1 Introduction
Carbon–carbon bond-forming reactions, involving carbon–hydrogen activation, are valuable processes and have become a powerful tool for the formation of more complex building blocks [1]. Cross dehydrogenative coupling reaction is a particularly attractive challenge in organic synthesis as it will not only improve atom economy but also increase the overall efficiency of multi-step synthetic sequences. An efficient coupling has been developed by Fujiwara and Moritani, involving arenes and activated alkenes through an oxidative palladium(II)-catalyzed process [2]. Although a great number of aromatic substrates have been employed in the last few years [3], only a limited substrate scope is reported concerning non-aromatic substrates with alkenes [4].
The nitrogen atom is ubiquitous in nature and life sciences and π-electron-rich olefins, such as enamides, are important motifs that have been widely used as synthons in the synthesis of various compounds [5]. Thus, the development of convenient as well as step-economical methodologies for the construction of small but complex nitrogen-containing molecules, following the Diversity-Oriented Synthesis (DOS) program [6], is currently an ongoing project in our laboratory [7]. Indeed, biological screening requires the upstream rapid synthesis of large numbers of structurally similar compounds. Within this area, we focused our attention on octa- or tetrahydroquinoline derivative systems, which are omnipresent in various alkaloids, pharmaceutical agents or dye sensitizers (Fig. 1) [8].
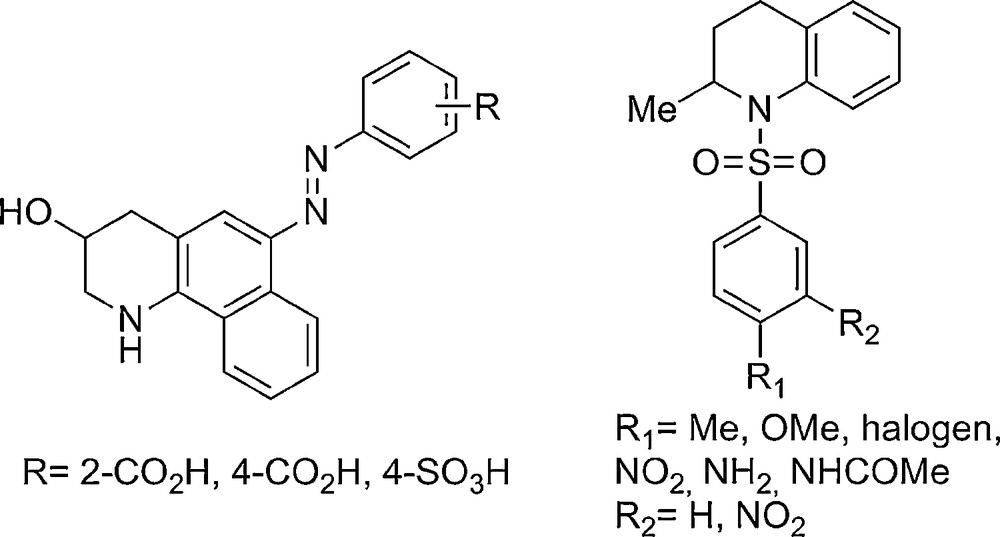
Representative examples of tetrahydroquinoline derivatives.
In the light of our recent results in this area [7], we speculated that we could readily obtain polyfunctionalized tetrahydroquinoline derivatives from conjugated enamides via Diels–Alder cycloadditions (Fig. 2). The latter could be generated according to a palladium-catalyzed Fijuwara–Moritani reaction, starting from non-aromatic enamides and activated alkenes. While C-2 functionalization was recently developed by direct arylation, especially in the case of acyclic compounds [9], to the best of our knowledge, the regioselective C-3 functionalization of non-substituted enamides has not yet been described, except in our work [7b].

Retrosynthetic strategy.
