1 Introduction
Oxidation of bis(2-hydroxy-1-naphthyl)methanes as a subunit of calix[n]arenes (i) has been used for the preparation of spirodienones [1] (ii), which is an important reaction in the biosynthesis of certain plant products (Fig. 1) [2–4].
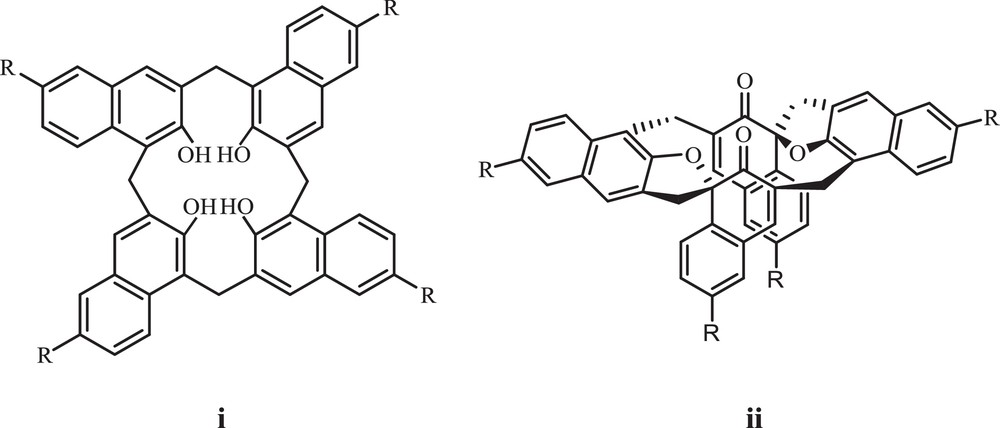
Calix[n]arenes (i) and spirodienones (ii).
Abel, in 1892, reported the oxidation of bisnaphthols to spirodienones with Br2 in alkaline solution [3]. The product was believed to be a peroxide, but on the basis of chemical evidence its structure was assigned as spirodienone [4,5]. Kasturi et al. perfectly studied the chemistry of spirodienone family [6]. According to Kasturi's reports, Abel's ketone derivatives have two sets of diastereomers which are assigned by the fact that one diastereomer, 3, shows in its 1HNM-R spectrum a doublet near δ6.1 ppm (vinylic H-3’); while for the other one, 4, this hydrogen appears at about δ5.4 ppm, the up-field shift being due to the shielding effect of the μ-phenyl ring (Fig. 2) [7]. It has been reported that different oxidants give either one isomer (3 or 4) or a mixture of the two isomeric products. For example, hexacyanoferrate (in benzene and pyridine) [7], 2,4-di-t-butyl-6-phenylphenoxyl [8], 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) [9] and hydrogen peroxide/MoO3 [10] give a mixture of two isomeric spiro products. Trichloroisocyanuric acid (TCCA) [11], potassium hypobromite and persulphate give 3 [7], whereas aerial oxidation in the presence of Ph3Bi [12], periodic acid or its sodium salt, and (diacetoxyiodo)benzene in benzene is specific for 4 [8].

Abel's ketone derivatives (3 and 4).
Most of these procedures have certain limitations, such as tedious procedure, long reaction time, harsh reaction conditions, poor yields and the use of toxic or expensive reagents (Fig. 2). In continuation of our research area for the oxidation of bisnaphthols [11,12], we were interested in the use of TEMPO as a catalyst. Based on literature survey and our previous experience, it was concluded that a suitable candidate might be 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) [a stable free nitroxyl radical], which has been used in many areas of synthetic organic chemistry as a safe, weakly toxic and highly efficient catalyst with the possible achievement of chemoselectivity in the oxidation processes [13–20]. TEMPO is quite an expensive reagent from which the separation of oxidation products requires lengthy work-up procedures. In order to fulfill the recovery problem, TEMPO has been immobilized on either inorganic or organic supports such as silica [15], organic polymers [16], mesoporous silica [17], functionalized ionic liquids [18], and perfluoroalkyl systems with multiple triazole moieties [19].
Recently, a good strategy has been proposed by Karimi et al. for the aerial oxidation of alcohols using magnetic core-shell nanoparticle-supported TEMPO as a high activity catalyst, which is easily separated with an external magnetic field [20]. In addition, much attention has been paid to the use of molecular oxygen or air as a highly green oxidant [14,21,22]. Moreover, taking into account the fact that iron is an abundant inexpensive and environmentally friendly metal, iron chloride has attracted a great deal of attention in modern chemistry [23–25]. With the above consideration and the need for new and green procedures with minimum wastes, a key challenge for today's environment, we now report the aerial oxidative cyclization of bisnaphthols using MNST in the presence of FeCl3·6H2O (Scheme 1).
