaction were accomplished by thin-layer chromatography (TLC) on a silica-gel polygram SILG/UV 254 plates.
2.2 Preparation of rice husk
The rice sample which was used in this study was named as Hassani and was obtained from Rasht (Guilan Province) in the north of Iran. Prior to use, the rice husk sample was washed several times with distilled water to remove any adhering materials and dried at room temperature for 48 h. The dried RiH was smashed and sieved (80–170 mesh size), washed with distilled water and dried at 110 °C for 4 h. We have determined the main components of the rice husk sample as follows, using the methods reported in the literature [55,56]; silica (14.2%), cellulose (27.4%), hemicelluloses (18.3%), lignin (25.8%), inorganic residue (5.8%), solubles (3.5%) and moisture (5%).
The catalyst is also characterized using different methods, including infrared (IR), scanning electron microscopy (SEM), X-ray diffraction (XRD) and X-ray fluorescence (XRF) analysis, as reported in the literature [57].
2.3 Trimethylsilylation of alcohols and phenols; general procedure
To a stirring mixture of the substrate (alcohol and/or phenol) (1 mmol), and RiH (0.08 g) in CH3CN (3 mL), HMDS (0.75 mmol, 0.120 g) was added at room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was filtered and the residue was washed with acetonitrile (5 mL). Evaporation of the solvent gave almost pure product(s). Further purification proceeded by bulb-to-bulb distillation under reduced pressure or re-crystallization to afford pure silyl ether.
2.4 N-Boc protection of amines; general procedure
The substrate (1 mmol) was added to a magnetically stirred mixture of RiH (0.05 g) and (Boc)2O (1 mmol, 0.218 g) at room temperature. After completion of the reaction (TLC), the mixture was diluted with EtOAc (10 mL) and filtered. Evaporation of the solvent by column chromatography (silica-gel) followed; eluting with EtOAc in n-hexane (5–15%) gave the desired product in good to high yields.
2.5 Acetylation of alcohols, phenols and thiols; general procedure
A mixture of the substrate (alcohol, phenol and/or thiol) (1 mmol), acetic anhydride (3 mmol) and RiH (0.3 g) was stirred at 80 °C. After completion of the reaction (TLC), EtOAc (15 mL) was added and the catalyst was filtered The organic layer was washed with saturated NaHCO3 and water (3 × 15 mL), and dried over anhydrous MgSO4. Evaporation of the solvent under reduced pressure gave the almost pure acetates.
2.6 Acetylation of amines; general procedure
A mixture of the substrate (1 mmol), acetic anhydride (3 mmol) and RiH (0.15 g) was stirred at room temperature. After completion of the reaction (TLC), EtOAc (15 mL) was added and the catalyst was filtered. The organic layer was washed with saturated NaHCO3 and water (3 × 15 mL), and dried over anhydrous MgSO4. Evaporation of the solvent under reduced pressure gave the almost pure acetates.
3 Results and discussion
Very recently, we reported the preparation and applicability of rice-husk-supported FeCl3 nano particles in the promotion of the acylation of aldehydes and deprotection of the obtained 1,1-diacetates [57]. In continuation of this study and on the basis of our ongoing research program on the development of the application of silica-based reagents in organic transformations [58–62], we were interested to investigate the applicability of RiH in the promotion of the other organic reactions. Our investigations clarified that RiH is efficiently able to catalyze the trimethylsilyl protection of alcohols and phenols with HMDS, Boc protection of amines with (Boc)2O and acetyl protection of alcohols, phenols, thiols and amines with Ac2O.
Initially, the standardization of the silylation reaction conditions was studied with investigating the effect of different molar ratio of reactants and different solvents (CH2Cl2, n-hexane, acetone, CHCl3, Et2O, CCl4, H2O and CH3CN) on the reaction, in terms of time and product yield. Our investigations clarified that the best results can be obtained under the conditions showed in Scheme 1.
