1 Introduction
Multi-component reactions (MCRs) are powerful and useful synthetic tools to produce complex molecules from simple precursors by a one-pot procedure [1]. Since they are performed without the need to isolate any intermediate during their processes, this reduces time and saves both energy and raw materials [2]. Therefore, the design of novel MCRs has attracted great attention from research groups working in the field of medicinal chemistry, drug discovery, and materials science [3].
Indole and its derivatives are known as an important class of heterocyclic compounds and bioactive intermediates in R&D and pharmaceutical industry [4–12]. 3-Substituted indoles are important building blocks for the synthesis of various biologically active molecules [13–18]. The immense potential of indole nucleus as drug candidates prompted the synthetic chemists to explore different methods suitable for the synthesis of 3-substituted indoles. One such method is MCR. The atom-economy, convergent character, operational simplicity, structural diversity, and complexity of the molecules are the major advantages associated with MCRs.
2 Experimental
All commercially available chemicals were obtained from Merck and Fluka companies, and used without further purification unless otherwise stated. Nuclear magnetic resonance, 1H- and 13C-NMR spectra were recorded on Bruker Avance 300 FT NMR spectrometers. Infrared (IR) spectroscopy was conducted on a Perkin Elmer GX FT-IR spectrometer. Mass spectra were recorded on a Shimadzu QP 1100 BX Mass Spectromet. Elemental analyzes (C, H, N) were performed with a Heraeus CHN-Rapid analyzer.
2.1 Typical procedure for the preparation of N-((1H-indol-3-yl)(p-tolyl)methyl)2-amino-4,6-dimethylpyrimidin
To a stirred mixture of 4-methylbenzaldehyde (1 mmol, 0.120 g) and N,N,N’,N’-tetrabromobenzene-1,3-disulfonamide [TBBDA] (0.05 g, 0.09 mmol) or poly(N-bromo-N-ethyl-benzene-1,3-disulfonamide) [PBBS] (0.12 g) at room temperature, was added 2-amino-4,6-dimethylpyrimidin (1 mmol, 0.123 g). Then, indole (1 mmol, 0.12 g) was added to the mixture after 10 min. The progress of the reaction was monitored by TLC (n-hexane/acetone, 17:2). After completion of the reaction, EtOH (5 mL) was added to the reaction mixture and the colorless precipitate was filtered (after evaporation of the ethanol, cool methylene dichloride (2 mL) was added, and the catalyst was recovered), washed with EtOH and dried and purified by recrystallization from ethanol.
2.2 Physical and spectroscopic data
2.2.1 N-((1H-indol-3-yl)(p-tolyl)methyl) 2-amino-4,6-dimethylpyrimidine (Table 2, 4b)
Mp: 154–156 °C. Yield: 93%. IR (KBr): 3427, 1631, 1587, 1568, 1511, 1456, 1384, 1340, 1121, 743, 802, 743, 663 cm−1. 1H-NMR (FT-400 MHz, DMSO-d6): δ (ppm) 2.20 (9H, s), 6.36 (1H, s), 6.60 (1H, s), 6.62–7.63 (10H, m), 10.89 (1H, s). 13C-NMR (FT-400 MHz, DMSO-d6): δ (ppm) 21.1, 24.1, 51.1, 109.3, 111.9, 117.9, 118.9, 119.4, 121.5, 123.5, 123.6, 126.5, 127.7, 129.0, 135.8, 136.7, 136.9, 141.6, 162.0, 167.2. MS, m/z: 342 (M+, 27), 220 (73), 117 (50), 42 (100), 28 (40). Anal. Calcd. for C22H22N4: C, 77.16; H, 6.48; N, 16.36%. Found: C, 77.04; H, 6.36; N, 16.35%.
Synthesis of various 3-substituted indoles using TBBDA and PBBS at room temperature.
| Structure | Aldehyde | Amine | Producta | TBBDA | PBBS | Reference | ||
| Time (min) | Yield (%) | Time (min) | Yield (%) | |||||
| 4a | 25 | 98 | 40 | 85 | [18] | |||
| 4b | 25 | 93 | 45 | 90 | – | |||
| 4c | 40 | 87 | 60 | 88 | – | |||
| 4d | 30 | 80 | 60 | 75 | – | |||
| 4e | 20 | 92 | 35 | 87 | [18] | |||
| 4f | 55 | 87 | 90 | 85 | [18] | |||
| 4g | 70 | 63 | 90 | 66 | [18] | |||
| 4h | 40 | 83 | 60 | 76 | – | |||
| 4i | 40 | 76 | 70 | 70 | – | |||
| 4j | 35 | 86 | 50 | 63 | [18] |
a Products were characterized from their physical properties, by comparison with authentic samples, and by spectroscopic methods.