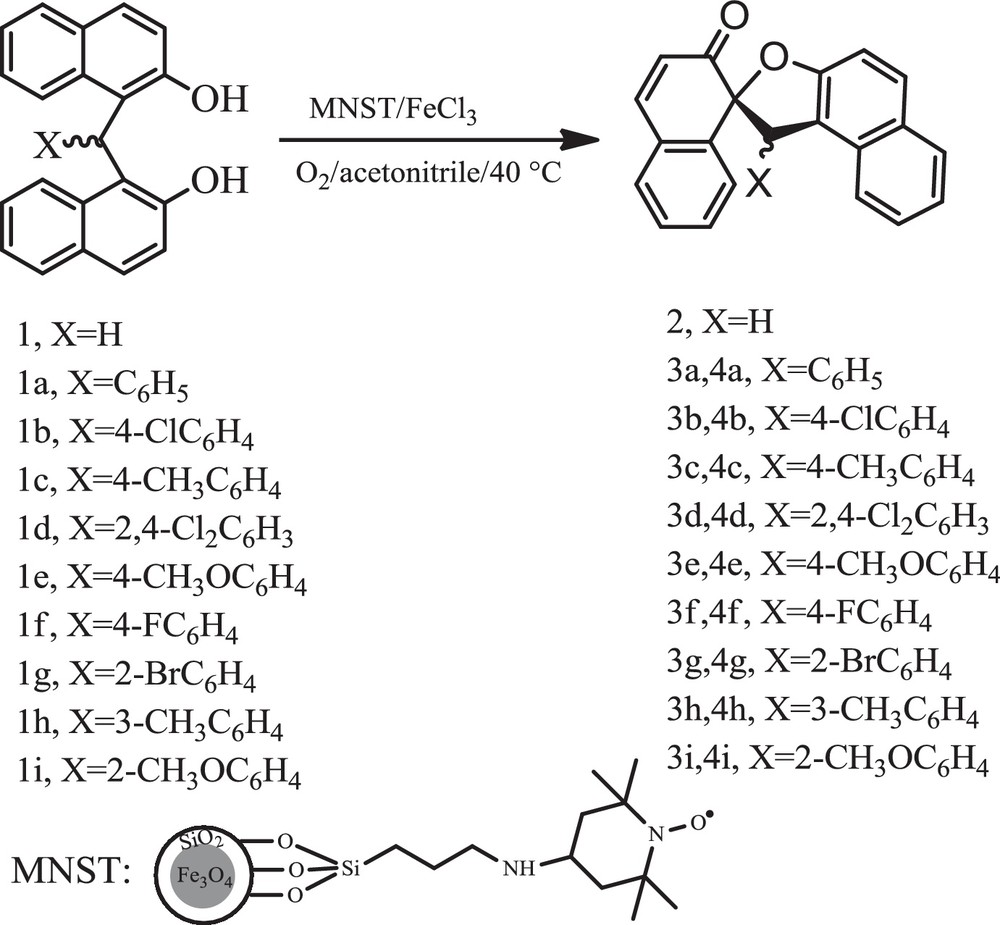
Oxidation of bisnaphthols.
2 Results and discussion
At the outset, to optimize the reaction conditions, the aerial oxidation of bisnaphthol 1 was run using MNST in the presence of different transition metal salts, such as Fe (III), Mn (II), Cu (II), Co (II) as well as Nano–Fe3O4 as a co-catalyst in acetonitrile at 40 °C, with bubbling air into the reaction vessel (Table 1). As is clear from this Table, the best results were obtained with FeCl3·6H2O as a co-catalyst (Table 1, Entries 6-8). The amount of FeCl3·6H2O was optimized at 1 mol% in order to reduce the reaction time and to increase the product yield (Table 1, Entry 6).
Aerial oxidation of bisnaphthol 1 in the presence of different co-catalysts.
| Entry | Co-catalyst (mol%) | Time (h) | Yielda (%) |
| 1 | CuCl2·4H2O (1) | 10 | Trace |
| 2 | Mn(OAc)2·4H2O (1) | 7 | Trace |
| 3 | Co(OAc)2·4H2O (1) | 10 | Trace |
| 4 | M*(acac)2 (1) | 8 | 25 |
| 5 | Nano–Fe3O4 (1) | 10 | – |
| 6 | FeCl3·6H2O (1) | 3 | 80 |
| 7 | FeCl3·6H2O (3) | 15 | 60 |
| 8 | FeCl3·6H2O (0.3) | 8 | 45 |
a Separated yields; Reaction conditions: bisnaphthol 1 (1 mmol), MNST (0.005 g, 0.001 mmol with respect to the TEMPO), co-catalyst, acetonitrile (20–25 ml), 40 °C, bubbling air.
