1 Introduction
Gorse (Ulex Europaeus, Linn.) is an evergreen shrub of the Leguminosae family, having spiny branches and bright yellow flowers with a strong and characteristic scent. The medicinal and insecticidal applications have been limited, and mainly restricted to the seeds, which contain alkaloids, studied by different authors [1,2]. Terpenoids and glycosides are the main constituents of flowers [3], and the phenolic content in the flowers is higher than that in other parts of the plant [4]. Volatile organic compound emission was higher for flowering branches than for other plant parts, and isoprene represented 90% of the total. Trans-ocimene and alpha-pinene are the main monoterpenes, accounting for 48% and 37% of the total monoterpenes, and other minor monoterpenes are camphene, sabinene, beta-pinene, myrcene, limonene and gamma-terpinene [5].
The aroma, antioxidant properties and color of the flowers could facilitate their utilization in cosmetics or perfume industry, either as a whole or as extracts. Compounds responsible for these attributes have been also widely employed as ingredients, additives or food flavors or in packaging materials [6]. Extraction of natural products can be carried out by several methods, from the use of different organic solvents (acetone, methanol, ethanol, hexane, etc.) to conventional methodologies, such as steam distillation, hydrodistillation and hydrodiffusion. In order to minimize the use of organic solvents, green chemistry was applied to the extraction of essential oils and antioxidants, by using supercritical fluid extraction (principally with carbon dioxide), subcritical water, ionic liquids, etc. [7,8]. Recently, microwave-assisted techniques have been successfully applied to the extraction of natural compounds.
The microwave-assisted extraction has also been useful to recover phenolic compounds from flowers with ethanol, i.e. the extraction of chlorogenic acid from flowers of Lonicera japonica Thunb, in shorter time and with higher yields than with conventional heat-reflux extraction [9]. But this technique shows additional advantages for the extraction of essential oils, offering energy and time savings, clean processes, higher product yields and better sensory, antimicrobial and antioxidant properties of the extracts [10,11]. Microwave-assisted techniques were successfully proposed for the isolation and concentration of volatile compounds and essential oils from flowers of fresh mango [12], Damasc rose [13], lavandin [14] and chamomille [15], without adversely influencing the composition of the essential oils and the extracts showed DPPH radical scavenging activity and reducing power.
Among the variety of microwave aided processes available, one method which can offer good results is microwave hydrodiffusion and gravity (MHG) [14]. In addition, it can be applied to the green extraction of natural products according to the biorefinery concept, offering operational advantages derived from the reduction of solvent, energy, wastes… [16]. The extraction and recovery are performed in a single stage involving the application of microwave irradiation and earth gravity [17,18]. The hydrodiffusion allows the extract to diffuse outside the plant material and the extract is dropped on a spiral condenser outside the microwave cavity; water and essential oil are collected and can be separated by density. MHG was successfully applied to the extraction of volatile compounds from fresh plant materials with a minimum 60% of initial moisture [17–19]. To avoid chemical modifications caused by temperature and prolonged extraction time on the aromatic and volatile molecule components, solvent free microwave extraction was proposed [20]. This technique can offer products with enhanced aromatic and antioxidant properties due to the higher content in oxygenated monoterpenes. MHG has also been proposed for the extraction of water soluble fractions with antioxidant properties from different sources, including brown algae [21] and mushrooms [22]. In addition, many natural colors also display antioxidant activity and solvent free microwave extraction techniques can overcome the limitations of other conventional techniques used for their extraction [16,20].
The present study is aimed at selecting the conditions during microwave hydrogravity to maximize the extraction yield from Ulex europaeus flowers, and to compare the antioxidant and aromatic properties of the extracts with those produced by steam distillation.
2 Materials and methods
2.1 Materials
The flowers of U. europaeus L. were manually collected in April and May 2013 from San Xoán de Río (Ourense, NW Spain). Flowers were separated from leaves and stems and stored at −18 ºC.
All standards and reagents were of analytical grade and were purchased from suppliers, such as Merck, Sigma–Aldrich, Panreac and Fluka. Stock solutions were prepared and stored in a freezer at 4 ºC and the working standard solution or reagents were prepared by dissolving a required amount of specific reagent in double-distilled water.
2.2 Extraction
The flower samples used for the study were extracted at least three times with two different extraction techniques.