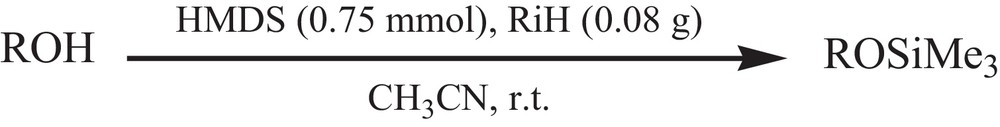
Trimethylsilylation of alcohols and phenols.
After optimization of the reaction conditions, different types of alcohols were subjected to trimethylsilylation using this method (Table 1).
Trimethylsilylation of alcohols and phenols catalyzed by rice huska,b.
| Entry | Substrate | Product | Time (min) | Yield (%) |
| 1 | PhCH2OH | PhCH2OSiMe3 | 5 | 94 |
| 2 | 2-ClC6H4CH2OH | 2-ClC6H4CH2OSiMe3 | 15 | 91 |
| 3 | 4-ClC6H4CH2OH | 4-ClC6H4CH2OSiMe3 | 8 | 93 |
| 4 | 3,4-Cl2C6H4CH2OH | 3,4-Cl2C6H4CH2OSiMe3 | 9 | 97 |
| 5 | 4-BrC6H4CH2OH | 4-BrC6H4CH2OSiMe3 | 19 | 92 |
| 6 | 2-BrC6H4CH2OH | 2-BrC6H4CH2OSiMe3 | 19 | 94 |
| 7 | 2-NO2C6H4CH2OH | 2-NO2C6H4CH2OSiMe3 | 30 | 92 |
| 8 | 4-NO2C6H4CH2OH | 4-NO2C6H4CH2OSiMe3 | 20 | 91 |
| 9 | 2-MeC6H4CH2OH | 2-MeC6H4CH2OSiMe3 | 26 | 90 |
| 10 | 4-Me2CHC6H4CH2OH | 4-Me2CHC6H4CH2OSiMe3 | 8 | 93 |
| 11 | 3-MeOC6H4CH2OH | 3-MeOC6H4CH2OSiMe3 | 12 | 95 |
| 12 | 4-MeOC6H4CH2OH | 4-MeOC6H4CH2OSiMe3 | 22 | 94 |
| 13 | 9 | 96 | ||
| 14 | 15 | 96 | ||
| 15 | 15 | 93 | ||
| 16 | 30 | 90 | ||
| 17 | PhCH2CH2OH | PhCH2CH2OSiMe3 | 15 | 93 |
| 18 | PhCH(Me)CH2OH | PhCH(Me)CH2OSiMe3 | 8 | 97 |
| 19 | 15 | 92 | ||
| 20 | 15 | 93 | ||
| 21 | 45 | 90 | ||
| 22 | PhCHCHCH2OH | PhCHCHCH2OSiMe3 | 15 | 90 |
| 23 | 20 | 65 | ||
| 24 | 16 | 93 | ||
| 25 | 6 | 80 | ||
| 26 | 10 | 84 | ||
| 27 | 10 | 90 | ||
| 28 | 6 | 98 | ||
| 29 | 8 | 93 | ||
| 30 | 20 | 95 | ||
| 31 | 15 | 90 | ||
| 32 | 4 | 94c | ||
| 33 | 18 | 87 | ||
| 34 | 120 | 0d | ||
| 35 | 120 | 0d | ||
| 36 | 180 | 0d | ||
| 37 | PhCH2NH2 | PhCH2NHSiMe3 | 120 | 0d |
| 38 | PhNH2 | PhNHSiMe3 | 150 | 0d |
| 39 | PhNHMe | PhN(SiMe3)Me | 120 | 0d |
| 40 | 120 | 0d | ||
| 41 | PhSH | PhSSiMe3 | 120 | 0d |
| 42 | 120 | 0d | ||
| 43 | 120 | 0d | ||
| 44 | PhCH2OH + | PhCH2OSiMe3 + | 7 | 100d |
| 0d | ||||
| 45 | PhCH2CH2OH + | PhCH2CH2OSiMe3 + | 20 | 100d |
| PhNHMe | PhN(SiMe3)Me | 0d | ||
| 46 | 4-ClC6H4CH2OH + | 4-ClC6H4CH2OSiMe3 + | 12 | 100d |
| PhSH | PhSSiMe3 | 0d |
a Products were identified spectroscopically and in comparison with authentic samples [11–13,61,63–72].