2.2.2 N-((2,3-dichlorophenyl)(1H-indol-3-yl)methyl)2-amino-4,6-dimethylpyrimidine (Table 2, 4c)
Mp: 209–211 °C. Yield: 87%. IR (KBr): 3424, 1588, 1563, 1507, 1451,1341, 1194, 1123, 830, 793, 740, 580 cm−1. 1H-NMR (FT-400 MHz, DMSO-d6): δ (ppm) 2.20 (6H, s), 6.40 (1H, s), 6.41 (1H, s), 6.79–7.79 (9H, m), 10.95 (1H, s). 13C-NMR (FT-400 MHz, DMSO-d6): δ (ppm) 24.0, 59.6, 109.8, 112.1, 115.7, 119.1, 119.2, 121.8, 124.1, 124.3, 126.6, 127.7, 128.4, 129.1, 130.9, 131.9, 136.8, 136.9, 144.9, 161.7, 167.3. MS, m/z: 396 (M+, 12), 361 (58), 238 (50), 173 (100), 29 (38).
2.2.3 N-((1H-indol-3-yl)(phenyl)methyl)2-amino-4,6-dimethylpyrimidine (Table 2, 4d)
Mp: 200–201 °C. Yield: 80%. IR (KBr): 3426, 3240, 1586, 1567, 1508, 1489, 1455, 1340, 1121, 1013, 832, 806, 744, 619, 479 cm−1. 1H-NMR (FT-400 MHz, DMSO-d6): δ (ppm) 2.21 (6H, s), 6.38 (1H, s), 6.60 (1H, s), 6.62–7.76 (10H, m), 10.95 (1H, s). 13C-NMR (FT-400 MHz, DMSO-d6): δ (ppm) 23.9, 50.9, 109.6, 112.0, 117.1, 119.0, 119.3, 119.9, 121.6, 123.8, 123.8, 126.3, 130.0, 131.3, 136.9, 144.1, 161.9, 167.2. MS, m/z: 328 (M+, 18), 251 (5), 206 (38), 118 (50), 42 (100), 28 (49). Anal. Calcd. for C21H20N4: C, 76.80; H, 6.14; N, 17.06%. Found: C, 76.56; H, 6.14; N, 17.08%.
2.2.4 N-((2,6-dichlorophenyl)(1H-indol-3-yl)methyl)2-aminopyrimidine (Table 2, 4h)
Mp: 161–162 °C Yield: 83%. IR (KBr): 3428, 3181, 1586, 1567, 1509, 1485, 1455, 1340, 1121, 1070, 1009, 804 cm−1. 1H-NMR (FT-90 MHz, DMSO-d6): δ (ppm) 6.66 (1H, s), 6.88–8.30 (12H, m), 11.02 (1H, s). 13C-NMR (FT-400 MHz, DMSO-d6): δ (ppm) 49.7, 105.6, 111.5, 112.2, 112.5, 118.9, 119.3, 121.7, 124.1, 124.3, 126.3, 129.9, 137.0, 137.3, 158.5, 161.6, 162.5. MS, m/z: 368 (M+, 2), 238 (5), 176 (11), 175 (71), 94 (37), 28 (100), 26 (27). Anal. Calcd. for C21H18Cl2N4: C, 61.80; H, 3.82; N, 15.17%. Found: C, 54.73; H, 3.38; N, 25.23%.
2.2.5 N-((1H-indol-3-yl)(4-nitrophenyl)methyl)2-aminopyrimidine (Table 2, 4i)
Mp 184–185 °C. Yield: 76%. IR (KBr): 3415, 3225, 1590, 1515, 1449, 1414, 1344, 1252, 1106, 1012, 870, 802, 739, 605, 425 cm−1. 1H-NMR (FT-400 MHz, DMSO-d6): δ (ppm) 6.62 (1H, s), 6.64 (1H, s), 6.68–8.32 (12H, m), 10.05 (1H, s). 13C-NMR (FT-400 MHz, DMSO-d6): δ (ppm) 51.4, 111.2, 112.1, 116.1, 119.1, 119.3, 121.9, 123.9, 124.1, 124.3, 126.3, 128.7, 136.8, 146.7, 152.3, 158.5, 161.9. MS, m/z: 345 (M+, 4), 298 (2), 204 (10), 117 (39), 28 (100), 27 (46). Anal. Calcd. for C19H15N5O2: C, 66.08; H, 4.38; N, 20.28%. Found: C, 65.91; H, 4.24; N, 20.18%.
3 Results and discussion
In continuation of our interest in the application of TBBDA and [PBBS] [16] in organic synthesis [19–31], we wish to report here a facile and improved protocol for the preparation of new 3-substituted indoles from heteroarylamines, aromatic aldehydes, and indole in the presence of TBBDA and PBBS as catalysts under solid-state conditions at room temperature (Scheme 1).