2.2.1 Steam distillation (SD)
The traditional steam distillation without a cohobation system was applied to 50 g of fresh flowers treated with 250 mL of deionized water. Steam was passed through the sample kept in a bag. The distillation process lasted 160 min, including the time required by water to reach the boiling point in a 1 L glass vessel. For comparative experiments, fractions of 30 mL were collected and the last fraction was discarded.
2.2.2 Microwave hydrogravity extraction (MHG)
The MHG procedure for the extraction of flowers was carried out in an open vessel NEOS-GR (Milestone Srl, Italy) microwave extractor with a 1.5 L Pyrex extraction vessel. During experiments at selected irradiation power, time and temperature were recorded. The temperature was measured by using a fiber optic temperature sensor inserted in the microwave cavity. Fresh samples (100 g) were placed in the microwave cavity and subjected to several irradiation powers (25, 50, 100, 150 and 200 W) and fractions of 5 mL were collected, cooled down and analyzed.
The drained liquid phase was analyzed for total solubles, total phenolics, in vitro antioxidant properties, color and olfactory characteristics. Analysis was performed in triplicate and the mean values were reported.
2.3 Analytical methods
The extraction yield was gravimetrically determined. The total phenolic content was determined by the Folin-Ciocalteu assay [23] and expressed as gallic acid equivalents (GAE).
The ability to reduce the ferric 2,4,6-tripyridyl-s-triazine (TPTZ) complex under acidic conditions was determined by the FRAP assay [24]. The FRAP reagent was prepared with 25 mL of 300 mmol/L acetate buffer (pH 3.6) and 2.5 mL of a 10 mmol TPTZ/L solution in 40 mmol/L HCl and 20 mmol/L FeCl3·6 H2O in distilled water. A volume of 100 μL samples was mixed with 3 mL of the reagent, and the absorbance was monitored at 593 nm. Ascorbic acid was used as the standard.
The scavenging capacity against the ABTS radical (2,2′-azinobis-(3-ethyl-benzothiazoline-6-sulfonate)) was used [25]. The radical cation was produced by reacting 7 mM ABTS stock solution with 2.45 mM potassium persulfate. The mixture was diluted with phosphate buffer saline (PBS) and equilibrated at 30 °C. Ten μL of the extracts diluted in ethanol or in PBS were mixed with 1.0 mL of diluted ABTS+ solution and the absorbance was recorded for 6 min. A similar determination was done with a standard Trolox solution and the percentage of absorbance inhibition at 734 nm was expressed as Trolox equivalents.
The scavenging capacity against α,α-diphenyl-β-picrylhydrazyl (DPPH) radical scavenging was determined by mixing two milliliters of a 3.6 × 10−5 M methanolic solution of the radical with 50 mL of an antioxidant solution and the decrease in absorbance at 515 nm was recorded after 16 min. The inhibition percentage was compared with the value obtained for ascorbic acid.
Color analysis was carried out under D65 Illuminant by using a Konica Minolta CR-400 colorimeter equipped with a pulsed xenon lamp. The system employed was CIELab, based on the measure of the luminance (L) (changes from 0 for black to 100 for white) and the coordinates a* (red-green) and b* (yellow-blue) varying from 120 to −120. The parameters a*, b* and L* were intercorrelated with the chroma (C), calculated as C = (a*2 + b*2)1/2, with the hue angle (hº), defined as h = arctan (b*/a*), and with the saturation (S), calculated by the formula: S = C/L*.
Descriptive sensory analyses of MHG and distilled extracts were performed by several judges who had extensive experience in food and beverage tasting. Samples from MHG and distillation procedures were presented in glass cups to panel members in some sessions, having as the basic odor, that of the fresh flower. The panellists were asked to evaluate the color and aroma. The odour profiling terms were discussed and rated.
Scanning electron microscopy: Freeze-dried flower samples were fixed onto aluminium stubs. After coating with gold in Emitech K550X equipment, samples were examined on a FEI Quanta 200 scanning electron microscope (12.5 kV).
3 Results and discussion
3.1 Selection of irradiation power during MHG
The moisture content differs among species and also for different parts of the plant, with average values in the range of 5–10% in seeds, 30–40% in bark, 60–90% in leaves, 65–80% in roots and 80–90% in flowers [26]. The U. europaeus flowers used in the present study contained 78.98% moisture, a value lower than the average value reported by Moré et al. [26]. The moisture content of the matrix is a key variable in solvent free microwave extraction processes since the high dipole moment of water molecules influences the sample heating, the solubility and structural effects associated with solvent extraction.