Control tests in various solvents, such as acetonitrile, ethanol, acetone, dichloromethane and water showed that acetonitrile would be the best choice for the reaction to proceed. An additional experiment was also performed for three runs to test the reusability of the catalyst, using oxidation of bisnaphthol 1 as a model reaction. After each run, the catalyst was washed twice (2 × 10 ml) with hot ethanol and dried. The reused catalyst was found to be efficient, without significant loss in product yield (Table 2, Entry 1). It should be noted that the co-presence of MNST and FeCl3 was found to be necessary for the aerial oxidation of bisnaphthols to occur (Scheme 1, Table 2).
Aerial oxidation of bisnaphthols using MNST.
| Entry | X | Product | Time (h) | Yield (%)a | Melting point (°C) | Diast.b (%) | ||||
| Found | Lit [10–12] | |||||||||
| 3 | 4 | 3 | 4 | 3 | 4 | |||||
| 1 | H | 2 | 4 | 80, 76c 76d | 171–172 | 170–171 | – | – | ||
| 2 | C6H5 | 3a, 4a | 4 | 75 | 210–211 | 265–266 | 210–211 | 263–264 | 85 | 15 |
| 3 | 4-ClC6H4 | 3b, 4b | 3 | 68 | 264–265 | 262–263 | 262–263 | 262–263 | 20 | 80 |
| 4 | 4-CH3C6H4 | 3c, 4c | 4 | 70 | 198–199 | 228–210 | 198–200 | 227–229 | 50 | 50 |
| 5 | 2,4-Cl2C6H3 | 3d, 4d | 5 | 73 | 208–209 | 195–197 | 204–206 | 195–197 | 55 | 45 |
| 6 | 4-CH3OC6H4 | 3e, 4e | 3 | 82 | 200–202 | 219–221 | 199–200 | 217–220 | 75 | 25 |
| 7 | 4-FC6H4 | 3f, 4f | 3 | 78 | 214–216 | 145–146 | 214–215 | 145–146 | 55 | 45 |
| 8 | 2-BrC6H4 | 3g, 4g | 5 | 78 | 219–221 | 147–149 | 218–223 | 146–148 | 50 | 50 |
| 9 | 3-CH3C6H4 | 3h, 4h | 5 | 75 | 187–189 | 225–227 | 187–188 | 225–227 | 70 | 30 |
| 10 | 2-CH3OC6H4 | 3i, 4i | 4 | 73 | 228–231 | 194–196 | 229–2230 | 194–196 | 60 | 40 |
a Total yield (isomer 3 + isomer 4).
b Diastereomeric ratio. The ratio of the two diastereomers, 3 and isomer 4, was determined by 1H-NMR.
c Reuse of MNST in second run.
d Reuse of MNST in third run.
As shown in Table 2, the aerial oxidative cyclization of bisnaphthol derivatives works well with a variety of substituted phenyl moieties bearing both electron-withdrawing and electron-donating groups, such as OMe, Me, F, Cl and Br. In all the cases, with the exception of bisnaphthol 1, it was noticed that the oxidation reaction led to the synthesis of two sets of diastereomers, 3 and 4 (Table 2, Entry 1–10). As it was mentioned earlier, the distinctive feature of the two diastereomers is the different chemical shifts of the corresponding vinylic hydrogens (H-3’). Therefore, their percentage was obtained from the integral ratio of H3’ hydrogens. As it has been stated by Dean and co-workers, the diastereomeric ratio 3:4 is nearly independent of the nature of the substituents on X [8]. In addition, as reported in the literature, the colour of 3 is bright yellow, while 4 is cream or faintly yellow in colour [8].
A mechanistic rationalization for the aerial oxidation of bisnaphthols is shown in Scheme 2. As reported in literature [11,12,14,23,26,27], at the first step, Fe (III) and MNST give active Fe (III)–MNST complexes. The next step is the coordination of bisnaphthol to Fe (III)–MNST complex to produce intermediate A. Abstraction of the hydrogen of O–H by MNST then results in a radical intermediate B, which affords diradical species C, MNSTH and Fe (II) species after intramolecular one-electron transfer reaction. Finally, diradical species C leads to the formation of spiro products. The resulting Fe (II) species will then react with MNST via another one-electron transfer to give Fe (III). MNSTH is finally oxidized by molecular oxygen to MNST and water.