b Isolated yield.
c 0.16 g of rice husk was used.
d Identified by thin layer chromatography or gas chromatography.
The obtained results showed that the trimethylsilylation of benzylic alcohols, including different types of substituents, proceeded efficiently with high isolated yields under the selected conditions (Table 1, entries 1–16). Primary and less hindered secondary aliphatic alcohols were also successfully converted to the corresponding silyl ethers in almost quantitative yields at room temperature (Table 1, entries 17–21). This method was also found to be useful for trimethylsilylation of allylic alcohols, diols and acyloins (Table 1, entries 22 and 23). No elimination and rearrangement by-products were observed at all. Phenols also undergo silylation easily using this method, and their corresponding silyl ethers can be isolated in good to high yields (Table 1, entries 24–33).
As shown in Table 2, more hindered secondary and tertiary alcohols, amines, and thiols are resistant to this reagent and remain intact in the reaction mixture (Table 1, entries 34-43). Therefore, this methodology shows selectivity, and is suitable for the selective trimethylsilylation of benzylic and primary and less hindered secondary aliphatic alcohols and phenols in the presence of the above-mentioned substrates (Table 1, entries 44–46).
Comparison of some of the results obtained by the silylation of alcohols with HDMS in the presence of rice husk (1), with some of those reported by LaCl3a(2) [73], trichloroisocyanuric acidb (3) [74], and PBBSc (4) [75].
| Entry | Substrate | Time (min)/Yield (%) | |||
| 1 | 2 | 3 | 4 | ||
| 1 | PhCH2OH | 5/94 | 180/91 | 240/90 | 90/90 |
| 2 | PhCH(OH)Ph | 30/90 | 210/93 | 180/95 | – |
| 3 | (-)-Menthol | 45/90 | 300/84 | 180/95 | – |
| 4 | 4-NO2C6H4CH2OH | 20/91 | – | 180/90 | 60/90 |
a Reaction conditions: room temperature, methylenedichloride as the solvent.
b Reaction conditions: room temperature, dichloromethane as the solvent.
c Reaction conditions: room temperature, dichloromethane as the solvent.
To illustrate the efficiency of the proposed method, Table 2 compares some of our results with some of those reported for the relevant reagents in the literature, which demonstrates its significant superiority [73–75].
RiH was then used for the protection of amines as their N-tert-butylcarbamates with (Boc)2O under similar reaction conditions. N-Boc protection of aniline was chosen as a probe reaction. However, the reaction was not as efficient as the O-silylation of alcohols under the pre-described conditions. Therefore, the reaction was performed in the absence of solvent at room temperature using 0.05 g of RiH. In this condition, aniline was N-Boc protected for 10 min to furnish the product in excellent yield (95%) (Scheme 2).

N-Boc protection of aniline.
Then, the protocol was followed using a series of aliphatic, aromatic and heterocyclic amines and aminols for generalization and the results are shown in Table 3. Regardless of the nature of the substituent attached to them, all of the above-mentioned substrates produced excellent yields in relatively short reaction times. However, the reaction with sterically hindered di-phenylamine and di-cyclohexyl amine was sluggish (Table 3, entries 8 and 14). No isocyanate or urea formation was observed at all (IR).