The influence of the irradiation power during MHG on the extraction time and temperature and the volume of the drained fractions are shown in Fig. 1. Information for steam distillation is also presented. During distillation the extraction temperature was close to 100 ºC, which was also reached in the extraction vessel during microwave irradiation at 100–200 W. However, during operation at lower microwave power the water boiling temperature at atmospheric pressure was not reached, up to 50 ºC at 25 W and up to 80 ºC at 50 W were recorded.
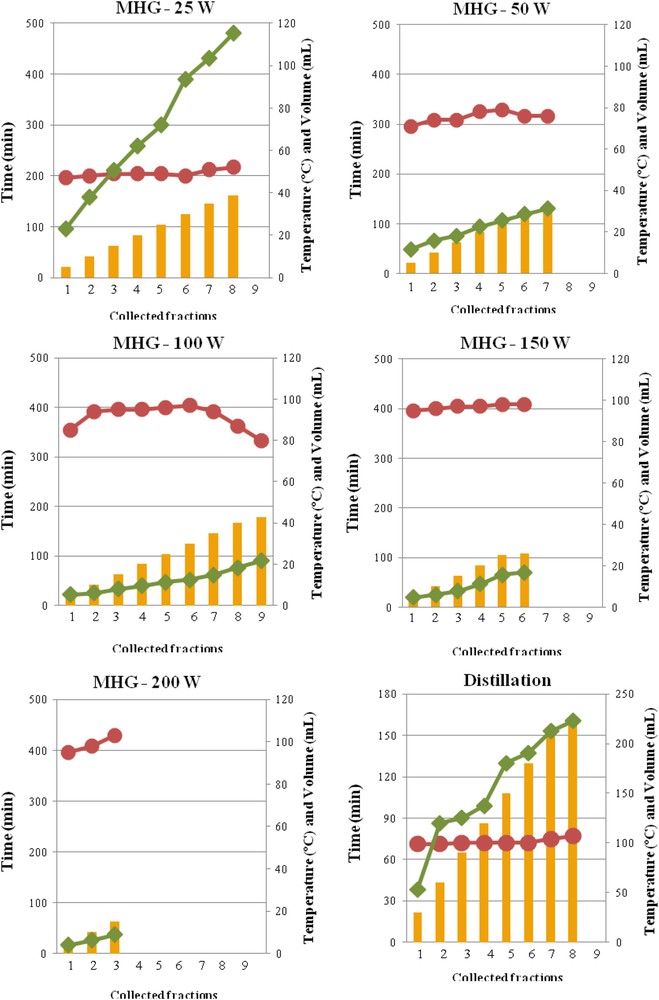
Effect of microwave irradiation power on the extraction time (), and temperature () and the volume collected () during MGH, and comparison with the distillation process.
Distillation provided considerable higher extract volumes (226 mL), whereas, as expected, MHG provided more concentrated extracts, with 38.8 mL at 25 W, 32.0 mL at 50 W, 42.8 mL at 100 W, 26.1 mL at 150 W and only 15.0 mL at 200 W.
The distillation process lasted up to 160 min, similar to the value required for MHG at 50 W, but considerably lower than the time needed for operating with 100–200 W, although at 25 W the process was performed for up to 475 min. The extraction periods are relatively long, compared to those used with other vegetal materials, such as rosemary leaves and citrus peels, with a maximum of 15 min [17,18] or 30 min for myrtle leaves [10] and for lavandin flowers [14]. The irradiation power in the present work was kept in a lower range to avoid undesirable olfactory sensations. Similarly, the optimal extraction of essential oil from Rosa damascene flowers requiring low times, 15 min at 650 W, decreased monoterpene alcohol content [13]. The application of a phase-power controlled microwave hydrodiffusion and gravity extraction with adjustable power could be more suited for better yield and quality of essential oils from flowers, since it could avoid the overheating of the material occurring in conventional systems. This technique was tried for jasmine flowers [27].
The phenolic concentration in the individual fractions collected during MHG is shown in Fig. 2a. At lower irradiation power, more fractions are obtained and the concentration of gallic acid equivalents was under 0.1 g/L. However, fractions 5 and 6 from MHG at 150 W showed higher values and fraction 8 from SD reached 0.25 g/L. The mixture of fractions obtained with MHG 150 W showed higher content than those obtained at other irradiation power and slightly higher than the mixture of distillates (Fig. 2b). Other authors reported an optimal potency, leading to higher extraction yields, but also higher phenolics or flavonoids content in the extracts [19,28].
