1 Introduction
Heteroaromatic rings containing nitrogen atoms often play important roles as the scaffolds of bioactive substances. [1] The pyridine ring system is one of the most popular N-heteroaromatics incorporated into the structure of many pharmaceuticals. Among them, 2-amino-3-cyanopyridine derivatives are known to have multiple biological activities, such as anti-microbial [2], cardiotonic, [3] anti-inflammatory [4,5], anti-parkinsonism [6] and anti-tumor properties. [7] Also, they have been identified as novel IKK-β inhibitors [4], A2A adenosine receptor antagonists [6] and potent inhibitor of HIV-1 integrase (Fig. 1) [8]. Despite the existence of extensive literature for the synthesis of 2-amino-3-cyanopyridines, the most common procedures need multiple steps [9], toxic benzene or toluene as the solvent [7,10], microwave assistance [11], resulting in unsatisfactorily low yields. Due to the interesting properties of pyridines, the development of synthetic methods that enable a facile access to these heterocycles is desirable.
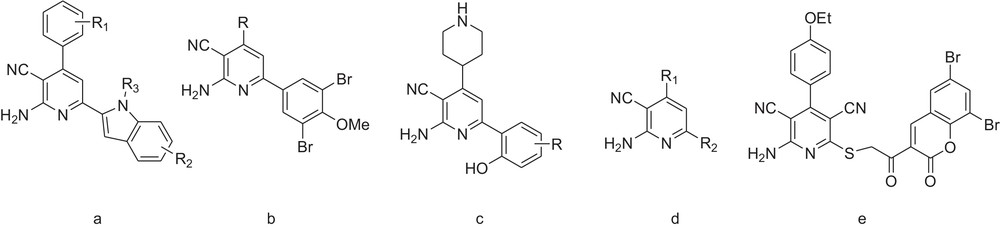
a: anti-tumor; b: anti-microbial; c: novel IKK-β inhibitors; d: A2A adenosine receptor antagonists; e: HIV-1 integrase.
2 Experimental
All commercially available chemicals were obtained from Merck and Fluka companies, and used without further purification unless otherwise stated. Nuclear magnetic resonance (NMR) spectra were recorded in DMSO-d6 on Bruker Avance 300 MHz FT and Varian 90 MHz NMR spectrometers using TMS as an internal standard. Chemical shifts were expressed in parts per million (ppm). Infrared (IR) spectroscopy was conducted on a PerkinElmer GX FT–IR spectrometer. Mass spectra were recorded on a Shimadzu QP 1100 BX Mass Spectrometer. Elemental analyses (C, H, N) were performed with a Heraeus CHN-O-Rapid analyzer.
2.1 General procedure for the synthesis of 2-amino-3-cyanopyridines
A mixture of aldehyde (2 mmol), substituted acetophenone (2 mmol), malononitrile (2 mmol), ammonium acetate (2.5 mmol) and TBBDA or PBBS (0.05 g) was heated under stirring at 100 °C for appropriate times (Table 1). The progress of the reaction was monitored by TLC (10:4, n-hexane/acetone). After completion of the reaction, the reaction mixture was allowed to cool to room temperature, and 95% cold EtOH (5 mL) was added. The precipitate was filtered off and washed with cold ethanol. After drying, the pure product was obtained. Removal of the solvent under reduced pressure gave back the catalysts.
Synthesis of 2-amino-3-cyanopyridine derivatives.
| Entry | A | B | Product | TBBDA | PBBS | Mp (°C) | ||
| Time (min) | Yield (%) | Time (min) | Yield (%) | |||||
| 5a | 10 | 90 | 10 | 85 | 170–173 | |||
| 5b | 10 | 92 | 10 | 80 | 222–224 | |||
| 5c | 5 | 94 | 5 | 90 | 248–250 | |||
| 5d | 30 | 90 | 40 | 90 | 198–200 | |||
| 5e | 30 | 88 | 40 | 85 | 235–237 | |||
| 5f | 25 | 94 | 25 | 90 | 186–189 | |||
| 5g | 15 | 86 | 15 | 85 | 172–174 | |||
| 5h | 35 | 85 | 40 | 85 | 235–237 | |||
| 5i | 10 | 89 | 10 | 85 | 207–210 | |||
| 5j | 15 | 85 | 15 | 79 | 269–273 | |||
| 5k | 25 | 89 | 25 | 80 | 149–151 |
2.2 Physical and spectroscopic data
2.2.1 2-Amino-4,6-bis(3-chlorophenyl)nicotinonitrile
Cream solid, mp: 170–173 °C. IR (KBr): 3435, 33.9, 3215, 2210, 1646, 1567, 1477, 1081, 784 cm−1; 1H NMR (90 MHz, DMSO-d6): δH (ppm) 7.11 (s, NH2, 2H), 7.57–8.19 (m, CH aromatic, 9H). 13C NMR (75 MHz, DMSO-d6): δc (ppm) 87.72, 109.89, 117.05, 126.35, 127.41, 127.69, 128.70, 129.96, 130.34, 130.99, 133.87, 134.13, 139.23, 139.95, 154.03, 157.46, 161.15. Anal. calcd for C18H11Cl2N3: C, 63.55; H, 3.26; N, 12.35. Found: C, 63.93; H, 2.95; N, 12.21. MS: m/z 339 (Table 1, 5a).
