1 Introduction
Nowadays synthesis in organic chemistry oriented towards “green chemistry” arouses increasing interest [1] resulting in new environmentally benign procedures, such as solvent-free synthesis, multicomponent reactions and reusable heterogeneous catalysts to save resources and energy. These organic reactions possess advantages over traditional reactions in organic solvents, such as solvent-free, multicomponent reactions with reusable heterogeneous catalysts reduce the consumption of environmentally unfriendly solvents and utilize scaled-down reaction vessels. Recently, several techniques for the efficient use of solvent-free reactions [2], reusable heterogeneous catalyzed reactions [3] and multicomponent reactions [4] have been developed individually, but when these three wings of green chemistry can be combined, an excellent green chemistry protocol is expected [5]. In this regard, development of solvent-free alternative processes are the best solution, especially when either one of the substrates or the products is a liquid and can be used as the solvent of the reaction [6].
Friedel–Crafts alkylation reaction is a powerful tool for C–C bond formation in organic synthesis for building molecular architectures [7]. Friedel–Crafts reaction needed electron-rich arenes. Indole and naphthols have been shown to be good donors. The interesting thing is that the reported reaction worked via Mannich-type Friedel–Crafts alkylation; here, we use an aromatic tertiary amine that plays a role of secondary amine for the activation of formaldehyde, to form aza quinone methide as the key intermediate further β-naphthol/indole used as a nucleophile to afford substituted diaryl methane derivative (4a and 7a) with high yield. The compound contains β-naphthol and indole have shown a wide range of biological active ingredients, medicaments [8] and venerable pharmacophores [9] in drug discovery as well as found in various ranges of natural products [10] respectively. Few reports on the synthesis of several diarylmethanes derivative using β-naphthol [11] and indole [12,13] have been published. However, these methods suffer from one or more drawbacks, such as long reaction times, use of organic solvents, expensive and homogeneous catalyst, also they failed to show one reaction condition for two nucleophiles (β-naphthol and indole).
In the course of the last few years, silica-supported solid acid catalysts have established numerous applications in modern organic synthesis, as they may be easily recovered and recycled [14]. In this connection, silica-supported tungstic acid (STA) has attracted tremendous attention as a green and solid acid catalyst to construct carbon–carbon and carbon–heteroatom bonds in various organic transformations [15,16]. It has received significant notice due to its non-toxicity, low cost, air and water compatibility, ease of handling, greater selectivity, recyclable catalyst, enhanced reaction rates, experimental simplicity and ease of preparation.1
Our literature investigation at this stage discovered that there are no reports on the synthesis of diarylmethanes derivatives catalyzed by silica-supported tungstic acid (STA) in solvent-free condition. As part of our ongoing research program on the development of clean protocols as well as our interest in applications of silica-supported catalyst [17,18] in organic reactions, herein, we report a green and convenient protocol for the synthesis of diarylmethanes derivatives via a multicomponent reaction of a tertiary aromatic amine, formaldehyde with β-naphthol/indole in the presence of a catalytic amount of silica-supported tungstic acid (STA) in solvent-free conditions at room temperature (Scheme 1).
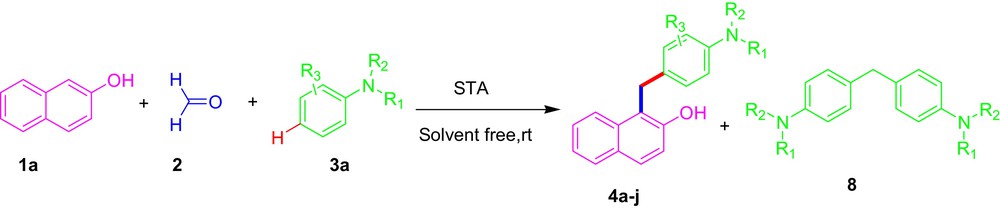
Synthesis of diarylmethanes derivative catalyzed by silica-supported tungstic acid (STA).