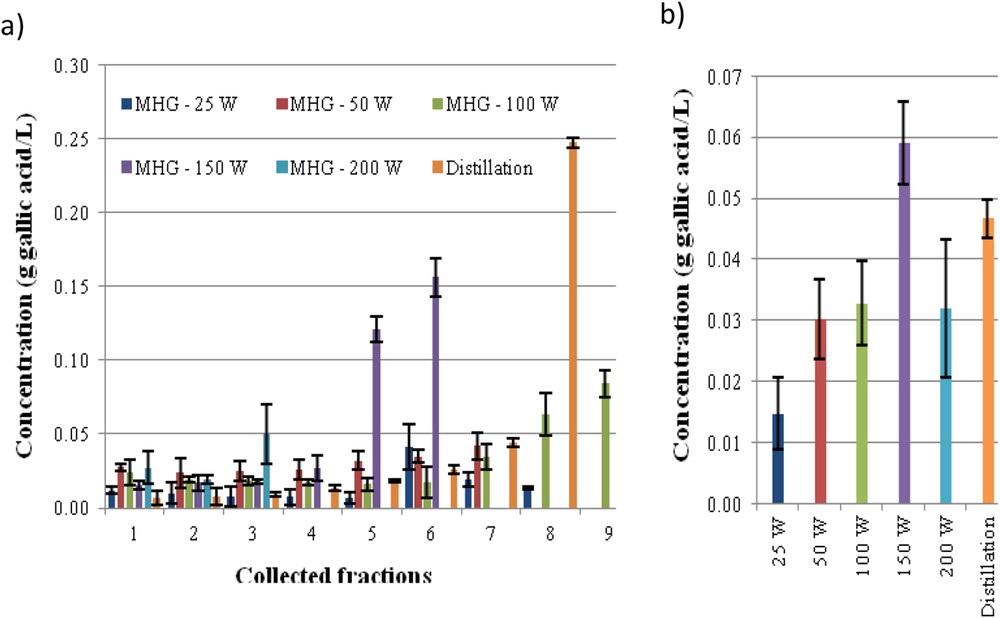
Phenolic content, expressed as gallic acid equivalents, for each of the fractions (a) and for the mixed fractions (b) collected during MGH in comparison with SD extract of Ulex europaeus flowers.
The ABTS radical scavenging activity is shown in Fig. 3, both for each of the individual collected fraction and for the mixture of fractions obtained during operation at each irradiation power. A slight increase in antiradical activity was observed in the latter fractions regardless of the irradiation power. A behaviour similar to that reported obtained with MHG at 150 W were less active than the mixture of fractions in the distillate, 0.5 and 0.7 mM Trolox, respectively. The antiradical capacity of extracts and distillate is lower than of other flower extracts, such as Anthyllis aurea Welden, Anthyllis vulneraria L., Cassia auriculata L., Cassia fistula L., Chrysanthemum morifolium Ramat., Lavandula pedunculata (Mill.) Cav., Litchi chinensis Sonn., Paeonia suffruticosa Andrews and Rosa chinensis Jacq., produced by solvent extraction with organic solvents [29–33].
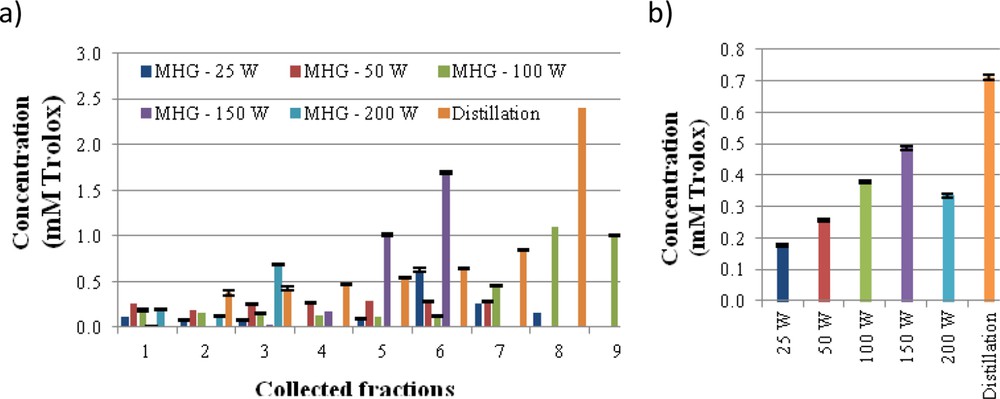
ABTS radical scavenging capacity, expressed as TEAC values, for each of the fractions (a) and for the mixed fractions (b) collected during MGH in comparison with SD extract of Ulex europaeus flowers.