2.2.2 2-Amino-4-(3-chlorophenyl)-6-(4-chlorophenyl)nicotinonitrile
Yellow solid, mp: 222–224 °C. IR (KBr): 3449, 3327, 3214, 2209, 1639, 1568, 1549, 1481, 1082, 786 cm−1; 1H NMR (90 MHz, DMSO-d6): δH (ppm) 7.09 (s, NH2, 2H), 7.35–8.23 (m, CH aromatic, 9H). 13C NMR (75 MHz, DMSO-d6): δc (ppm) 87.28, 109.60, 117.18, 127.62, 128.65, 129.08, 129.50, 129.92, 130.98, 133.88, 135.50, 136.63, 139.28, 153.85, 157.87, 161.19. Anal. calcd for C18H11Cl2N3: C, 63.55; H, 3.26; N, 12.35. Found: C, 63.71; H, 2.97; N, 12.16. MS: m/z 339 (Table 1, 5b).
2.2.3 2-Amino-4,6-bis(4-chlorophenyl)nicotinonitrile
Yellow solid, mp: 248–250 °C. IR (KBr): 3507, 3701, 2204, 1614, 1578, 1567, 1549, 1088, 829 cm−1; 1H NMR (90 MHz, DMSO-d6): δH (ppm) 7.06 (s, NH2, 2H), 7.35–8.24 (m, CH aromatic, 9H). 13C NMR (75 MHz, DMSO-d6): δc (ppm) 87.65, 109.86, 117.09, 126.36, 127.41, 127.71, 128.72, 129.74, 129.97, 130.36, 130.99, 133.86, 134.12, 139.22, 139.93, 154.03, 157.43, 161.16. Anal. calcd for C18H11Cl2N3: C, 63.55; H, 3.26; N, 12.35. Found: C, 63.86; H, 2.89; N, 12.46. MS: m/z 339 (Table 1, 5c).
2.2.4 2-Amino-6-(4-fluorophenyl)-4-p-tolylnicotinonitrile
Yellow solid, mp: 198–200 °C. IR (KBr): 3495, 3394, 2207, 1610, 1583, 1549, 1510, 1223, 1161, 819 cm−1; 1H NMR (90 MHz, DMSO-d6): δH (ppm) 2.38 (s, CH3, 3H), 6.95 (s, NH2, 2H), 7.29–8.18 (m, CH aromatic, 9H). 13C NMR (75 MHz, DMSO-d6): δc (ppm) 21.33, 86.91, 109.36, 115.84, 116.13, 117.59, 128.72, 129.73, 129.96, 130.08, 134.49, 139.85, 155.40, 157.86, 161.31, 162.19. Anal. calcd for C19H14FN3: C, 75.23; H, 4.65; N, 13.85. Found: C, 75.23; H, 4.16; N, 13.61. MS: m/z 303 (Table 1, 5d).
2.2.5 2-Amino-6-(4-bromophenyl)-4-p-tolylnicotinonitrile
Yellow solid, mp: 235–237 °C. IR (KBr): 3496, 3391, 2207, 1608, 1583, 1547, 1009, 815 cm−1; 1H NMR (90 MHz, DMSO-d6): δH (ppm) 2.40 (s, CH3, 3H), 7.02 (s, NH2, 2H), 7.30–8.13 (m, CH aromatic, 9H). 13C NMR (75 MHz, DMSO-d6): δc (ppm) 21.34, 87.34, 109.47, 117.53, 124.24, 128.73, 129.73, 132.05, 134.41, 137.20, 139.89, 155.49, 157.70, 161.32. Anal. calcd for C19H14BrN3: C, 62.65; H, 3.87; N, 11.54. Found: C, 62.75; H, 3.41; N, 11.29. MS: m/z 363 (Table 1, 5e).