Recent advances in this area have shown that an oxidant (Cu(OAc)2·H2O), a co-oxidant (O2) and an acidic source are required to perform the direct Fijuwara–Moritani cross-coupling reaction efficiently. Having established the optimal conditions [7b], a variety of enamides and activated alkenes were found to undergo direct alkenylation in fair to good yields (Scheme 1). A first series of ester acrylates was investigated, affording the desired conjugated enamides 3a–c in good yields. These newly formed enamides were isolated with an absolute regio- and stereoselectivity control (E-isomer). Polar functional groups (sulfonyl, aldehyde, ketone, phosphonate or amide) are also tolerated, giving scaffolds 3d–i in moderate to high yields and demonstrating the potential of this protocol. More remarkably, starting from methyl metacrylate, the compound 3j was unfortunately obtained as an inseparable mixture of regioisomers. The dynamic β-hydride elimination derivative was, thus, in fact observed when the methyl group was present at the α-position of the acrylate. The substrate scope was increased by using various protecting groups on the nitrogen atom and the nature of the cyclic enamide. Pleasingly, the original conjugated enamides, 3k–m, were synthesized in good yields and the protocol was also amenable to endo-enamides (3n–o). In contrast, the introduction of a C2-electron-rich phenyl substituent to the cyclic enamide or the presence of a heteroatom (O or S) in the beta position significantly decreased the yield of the coupling reaction (Scheme 2). We speculated that the use of Ag2CO3 in pure pivalic acid would facilitate the desired C–H functionalization. Indeed, as reported in the literature, the preference for pivalic acid in comparison to acetic acid, which is less voluminous, is explained by a greater steric encumbrance as well as a higher basicity of its conjugate base [10]. Finally, these conditions led to the products 3p–r in moderate to good yields.
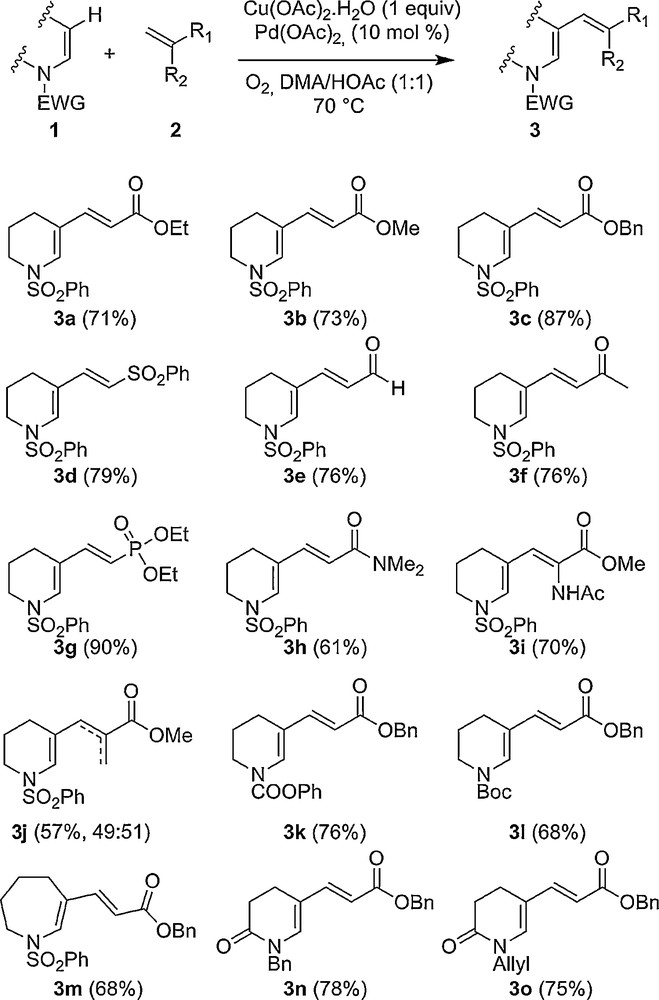
Direct coupling involving enamides 1 and alkenes 2.

Direct coupling involving complex enamides 1 and alkenes 2.