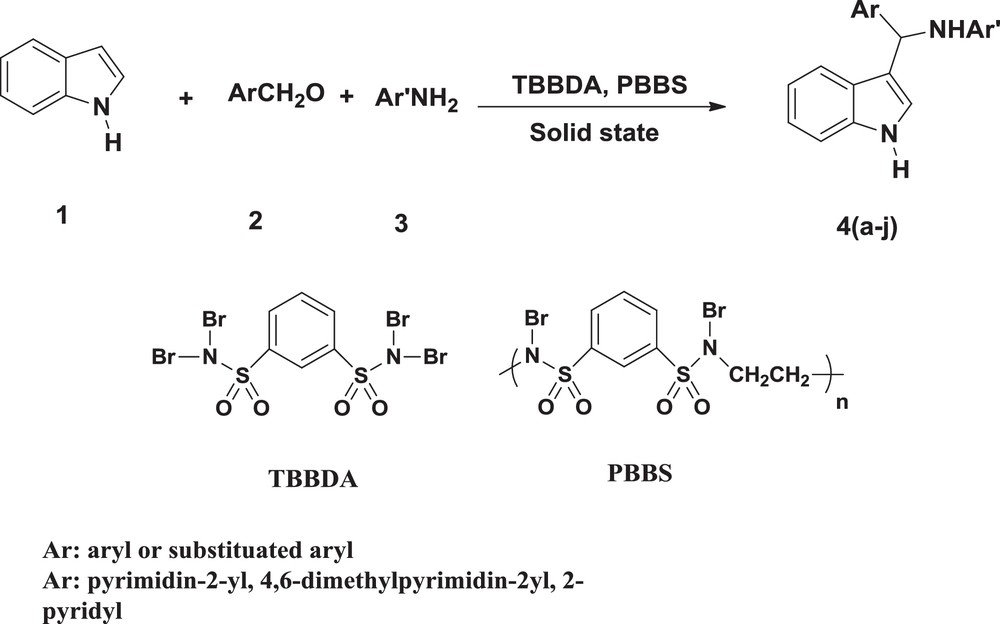
Three-component synthesis of 3-substituted indoles.
The advantages of TBBDA and PBBS are as follows:
- • the preparation of TBBDA and PBBS is easy;
- • TBBDA and PBBS are stable under atmospheric conditions for two months;
- • after completion of the reaction, the catalysts are recovered and can be reused several times without decreasing the yield.
Initially, we decided to explore the role of our catalyst in EtOH as a solvent system for the preparation of 3-substituted indoles. In the absence of catalyst, no 3-substituted indole was observed, even after prolonged reaction time. Since the synthesis of 3-substituted indole failed in the absence of catalyst, the effect of the catalyst was also investigated in various conditions, and the results are presented in Table 1.
Optimization of reaction conditions for the synthesis of 3-substituted indoles.
| Entry | Solvent/Condition | Catalyst TBBDA (g) | Time (min) | Yield (%)a |
| 1 | EtOH/rt | No Catalyst | 380 | 27 |
| 2 | EtOH/rt | 0.05 | 70 | 52 |
| 3 | EtOH/rt | 0.08 | 70 | 65 |
| 4 | EtOH/rt | 0.1 | 70 | 76 |
| 5 | Neat/rt | No Catalyst | 340 | 30 |
| 6 | Neat/rt | 0.02 | 60 | 56 |
| 7 | Neat/rt | 0.03 | 55 | 67 |
| 8 | Neat/rt | 0.04 | 35 | 78 |
| 9 | Neat/rt | 0.05 | 25 | 98 |
| 10 | Neat/rt | 0.06 | 25 | 96 |
| 11 | Neat/rt | 0.07 | 25 | 88 |
| 12 | Neat/rt | 0.08 | 25 | 95 |
a Standardization of reaction conditions: 2-chlorobenzaldehyde (1 mmol, 0.140 g), 2-amino-4,6-dimethylpyrimidine (1 mmol, 0.12 g), indole (1 mmol, 0.12 g).
In the solvent system, the best results were achieved using 0.1 g, 0.18 mmol of catalyst (Table 1, entry 4). In recent years, there has been an increasing interest in reactions that proceeded in the absence of a solvent due to reduced pollution, low cost, simplicity in process and handling. Therefore, we decided to test this reaction in the solid state and in various ratio of catalyst. We found that the reaction was rapid and gave excellent yields of the product when catalyzed by TBBDA (25 min, 98%, entry 9).