A plausible mechanism for the aerial oxidation of bisnaphthols.
3 Conclusions
In conclusion, we have developed the oxidation of bisnaphthols to their corresponding spirans (a mixture of two diastereomers, 3 and 4) in the presence of MNST/FeCl3·6H2O/O2 as a highly efficient and environmentally friendly catalyst. This work consistently has several advantages, such as using magnetically separable TEMPO catalyst, the reusability of MNST, small amounts of FeCl3·6H2O as an inexpensive co-catalyst, air as a green oxidant, fairly short reaction times, moderate to good yields, mild reaction conditions, highly economic and simple experimental procedure.
4 Experimental
4.1 General procedures and materials
All solvents and reagents were obtained from Merck and used without further purification. Arylbisnaphthols (Hewitt's method) [8,28,29], methylenbisnaphthol (Mironove's method) [30] and magnetic core-shell nanoparticle-supported TEMPO (MNST) [20] were prepared according to the reported procedures. The NMR spectra were run on Jeol 90 MHz and Bruker 500 MHz instruments. Melting points were determined on a Stuart Scientific SMP3 apparatus and are uncorrected. Chromatographic separations were performed on silica gel 60 (230–400 mesh). The desired pure products were identified by comparison of their physical and spectroscopic data with those of known compounds [10–12].
4.2 A typical procedure for the oxidation of bisnaphthols
Into a round bottom flask (50 ml), a mixture of bisnaphthol (1 mmol), FeCl3·6H2O (0.01 mmol) and MNST (0.005 g) was poured in acetonitrile (20–25 ml). Into the resulting mixture air was bubbled at 40 °C (for the indicated time in Table 2). The progress of the reaction was followed by TLC. After completion of the reaction, the catalyst (MNST) was separated by an external magnet, the excess solvent concentrated by evaporation and the crude mixture was purified by column chromatography (ethyl acetate:n-hexane, 2:10) to obtain the pure product. The Rf values were also determined using the ratio of ethyl acetate to hexane (see Table 3). All of the desired products were characterized by comparison of their physical and 1H-NMR data with those of known compounds [10–12].
Rf values of products.
| Entry | X | Producta | Rf | |
| 3 | 4 | |||
| 1 | H | 2 | 0.55 | |
| 2 | C6H5 | 3a, 4a | 0.68 | 0.58 |
| 3 | 4-ClC6H4 | 3b, 4b | 0.54 | 0.42 |
| 4 | 4-CH3C6H4 | 3c, 4c | 0.74 | 0.65 |
| 5 | 2,4-Cl2C6H3 | 3d, 4d | 0.48 | 0.37 |
| 6 | 4-CH3OC6H4 | 3e, 4e | 0.77 | 0.67 |
| 7 | 4-FC6H4 | 3f, 4f | 0.58 | 0.42 |
| 8 | 2-BrC6H4 | 3g, 4g | 0.71 | 0.54 |
| 9 | 3-CH3C6H4 | 3h, 4h | 0.62 | 0.55 |
| 10 | 2-CH3OC6H4 | 3i, 4i | 0.71 | 0.61 |
a In each case, the two isomers 3 and 4 could be separated by column chromatography (ethyl acetate:n-hexane, 2:10).
4.2.1 Spiro{naphthalene-1(2H),2′(1′H)-naphtho[2,1-b]furan}-2-one (2)
Yellow solid, yield 80%; IR (νmax, cm−1): 1685 (CO). 1H-NMR (90 MHz, CDCl3): δ3.50 and 4.1 (2H, dd, J = 16 Hz due to hydrogens number 1); δ6.30 (1H, J = 9.9 Hz due to hydrogen number 3′); δ6.90–7.99 (11H, Ar and hydrogen number 4′).
4.2.2 1′-Phenyl-spiro{naphthalene-1(2H),2′(1′H)-naphtho[2,1-b]furan}-2-one (3a and 4a)
Yellow solid, yield 75%; IR (νmax, cm−1): 1687 (CO). 1H NMR (90 MHz, CDCl3): δ5.21 and 5.40 (2H, s, hydrogen number 1); δ5.53 and 6.28 (1H, J = 10 Hz and J = 9.9 Hz due to hydrogen number 3′); δ6.95–7.99 (11H, Ar and hydrogen number 4′).
4.2.3 1′-(4-Chlorophenyl)-spiro{naphthalene-1(2H),2′(1′H)-naphtho[2,1-b]furan}-2-one (3b and 4b)
Yellow solid, yield 68%; IR (νmax, cm−1): 1678 (CO). 1H-NMR (90 MHz, CDCl3): δ5.17 and 5.36 (1H, s, hydrogen number 1); δ5.58 and 6.27 (1H, d, J = 9.8 Hz and J = 10 Hz due to hydrogen number 3′); δ6.87–7.98 (15H, Ar and hydrogen number 4′).