Keeping in mind that molecular diversity is a perpetual challenge and having synthesized a range of simple conjugated enamides, we wished to take advantage of their reactivity towards the Diels–Alder cycloaddition reaction in order to generate, in a one-step process, a range a polyfunctionalized quinoline scaffolds. Diene 3c was then submitted to a series of dienophiles (Scheme 3). The use of either N-methylsuccinimide or succinimide afforded the two cycloadducts, 4a and 4b, in high yields, giving access to the corresponding original functionalized octahydroquinoline skeleton. Performing the cycloaddition reaction in the presence of maleic anhydride was also efficient, leading to the desired cycloadduct in 77% yield. Unfortunately, using 1,4-benzoquinone as dienophile was unreactive in these conditions, only slight traces of the desired product were isolated, accompanied by degradation products.
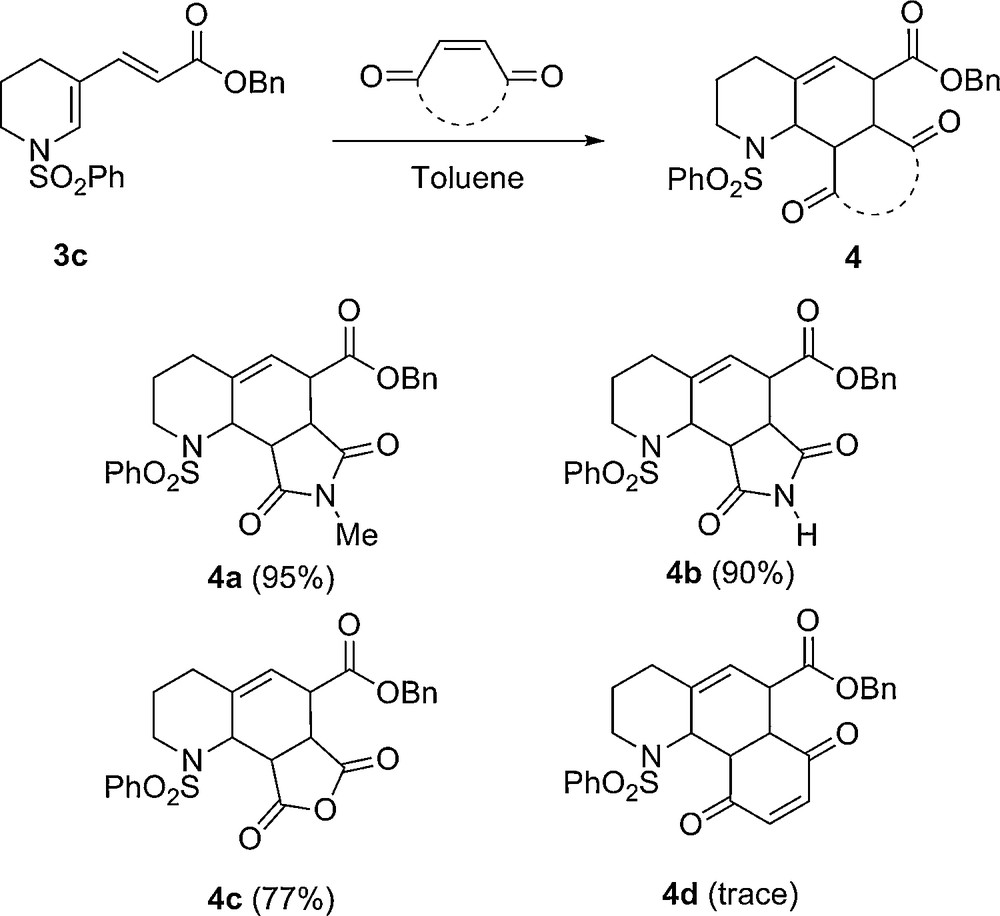
Diels–Alder cycloaddition reactions.
Finally, in order to obtain tetrahydroquinoline derivatives, cycloadduct 4a, chosen as a model, was submitted to an oxidative step. Unfortunately, a total lack of reactivity was observed by using DDQ as the oxidant even when the loading of oxidant, the reaction time or the temperature were increased. Using MnO2 led to the aromatized product 5 in good yield (Scheme 4). These results again highlight the potential of this process in combination with further conventional transformations.