2 Result and discussion
Initially, we investigated the model reaction of equimolar amounts of β-naphthol (1 mmol) 1a, 37% aqueous formaldehyde (1 mmol) 2, and tertiary aromatic amine (1 mmol) 3a in the presence of different catalysts under solvent-free conditions at room temperature. The solvent-free and catalyst-free reaction gave a mixture of products that include only trace amounts of the formed products 4a (Table 1, entry 1). The model reaction in the presence of SiO2, ZnCl2·H2O, AlCl3 and FeCl3 under solvent-free conditions afforded 37, 48, 60 and 73% yield of 4a, respectively (Table 1, entry 2–5). Therefore, we focused on the search for a suitable silica-supported catalyst. We attempt with different silica-supported catalysts, such as CAN·SiO2, TiO2·SiO2, STA, BF3·SiO2 and FeCl3·SiO2 were screened in our model reaction at room temperature. Among them STA proved to be the most efficient since the reaction could be carried out and the desired product was obtained in excellent yield and high purity (96% Table 1, entry 9). This result showed that the catalyst exhibited a good catalytic activity in this transformation.
Influence of the catalyst and solvent in the synthesis of 4aa.
| Entry | Catalyst (10 mol %) | Solvent | Time | Yield 4a (%)b | Yield 8 (%)b |
| 1 | None | – | 8 h | 23 | 11 |
| 2 | SiO2 | – | 9 h | 37 | 13 |
| 3 | ZnCl2·H2O | – | 7 h | 48 | 23 |
| 4 | AlCl3 | – | 5 | 60 | 13 |
| 5 | FeCl3 | – | 8 h | 73 | 12 |
| 6 | STA | Ethanol | 6 h | 80 | 11 |
| 7 | BF3·SiO2 | Ethanol | 4 h | 56 | 20 |
| 8 | TiO2·SiO2 | Ethanol | 9 h | 48 | 23 |
| 9 | STA | Solvent-free | 20 min | 96 | Trace |
| 10 | FeCl3·SiO2 | Solvent-free | 1 h | 84 | Trace |
| 11 | STA | Dioxane | 2 h | 65 | 19 |
| 12 | STA | Acetonitrile | 1 h | 75 | 16 |
| 13 | STA | DMF | 2 h | 65 | 18 |
| 14 | STA | Methanol | 3 h | 75 | 12 |
| 15 | STA | THF | 3 h | 63 | 21 |
| 16 | STA (5) | Solvent-free | 40 min | 90 | Trace |
| 17 | STA (20) | Solvent-free | 20 min | 94 | Trace |
a Reaction of β-naphthol 1a, 37% aqueous formaldehyde 2a, N,N-dimethylaniline 3a and catalyst (10 mol%) at room temperature.
b Isolated yield.
Next, we investigated the effects of solvents on the model reaction, we screened different solvents, such as methanol, DMF, dioxane, THF and acetonitrile in the presence of a catalytic amount of STA at room temperature. It was observed that in the presence of solvent the reaction takes a longer time to give an even lower yield of product. This may be due to the competitive adsorption of the solvent with the substrate molecule on the catalyst surface; hence, reaction under solvent-free condition gives high yields in less time (Table 1 entry, 9). Moreover, we found that the yields were not that much affected by the amount of STA loaded. When 5, 10, and 20 mol% of STA were used, the yields were 90, 96, and 94%, respectively (Table 1, entries 16, 9, and 17). Therefore, 10 mol% of STA were a sufficient and optimal quantity for the completion of the reaction.
The reusability of the catalyst was also examined in the synthesis of 4a (diarylmethanes derivatives). The catalyst was recovered after each run, washed with ethyl acetate and acetone, dried under vacuum at 120 °C and tested for its activity in subsequent runs. It was found that the catalyst could be reused four times without loss of activity (Table 2, entry 1–4).2
The plausible mechanism of the reaction is given in Scheme 2. The tertiary aromatic amine reacts with formaldehyde to generate an intermediate aza quinone methide 3, which through addition of β-naphthol gave the desired N,N-dialkyl amino arylated β-naphthol 6. Compound 8 was also formed by the attack of tertiary aromatic amine 1 on intermediate 3.3
Gratifyingly, we found that this STA catalyzed three-component reaction worked well for a different naphthalene (Table 3)/indole (Table 4) and tertiary aromatic amine provided easy access to annulated diarylmethanes derivatives in good to excellent yields.