2.2.6 2-Amino-6-(4-fluorophenyl)-4-(4-methoxyphenyl)nicotinonitrile
Brown solid, mp: 186–189 °C. IR (KBr): 3491, 3390, 2209, 1609, 1584, 1548, 1509, 1220, 826 cm−1; 1H NMR (90 MHz, DMSO-d6): δH (ppm) 3.82 (s, CH3, 3H), 6.94–7.69 (m, CH aromatic, 9H), 8.07 (s, NH2, 2H). 13C NMR (75 MHz, DMSO-d6): δc (ppm) 55.78, 86.81, 109.48, 114.59, 117.83, 127.68, 129.07, 129.52, 130.32, 130.47, 138.11, 154.92, 158.92, 160.86, 161.44. Anal. calcd for C19H14FN3O: C, 71.46; H, 4.42; N, 13.16. Found: C, 71.08; H, 4.16; N, 13.25. MS: m/z 319 (Table 1, 5f).
2.2.7 2-Amino-6-(4-fluorophenyl)-4-(3-methoxyphenyl)nicotinonitrile
Brown solid, mp: 172–174 °C. IR (KBr): 3475, 3378, 2207, 1634, 1601, 1575, 1510, 1258, 834 cm−1; 1H NMR (90 MHz, DMSO-d6): δH (ppm) 3.83 (s, CH3, 3H), 7.01 (s, NH2, 2H), 7.21–8.27 (m, CH aromatic, 9H). 13C NMR (75 MHz, DMSO-d6): δc (ppm) 55.74, 87.09, 109.49, 114.26, 115.83, 116.12, 117.43, 120.98, 130.01, 130.12, 130.34, 134.48, 138.67, 155.27, 157.96, 159.75, 161.23. Anal. calcd for C19H14FN3O: C, 71.46; H, 4.42; N, 13.16. Found: C, 71.28; H, 4.30; N, 13.09. MS: m/z 319 (Table 1 5g).
2.2.8 2-amino-6-(4-fluorophenyl)-4-(3,4,5-trimethoxyphenyl)nicotinonitrile
Cream solid, mp: 235–237 °C. IR (KBr): 3497, 3370, 2213, 1635, 1555, 1508, 1130, 811 cm−1; 1H NMR (90 MHz, DMSO-d6): δH (ppm) 3.74 (s, CH3, 3H), 3.86 (s, CH3, 6H), 6.99–8.21 (m, CH aromatic and NH2, 11H). 13C NMR (75 MHz, DMSO-d6): δc (ppm) 56.53, 60.56, 87.01, 106.47, 109.42, 115.85, 116.13, 117.71, 130.02, 130.14, 132.63, 134.52, 138.91, 153.31, 155.36, 157.80, 161.27. Anal. calcd for C21H18FN3O3: C, 66.48; H, 4.78; N, 11.08. Found: C, 66.18; H, 4.54; N, 10.62. MS: m/z 379 (Table 1 5h).
2.2.9 2-amino-4-(3-chlorophenyl)-6-(4-fluorophenyl)nicotinonitrile
Cream solid, mp: 207–210 °C. IR (KBr): 3513, 3409, 2204, 1646, 1579, 1512, 1159, 834 cm−1; 1H NMR (90 MHz, DMSO-d6): δH (ppm) 6.83–8.19 (m, CH aromatic and NH2, 11H). 13C NMR (75 MHz, DMSO-d6): δc (ppm) 86.88, 94.50, 109.46, 115.87, 116.13, 118.97, 127.64, 128.66, 129.77, 130.06, 131.00, 133.86, 139.36, 148.61, 153.81, 154.49, 158.10, 161.19. Anal. calcd for C18H11ClFN3: C, 66.78; H, 3.42; N, 12.98. Found: C, 67.33; H, 3.16; N, 12.20. MS: m/z 323 (Table 1 5i).
2.2.10 2-amino-4-(4-fluorophenyl)-6-(pyridin-3-yl)nicotinonitrile
Cream solid, mp: 269–273 °C. IR (KBr): 3476, 3362, 2215, 1650, 1578, 1514, 1193, 838 cm−1; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 6.96 (s, CH, 1H), 7.03 (s, NH2,2H), 7.42–7.47 (t, CH, 2H), 7.61–7.65 (m, CH, 1H), 7.77–7.80 (m, CH, 3H), 8.14–8.17 (m, CH, 1H), 8.76–8.77 (m, CH, 1H). 13C NMR (100 MHz, DMSO-d6): δc (ppm) 115.55, 115.77, 115.81, 118.58, 123.51, 130.97, 131.06, 133.28, 133.97, 146.58, 148.79, 149.01, 150.28, 153.96, 154.03, 161.59, 164.05. Anal. calcd for C17H11FN4: C, 70.34; H, 3.82; N, 19.30. Found: C, 70.06; H, 3.99; N, 19.58. MS: m/z 290 (Table 1 5j).