Olfactory characteristics of the individual fractions collected by MHG were similar. Generally, the first fractions provided most intensely floral and fruity nuances, and the green and herbaceous notes were less intense; finally, in the two last collected fractions at each irradiation power, diminished these attributes and the toasty and undesirable roasted and burned smells were then detected.
Visually, the colors of extracts from MHG extraction were pale yellow and colorless compared to the sample obtained from steam distillation. Irradiation with the lower tested power (25 or 50 W) allowed to obtain fractions with the highest positive values of the chromaticity coordinate a* and the highest negative values of the b*. The highest saturation values were found in extracts from steam distillation, followed by those produced by MGH and irradiated with 100 and 25 W (data not shown).
3.2 Comparison of MHG and distilled extracts
The operation at 100 W was selected for the production of extracts from U. europaeus, based on the collected volume, the extraction yield and sensorial properties. Extraction time was 90 min, but the last fraction was discarded and finally 40 mL of the extract drained were collected in 76 min, cooled down and analyzed. The volume of the collected liquid phase accounted for 53.43% of the initial moisture content. The collected fractions were joined and homogenized to prepare a single extract. The last fractions were discarded, fraction 9 from MHG and fraction 8 from SD, based on their unfavorable olfactory properties.
The extracts produced by MHG showed higher DPPH radical scavenging capacity (0.005 mM ascorbic acid) than the extracts produced by conventional technology (0.002 mM ascorbic acid). The EC50 values were 0.557 g/L for MHG extracts, 0.853 g/L for SD extracts, and 0.24 g/L for BHA (butyl hydroxyanisole). These values obtained for gorse flowers are higher than the values found for highly potent extracts, such as those from Citrus aurantium L., Erica arborea L. and Schisandra sphenanthera Rehder&E.H. Wilson [34,35].
The extract produced by MHG shows higher reducing power than the extract produced by steam distillation, an opposite behaviour to that observed for the TEAC values (Fig. 3). As expected, the phenolic concentration in the mixtures and FRAP values showed a similar trend. The distillate has a superior phenolic content than the MHG extract (Fig. 2), but notably lower than the methanolic extracts from A. aurea Welden and A. vulneraria L. flowers [31], acetone/water (70:30 v/v) extracts from C. fistula L. flowers [29], the freeze-dried Crocus sativus L. flowers [36] and the acetone, methanol and water extracts of hot-air dried lychee (Litchi chinensis Sonn.) flowers [31].
The reducing power of Fe(III) observed for the selected extracts (0.102 ± 0.002 and 0.037 ± 0.001 mM FeSO4·7 H2O for MHG and distilled extract, respectively) was significantly lower than the values observed for other flower extracts, such as acetone/water (70:30 v/v) extracts from C. fistula L. flowers [28], the acetone, methanol and water extracts of hot-air dried lychee (L. chinenesis Sonn.) flowers [32], the methanolic extracts from Globularia alypum L. flowers [37], and dried Lavandula pedunculata (Mill.) Cav., P. suffruticosa Andrews and R. chinensis Jacq. [33].
3.2.1 Sensorial characterization
The olfactory profile of the samples was qualitatively described and is shown in Table 1. The flavor descriptors are listed following their identification order and intensity. The aromatic profile of U. europaeus extracts was not influenced by the extraction technique. The olfactory properties reveal floral and fruity nuances; vegetable and herbaceous nuances were less intense that the typical from the fresh flower. Some honey like and toasty smells are found as a consequence of heating during extraction.