STA catalyzed synthesis of N,N-dialkyl amino arylated β-naphthol derivativesa.
| Entry | Aromatic amine | Naphthalene | Product | Time (min) | Yieldb | Mp (°C) | |
| Found | Reported | ||||||
| 1 | 20 | 96 | 129–131 | 127–130 | |||
| 2 | 20 | 95 | 84–87 | – | |||
| 3 | 20 | 92 | 94–97 | – | |||
| 4 | 20 | 91 | 137–139 | 138–140 | |||
| 5 | 20 | 92 | 143–144 | – | |||
| 6 | 30 | 90 | 184–187 | – | |||
| 7 | 30 | 90 | 131–134 | 130–134 | |||
| 8 | 30 | 94 | 137–139 | – | |||
| 9 | 30 | 93 | 158–160 | 57–160 | |||
| 10 | 30 | 92 | 143–145 | – |
a Reaction condition: β-naphthol 1a (1 mmol), 37% aqueous formaldehyde 2 (1 mmol), N,N-dimethylaniline 3a (1 mmol) and STA (10 mol%) at room temperature.
b Isolated yield.
STA catalyzed synthesis of N,N-dialkyl amino arylated indole derivativesa
| Entry | Aromatic amine | Indole | Product | Time (min) | Yieldb | Mp (°C) | |
| Found | Found | ||||||
| 1 | 20 | 96 | 142–44 | 143 | |||
| 2 | 20 | 95 | 139 | 139 | |||
| 3 | 20 | 94 | 143–147 | ||||
| 4 | 25 | 94 | 161 | 159 | |||
| 5 | 25 | 96 | 134–135 | – |
a Reaction condition: indole 6 (1 mmol), 37% aqueous formaldehyde 2 (1 mmol), N,N-dimethylaniline 3a (1 mmol) and STA (10 mol%) at room temperature.
b Isolated yield.
The structures of all reported as well as new compounds were established by their 1H, 13C NMR and HRMS analysis (Scheme 2).
3 Conclusion
To summarize, a simple, faster, clean, green and atom-economical solvent-free protocol is established for the one-pot synthesis of diarylmethanes derivative in excellent yields and purity. The main advantage of the present methodology is the simple work-up, easy recovery of catalyst, no need for anhydrous condition, no base or any additional activator required. To the best of our knowledge, this is the first report for the synthesis of diarylmethane derivatives by using silica-supported tungstic acid (STA) as a heterogeneous catalyst under solvent-free conditions.
1 General procedure for the synthesis of silica tungstic acid: A silica tungstic acid was prepared by adopting the method of Karami et al. [15a]. To a mixture of silica chloride (6.00 g) and sodium tungstic (7.03 g) was added n-hexane (10 mL). The reaction mixture was stirred under refluxing conditions for 4 h. After completion of the reaction, the reaction mixture was filtered and washed with distilled water, and dried and then stirred in the presence of 0.1 N HCl (40 mL) for an hour. Finally, the mixture was filtered, washed with distilled water, and dried to afford STA.
2 General procedure for the synthesis of compound 4a–4j: A mixture of β-naphthol (1 mmol), 37% aqueous formaldehyde (1 mmol), N,N-dimethylaniline (1 mmol) and STA(10 mol%) without solvent was stirred at room temperature. After completion of the reaction (monitored by TLC), the reaction mixture was dissolved in ethyl acetate and the catalyst was recovered by filtration, after ethyl acetate evaporation, the crude product was obtained purified by column chromatography (silica gel, ethylacetate: hexane). The recycled catalyst was washed with ethanol and acetone, dried and reused.
| Entry | Reaction cycle | Yielda (%) |
| 1 | First (fresh run) | 96 |
| 2 | Second cycle | 94 |
| 3 | Third cycle | 90 |
| 4 | Fourth cycle | 90 |
a Isolated yield.
3 General procedure for the synthesis of compound 7a–7e: A mixture of Indole (1 mmol), 37% aqueous formaldehyde (1 mmol), N,N-dimethylaniline (1 mmol) and STA (10 mol%) without solvent was stirred at room temperature. After completion of the reaction (monitored by TLC), the reaction mixture was dissolved in methanol. The products were precipitated from the reaction mixture. The precipitate was filtered off, dissolved in hot methanol and the catalyst was removed by hot filtration. The filtrate was kept at room temperature and the resulting crystallized product was collected by filtration and washed with cold ethanol to get the title compound. There cycled catalyst was washed with ethanol and acetone, dried and reused.