4.2.4 1′-(4-Methylphenyl)-spiro{naphthalene-1(2H),2′(1′H)-naphtho[2,1-b]furan}-2-one (3c and 4c)
Yellow solid, yield 70%; IR (νmax, cm−1): 1680 (CO). 1H-NMR (500 MHz, CDCl3): δ2.12 and 2.27 (3H, s); δ5.18 and 5.37 (1H, s, hydrogen number 1); δ5.55 and 6.28 (1H, d, J = 9.99 Hz and J = 9.92 Hz due to hydrogen number 3′); δ6.66–7.89 (15H, Ar and hydrogen number 4′).
4.2.5 1′-(2,4-Dichlorophenyl)-spiro{naphthalene-1(2H),2′(1′H)-naphtho[2,1-b]furan}-2-one (3d and 4d)
Yellow solid, yield 73%; IR (νmax, cm−1): 1683 (CO). 1H-NMR (90 MHz, CDCl3): δ5.52 and 5.63 (1H, s, hydrogen number 1); δ5.58 and 6.23 (1H, d, both J = 10 Hz due to hydrogen number 3′); δ6.65–8.00 (14H, Ar and hydrogen number 4′).
4.2.6 1′-(4-Methoxyphenyl)-spiro{naphthalene-1(2H),2′(1′H)-naphtho[2,1-b]furan}-2-one (3e and 4e)
Yellow solid, yield 82%; IR (νmax, cm−1): 1684 (CO). 1H-NMR (90 MHz, CDCl3): δ3.64 and 3.75 (3H, s); δ5.19 and 5.37 (1H, s, hydrogen number 1); δ5.57 and 6.26 (H, d, J = 9.9 Hz and J = 10 Hz due to hydrogen number 3′); δ6.48–7.93 (15H, Ar and hydrogen number 4′).
4.2.7 1′-(4-Fluorophenyl)-spiro{naphthalene-1(2H),2′(1′H)-naphtho[2,1-b]furan}-2-one (3f and 4f)
Yellow solid, yield 78%; IR (νmax, cm−1): 1676 (CO). 1H-NMR (500 MHz, CDCl3): δ5.19 and 5.38 (1H, s, hydrogen number 1); δ5.57 and 6.27 (1H, d, J = 9.98 Hz and J = 9.92 Hz due to hydrogen number 3′); δ6.26–7.9 (15H, Ar and hydrogen number 4′).
4.2.8 1′-(2-bromophenyl)-spiro{naphthalene-1(2H),2′(1′H)-naphtho[2,1-b]furan}-2-one (3g and 4g)
Yellow solid, yield 78%; IR (νmax, cm−1): 1687(CO). 1H-NMR (90 MHz, CDCl3): δ5.48 and 5.59 (1H, s, hydrogen number 1); δ5.53 and 6.26 (1H, d, both J = 10 Hz due to hydrogen number 3′); δ6.57–7.98 (15H, Ar and hydrogen number 4′).
4.2.9 1′-(3-Methylphenyl)-spiro{naphthalene-1(2H),2′(1′H)-naphtho[2,1-b]furan}-2-one (3h and 4h)
Yellow solid, yield 75%; IR (νmax, cm−1): 1687(CO). 1H-NMR (90 MHz, CDCl3): δ2.14 and 2.29 (3H, s); δ5.19 and 5.38 (1H, s, hydrogen number 1); δ5.56 and 6.27 (H, d, J = 9.9 Hz and J = 10 Hz due to hydrogen number 3′); δ6.47–7.97 (15H, Ar and hydrogen number 4′).
4.2.10 1′-(2-Methoxyphenyl)-spiro{naphthalene-1(2H),2′(1′H)-naphtho[2,1-b]furan}-2-one (3i and 4i)
Yellow solid, yield 73%; IR (νmax, cm−1): 1681(CO). 1H-NMR (90 MHz, CDCl3): δ3.55 and 3.66 (3H, s); δ5.44 and 5.55 (1H, s, hydrogen number 1); δ5.35 and 6.23 (H, d, J = 9.9 Hz and J = 10 Hz due to hydrogen number 3′); δ6.63–7.96 (15H, Ar and hydrogen number 4′).
Acknowledgments
The authors gratefully acknowledge the financial support for this work from the Bu-Ali Sina University, Hamedan, Iran.