Oxidation of product 4a.
2 Conclusion
In summary, we have developed an efficient synthesis of conjugated enamides via the Fujiwara–Moritani reaction. This simple and efficient approach was performed under mild conditions, and tolerated a wide range of enamides as well as activated alkenes. The synthetic utility of the dienes obtained was demonstrated via the synthesis of octahydroquinoline scaffolds via Diels–Alder cycloaddition. One of the latter intermediates was finally oxidized, giving the corresponding polyfunctionalized tetrahydroquinoline derivative. Further applications of this methodology for the generation of suitably functionalized key scaffolds are in progress and the results of these investigations will be reported in due course.
3 Experimental
The infrared spectra of compounds were recorded on a Thermo Scientific Nicolet iS10. 1H-and 13C-NMR spectra were recorded on a spectrometer at 250 MHz (13C, 62.9 MHz) or 400 MHz (13C, 100 MHz). Chemical shifts are given in parts per million from tetramethylsilane (TMS) as an internal standard. Coupling constants (J) are reported in Hertz (Hz). An electronic ion spray methodology was used to record mass spectra. All the reagents were obtained from commercial suppliers unless otherwise stated.
3.1 General experimental procedure
3.1.1 Direct coupling
To a solution of enamide (0.22 mmol) in a mixture of dimethylacetamide/acetic acid (1:1, 0.60 mL) was added Pd(OAc)2 (10% mol) followed by Cu(OAc)2·H2O (0.22 mmol) and alkene (0.44 mmol). A slow O2 bubbling was injected into the solution. The mixture was heated to 70 °C until the disappearance of the starting material. The reaction was then hydrolyzed by the addition of water. The aqueous layer was extracted with AcOEt and the combined organic extracts were washed with a saturated aqueous solution of NaHCO3, with brine, dried over MgSO4, filtered and concentrated. The products were purified by flash chromatography with petroleum ether/ethyl acetate to yield the desired products.
3.1.2 Diels–Alder reaction
To a solution of diene (0.13 mmol) in dry toluene (2 mL) was added dienophile (0.20 mmol). The mixture was heated under reflux until the disappearance of the starting material (TLC control). After the evaporation of the solvent, the products were purified by flash chromatography with petroleum ether/ethyl acetate to yield the desired products.
3.1.3 Oxidation step
To a solution of octahydroquinoline derivative (0.07 mmol) in dry CHCl3 (2 mL) was added MnO2 (1.42 mmol). The mixture was heated under reflux until the disappearance of the starting material (TLC control). After cooling and filtration on Celite, the solvent was evaporated. The product was purified by flash chromatography with petroleum ether/ethyl acetate to yield the desired compound.
3.2 Spectral data of new and representative compounds
3.2.1 1-Benzenesulfonyl-8-methyl-7,9-dioxo-2,3,4,6,6a,7,8,9,9a, 9b-décahydro-1H-pyrrolo[3,4-h]quinoline-6-carboxylic acid benzyl ester (4a)
Yellow solid; m.p. 178 °C; IR (neat, cm(1) 2923, 1737, 1699, 1435, 1157; 1H-NMR (250 MHz, CDCl3) δ 1.30 (m, 1H), 1.57–1.72 (m, 1H), 1.95 (m, 1H), 2.17 (m, 1H), 2.90 (s, 3H), 3.19 (m, 1H), 3.55 (m, 2H), 3.59–3.73 (m, 2H), 4.58 (m, 1H), 5.24 (d, 1H, J = 12.1 Hz), 5.31 (d, 1H, J = 12.1 Hz), 6.09 (m, 1H), 7.35–7.43 (m, 5H), 7.48–7.62 (m, 3H), 7.88 (m, 2H); 13C-MR (100 MHz, CDCl3) δ 22.0, 25.2, 27.4, 39.8, 43.0, 44.1, 45.1, 53.8, 67.6, 118.9, 127.4, 128.6, 128.8, 128.8, 129.4, 133.0, 125.7, 137.1, 139.5, 170.2, 176.3, 176.6; MS (IS) m/z 495.5 [M+H]+.