These results encouraged us to investigate the scope and generality of this new protocol for various 3-substituted indoles under optimized conditions in the presence of catalytic amounts of either TBBDA or PBBS. As shown in Table 2, a series of aromatic aldehydes containing either electron-withdrawing or electron-donating substituents successfully reacted with indole and various amines, such as 2-amino-4,6-dimethylpyrimidine, 2-aminopyrimidine, 2-aminopyridine, affording good to high yields of products with high purity, at room temperature under solid-state conditions. Our attempt for the reaction of aromatic aldehyde with OMe substituents failed.
All these reactions underwent smoothly to provide the corresponding 3-substituted indole, in moderate to good yields (Table 2, entries 1–10). The present method failed to furnish the expected 3-substituted indole derivative with 4-aminopyridine. In general, aromatic aldehydes bearing electron-donating or electron-withdrawing functional groups at different positions reacted smoothly with arylamine in the presence of TBBDA and indole to generate the corresponding products in good to excellent yields. The products were characterized by IR, 1H-NMR, 13C-NMR spectra and mass spectra.
TBBDA and PBBS are stable, non-volatile, inexpensive, and safe catalysts.
It is likely that these catalysts release Br+ in situ, which can act as an electrophilic species [19–31]. Therefore, the mechanism shown in Scheme 2 can be suggested for the conversion of the indole, aldehyde, and arylamine, to 3-substituted indole [4].
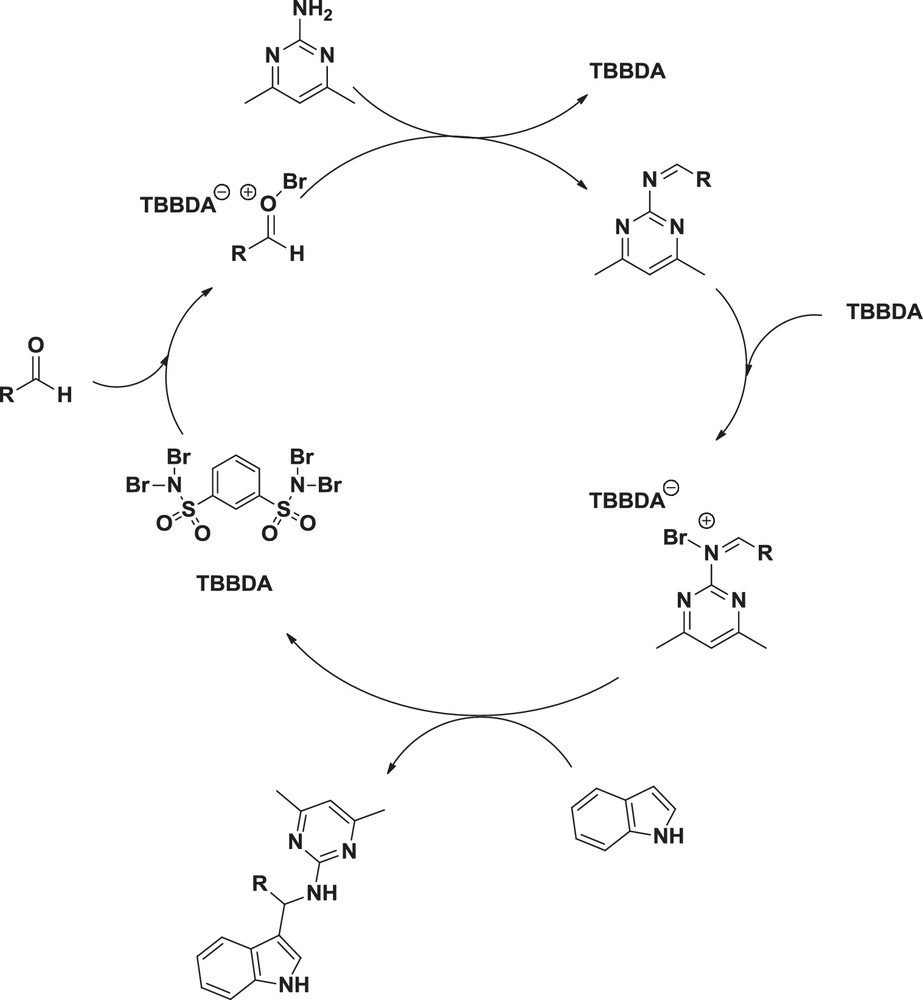
Suggested mechanism for synthesis of 3-substituted indoles.
4 Conclusions
In summary, we have developed a new facile protocol for the synthesis of new N-[(1H-indol-3-yl) arylmethyl] heteroarylamines derivatives from the reaction of aldehydes, indole, and arylamine compounds using TBBDA and PBBS under solvent-free conditions. The catalysts are heterogeneous, recyclable, non-corrosive and environmentally benign.
Acknowledgements
We are thankful to Bu-Ali Sina University, the Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for financial support.