2.2.11 2-amino-4-(furan-2-yl)-6-p-tolylnicotinonitrile
Cream solid, mp: 149–151 °C. IR (KBr): 3477, 3311, 2204, 1642, 1542, 1508, 1264, 805 cm−1; 1H NMR (400 MHz, DMSO-d6): δH (ppm) 2.38 (s, CH3, 3H), 6.78–6.80 (m, CH, 1H), 6.98 (s, NH2,2H), 7.31–7.33 (d, CH, 2H), 7.52–7.53 (d, CH, 2H), 8.01–8.04 (m, CH, 3H). 13C NMR (100 MHz, DMSO-d6): δc (ppm) 20.90, 103.95, 112.72, 113.00, 117.30, 127.00, 129.26, 134.63, 139.97, 141.18, 145.45, 145.46, 148.72, 158.7, 161.14. Anal. calcd for C17H13N3O: C, 74.17; H, 4.76; N, 15.26. Found: C, 74.72; H, 4.56; N, 15.58. MS: m/z 275 (Table 1 5k).
3 Results and discussion
In continuation of our interest in the application of N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide [TBBDA] and poly(N-bromo-N-ethylbenzene-1,3-disulfonamide) [PBBS] [12] in organic synthesis [13–22], we report a new and efficient method for the one-pot synthesis of 2-amino-3-cyanopyridine derivatives by the condensation of substituted acetophenone with various arylaldehydes, malononitrile and ammonium acetate in the presence of TBBDA and PBBS as the efficient catalysts at 100 °C in good yields, as shown in Scheme 1.

One-pot synthesis of 2-amino-3-cyanopyridine derivatives.
Initially, we decided to examine various catalysts for the synthesis of 2-amino-4,6-bis(4-chlorophenyl)nicotinonitrile as a model compound (Table 2). We investigated the effects of various catalysts, including ZnCl2, SnCl2, AlCl3 and N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide [TBBDA] under various conditions. The results are summarized in Table 2. The reaction without catalyst provided little amounts of product (Table 2, Entry 1). The best result in ethanol was achieved using N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide (Table 2, entry 5). In recent years, the synthesis of compounds under solvent-free conditions is an important challenge in heterocyclic synthesis. Therefore, we decided to test this solvent-free reaction with various ratios of TBBDA. We found that the reaction was rapid and gave excellent yield of the product when using N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide [TBBDA] (10 min, 92%, Entry 6). In the light of this, subsequent studies were carried out under the following optimized conditions, that is, with 4.53 mol% TBBDA at 100 °C.
Reaction times and yields in various conditions.
| Entry | Catalyst | Solvent | Amount of catalyst (mol%) | Temperature | Time (min) | Yield (%)a |
| 1 | None | Ethanol | – | Reflux | 720 | 38 |
| 2 | ZnCl2 | Ethanol | 12.83 | Reflux | 720 | 36 |
| 3 | AlCl3 | Ethanol | 13.12 | Reflux | 720 | 18 |
| 4 | SnCl2 | Ethanol | 9.22 | Reflux | 720 | 34 |
| 5 | TBBDA | Ethanol | 4.53 | Reflux | 90 | 80 |
| 6 | TBBDA | Solvent-free | 4.53 | 100 °C | 10 | 92 (92, 90, 89)b |
| 7 | TBBDA | Solvent-free | 3.62 | 100 °C | 10 | 88 |
| 8 | TBBDA | Solvent-free | 6.34 | 100 °C | 20 | 90 |
a Isolated yield.
b The catalyst was reused for three times.
These results encouraged us to investigate the scope and generality of this new protocol for various aromatic aldehydes and ketones under optimized conditions. Surprisingly, while para-substituted, the meta-substituted aromatic aldehydes, the heteroaromatic aldehyde and the heteroaromatic ketone gave 2-amino-3-cyanopyridines in good to excellent yields, the ortho-substituted aromatic aldehydes, the aliphatic aldehyde (hexanale) and the aliphatic ketone (acetone) gave no products. Obviously, the reactivity of aldehydes is the key factor for this one-pot transformation.
Mechanistically, it is likely that these catalysts release Br+ in situ, which can act as an electrophilic species and the mechanism shown in Scheme 2 is proposed for the synthesis of 2-amino-3-cyanopyridine derivatives [17,20].

Suggested mechanism for the synthesis of 2-amino-3-cyanopyridine derivatives.
4 Conclusion
In conclusion, we have developed a simple procedure for the synthesis of novel 2-amino-3-cyanopyridine derivatives from the reaction of various aryl aldehydes, substituted acetophenones, malononitrile and ammonium acetate in the presence of TBBDA and PBBS as the catalysts under solvent-free conditions. Moreover, the method has advantages in terms of product yields, recyclable catalyst, operational simplicity (easy work-up of reactions), environmental friendliness (non-corrosive catalyst) and short reaction times.
Acknowledgments
We are thankful to Bu-Ali Sina University, Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for financial support.