| Entry | Substrate | Product | Time | Yield (%) |
| 1 | PhNH2 | PhNH-Boc | 10 min | 93 |
| 2 | 4-EtC6H4NH2 | 4-EtC6H4NH-Boc | 25 min | 96 |
| 3 | 3-MeOC6H4NH2 | 3-MeOC6H4NH-Boc | 60 min | 93 |
| 4 | 3-ClC6H4NH2 | 3-ClC6H4NH-Boc | 25 min | 92 |
| 5 | 4-BrC6H4NH2 | 4-BrC6H4NH-Boc | 15 min | 95 |
| 6 | 2-HOC6H4NH2 | 2-HOC6H4NH-Boc | 70 min | 92 |
| 7 | 30 min | 94 | ||
| 8 | Ph2NH | Ph2N-Boc | 4 h | 92 |
| 9 | PhCH2NH2 | PhCH2NH-Boc | 1 min | 94 |
| 10 | 1 min | 96 | ||
| 11 | 1 min | 93 | ||
| 12 | 1 min | 95 | ||
| 13 | 1 min | 92 | ||
| 14 | 3.7 h | 92 | ||
| 15 | 5 min | 95 | ||
| 16 | 30 min | 94 | ||
| 17 | PhCH2OH | PhCH2O-Boc | 2 h | 0c |
| 18 | 2 h | 0c | ||
| 19 | PhCH2NH2 + | PhCH2NH-Boc + | 3 min | 100c + |
| PhCH2OH | PhCH2O-Boc | 0c | ||
| 20 | + | + | 2 min | 100c + |
| 0c |
a Products were identified spectroscopically and in comparison with authentic samples [76–81].
b Isolated yield.
c Identified by thin layer chromatography or gas chromatography.
Under the selected conditions, Boc protection of alcohols and phenols was not successful and the starting material was recovered unchanged after 2 h (Table 3 entries 17and 18). The selectivity of a method determined the importance of its application in organic reactions. Therefore, the chemoselectivity of this method was also investigated and the results are reported in Table 3 (Entries 6, 15, 16, 19 and 20). The obtained results clearly showed that this method could be efficiently used for the chemoselective protection of different types of amines in the presence of alcohols and phenols.
In order to show the merit of this method, Table 4 compares the results obtained from the N-Boc protection of aniline by our method with some of those reported in the literature [82–86].
Comparison of the results obtained from the N-Boc protection of aniline in the presence of various catalysts.
| Entry | Catalyst | Solvent | Time | Yield (%) | Reference |
| 1 | RiH | Neat | 10 min | 95 | This work |
| 2 | Zr(ClO4)2·6H2O | CH2Cl2 | 12 h | 92 | [81] |
| 3 | Iodine | Neat | 30 min | 95 | [82] |
| 4 | β-Cyclodextrine | H2O | 2.5 h | 75 | [83] |
| 5 | Thioglycolic acid | EtOH | 8 min | 95 | [84] |
| 6 | Yttria-zirconia | CH3CN | 14 h | 90 | [85] |
| 7 | Saccharin sulfonic acid | n-Hexane | 1 h | 97 | [86] |
After the above-mentioned reactions, we have tried to extend the applicability of RiH in organic transformations by studying the role of this catalyst in the promotion of the acetylation reactions. Our investigations clarified that RiH was also efficiently able to catalyze the acetyl protection of alcohols, phenols, thiols and amines with Ac2O. All reactions are performed under mild reaction conditions in good to high yields (Scheme 3, Table 5).
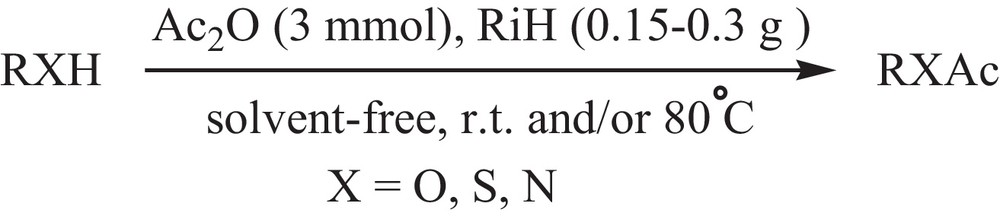
Acetylation reactions catalyzed by rice husk.