3.2.2 1-Benzenesulfonyl-7,9-dioxo-2,3,4,6,6a,7,8,9,9a,9b-decahy dro-1H-pyrrolo[3,4-h]quinoline-6-carboxylic acid benzyl ester (4b)
White solid; m.p. 104 °C; IR (neat, cm(1) 3240, 2955, 1779, 1447, 1330, 1157; 1H-NMR (400 MHz, CDCl3) δ 1.38 (m, 1H), 1.59 (m, 1H), 1.97 (m, 1H), 2.20 (m, 1H), 3.17 (m, 1H), 3.50 (m, 2H), 3.65–3.76 (m, 2H), 4.53 (m, 1H), 5.23 (d, 1H, J = 12.1 Hz), 5.27 (d, 1H, J = 12.1 Hz), 6.13 (m, 1H), 7.34–7.42 (m, 5H), 7.49–7.55 (m, 2H), 7.56-7.62 (m, 1H), 7.86 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ 21.9, 27.4, 39.7, 44.2, 46.4, 53.8, 67.6, 119.1, 127.3, 128.6, 128.8, 128.8, 129.4, 133.0, 135.7, 137.0, 139.4, 170.0, 176.3, 176.6; MS (IS) m/z 481.0 [M+H]+.
3.2.3 1-Benzenesulfonyl-7,9-dioxo-1,2,3,4,6,6a,7,9,9a,9b-decahy dro-furo[3,4-h]quinoline-6-carboxylic acid benzyl ester (4c)
White solid; m.p. 54 °C; IR (neat, cm(1) 3240, 2955, 1779, 1447, 1330, 1157; 1H-NMR (400 MHz, CDCl3) δ 1.59 (m, 1H), 1.67 (m, 1H), 2.02 (m, 1H), 2.29 (m, 1H), 3.23 (m, 1H), 3.51 (m, 2H), 3.94–4.03 (m, 2H), 4.57 (m, 1H), 5.26 (d, 1H, J = 12.4 Hz), 5.31 (d, 1H, J = 12.0 Hz), 6.22 (s, 1H), 7.35–7.42 (m, 5H), 7.54 (m, 2H), 7.61 (m, 1H), 7.87 (m, 2H); 13C-MR (100 MHz, CDCl3) δ 21.8, 27.4, 39.6, 43.8, 44.1, 46.1, 53.5, 68.1, 120.0, 127.4, 128.8, 128.9, 128.9, 129.5, 133.3, 135.3, 137.6, 139.9, 168.9, 170.1, 170.9; MS (IS) m/z 514.0 [M+Na]+.
3.2.4 1-Benzenesulfonyl-8-methyl-7,9-dioxo-2,3,4,7,8,9-hexahy dro-1H-pyrrolo[3,4-h]quinoline-6-carboxylic acid benzyl ester (5)
orange solid; m.p. 81 °C; IR (neat, cm(1) 2926, 1711, 1433, 1162; 1H-MR (250 MHz, CDCl3) δ 1.66 (m, 1H), 1.87 (m, 1H), 2.38 (m, 1H), 2.63 (m, 1H), 3.19 (s, 3H), 3.47 (m, 1H), 3.76 (m, 1H), 5.44 (s, 2H), 7.35–.64 (m, 9H), 7.88 (m, 2H); 13C-MR (62.5 MHz, CDCl3) δ 21.6, 24.5, 26.6, 45.4, 68.4, 126.7, 128.6, 128.8, 128.8, 129.0, 129.2, 129.5, 129.8, 133.6, 133.7, 135.3, 135.6, 138.5, 141.5, 165.5, 165.6, 166.0; MS (IS) m/z 491.0 [M+H]+.
Acknowledgments
The MESR (Ministère de l’Enseignement supérieur et de la Recherche) is gratefully acknowledged for the doctoral followsip to N.G., and the Region Centre and the University of Orleans for the doctoral followsip to R.R.-R.