| Entry | Substrate | Product | Time (h) | Yield (%)d |
| 1 | PhCH2OH | PhCH2OAc | 1 | 94 |
| 2 | 4-ClC6H4CH2OH | 4-ClC6H4CH2OAc | 1.5 | 92 |
| 3 | 4-BrC6H4CH2OH | 4-BrC6H4CH2OAc | 1.25 | 91 |
| 4 | 2-BrC6H4CH2OH | 2-BrC6H4CH2OAc | 1.25 | 91 |
| 5 | 4-Me2CHC6H4CH2OH | 4-Me2CHC6H4CH2OAc | 1.5 | 92 |
| 6 | 1.75 | 90 | ||
| 7 | 2.5 | 87 | ||
| 8 | PhCH(Me)OH | PhCH(Me)OAc | 2 | 85 |
| 9 | 1.5 | 90 | ||
| 10 | 1.75 | 88 | ||
| 11 | 1.75 | 84e | ||
| 12 | 10 | 60 | ||
| 13 | 1.5 | 87 | ||
| 14 | 1.75 | 85 | ||
| 15 | 3 | 80 | ||
| 16 | 0.8 | 92 | ||
| 17 | 1 | 83 | ||
| 18 | 1 | 92 | ||
| 19 | 1 | 90 | ||
| 20 | 1 | 93 | ||
| 21 | 2 | 81 | ||
| 22 | 0.5 | 90e | ||
| 23 | PhCH2SH | PhCH2SAc | 0.5 | 92 |
| 24 | 0.5 | 91 | ||
| 25 | 0.5 | 93 | ||
| 26 | 0.75 | 87 | ||
| 27 | PhCH2NH2 | PhCH2NHAc | 0.25 | 95 |
| 28 | PhNH2 | PhNHAc | 0.25 | 93 |
| 29 | 0.25 | 93 | ||
| 30 | 0.8 | 92 | ||
| 31 | 0.5 | 94 | ||
| 32 | 0.1 | 97 | ||
| 33 | 1.5 | 92 |
a Alcohols, phenols and thiols are acetylated at 80 ̊C.
b Amines are acetylated at room temperature.
c Products were identified spectroscopically and in comparison with authentic samples [26,27,31,36,40,43,87–97].
d Isolated yield.
e 0.6 g of rice husk was used.
From the context of green approach, the reusability of the catalyst was tested in the silylation and acetylation of 4-chlorobenzyl alcohol and N-Boc protection of aniline. After completion of the reaction, the catalyst was washed well with acetonitrile, acetone and water, then dried at 100 °C prior to use and tested for its activity in subsequent run and fresh catalyst was not added. It was seen that the catalyst displayed very good reusability (Fig. 1).

Reusability of rice husk in the silylation and acetylation of 4-chlorobenylalcohol (Table 1, entry 3 and Table 5, entry 2), and N-Boc protection aniline (Table 4, entry 1). The horizontal axis represents the usability times of the catalyst and the vertical one the conversion rate of the product.
4 Conclusions
In conclusion, we have developed an efficient method for the O-silyl protection of alcohols and phenols, N-Boc protection of amines and acetylation of alcohols, phenols, thiols and amines, producing remarkable high yields under very mild conditions. In contrast to some existing methods using potentially hazardous catalysts/additives, this new method offers the following advantages: (i) low cost, availability and reusability of the reagent, (ii) avoidance of the use of any base, metal, or Lewis acid catalysts, (iii) relatively short reaction times, (iv) easy and clean work-up, (v) high chemoselectivity, and (vi) no side reactions. We are exploring further applications of RiH for the other types of organic reactions in our laboratory.
Acknowledgments
We are thankful to the University of Guilan Research Council for the partial support of this work.