Sensorial qualitative description of the samples.
| Samples | Aromatic descriptors |
| Fresh flower | Floral (wallflower, orange blossom and yellow flowers), fruity (ripe banana, dried peach, apricot, sweet fruits and honey), citric, green, earthy and herbaceous. |
| MHG-100 | Yellow flowers, vegetable, honey, beeswax, herbaceous. Secondary notes showed sweet, honey, lactic and toasty and lightly smoked aromas. |
| SD | Yellow flowers, vegetable, honey, beeswax, herbaceous. Secondary notes showed sweet, honey, boiled vegetable and lightly toasty aromas. |
3.2.2 Color
The visual aspect of MHG extracts showed a progressively reduced intensity, from pale yellow to colorless, whereas the extract from SD did not show any change and remained colorless. The pale color was also found for extracts and distillates from Cananga odorata (Lam.) Hook.f.&Thomson, Chamaemelum nobile (L.) All., Lavandula angustifolia Mill., Lavandula latifolia Medik., Mentha arvensis L., Rosa damascena Herm., Ocimum basilicum L. and Piper nigrum L., which ranged between colorless and pale yellow [38].
Chroma (C), hue angle (h) and saturation (S) were determined from the chromaticity coordinates L*, a*, and b* determined by using a CIELab system (Table 2). L* values are similar for both extracts (high lightness or tone), although they are higher for MHG. The coordinates a* and b* are also more positive (less green) and negative (less yellow), respectively, for the distillation sample, leading to different chromas, but the same hue angle and color saturation or vividness. According to the chromaticity diagram, the flower extracts from MHG and SD were practically achromatic.
CIELab coordinates of the extract from MHG (100 W) and from steam distillation.
| Coordinates | MHG sample | SD sample |
| L* (lightness) | 88.16 | 88.79 |
| a* (chromaticity coordinate: green-red) | 1.43 | 1.55 |
| b* (chromaticity coordinate: blue-yellow) | −2.89 | −3.07 |
| h (hue angle) | −63.65 | −63.29 |
| C (chroma) | 3.23 | 3.44 |
| S (saturation) | 0.04 | 0.04 |
3.2.3 Microstructure
The structural damage caused by microwave irradiation was confirmed by microscopic observations. Fig. 4 shows a comparison of the intact U. europaeus flowers with the solids remaining after MHG at 100 W and the residual solids after steam distillation. Whereas the non-extracted flowers show an intact cell wall, similar to that observed in steam distillated samples, microwave irradiated flowers show shrinked cells. MHG is more severe at the tissular level than steam distillation. The cell destruction was also reported as a factor associated with the enhanced extraction of phenolic acids from L. japonica flowers [9], essential oils from orange peel [39], lavender flowers [40], or leaves from thyme [41] or rosemary [18].
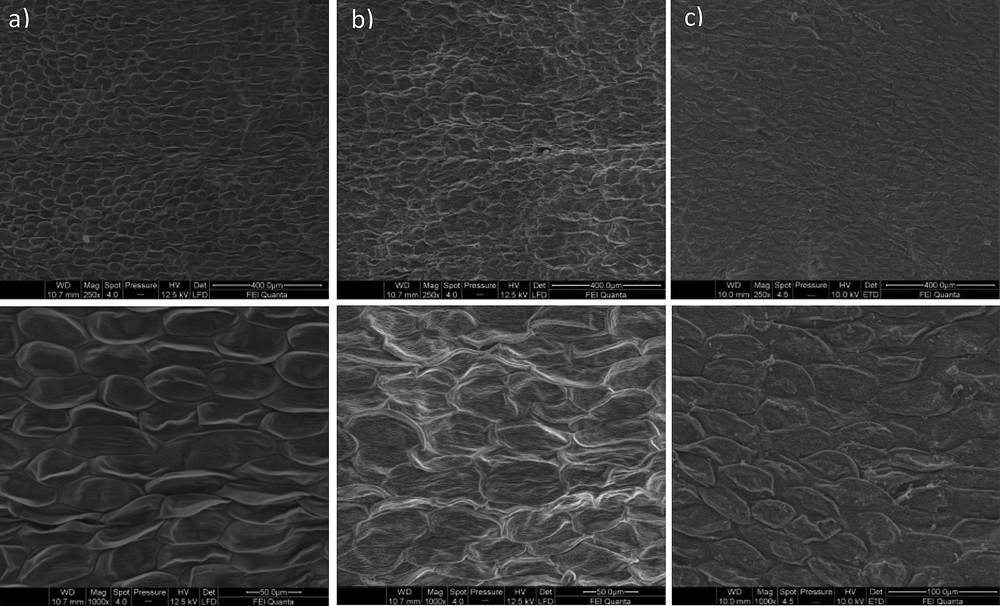
Microstructure of Ulex europaeus flowers (a), the solid residue after MHG at 100 W (b) and after steam distillation without cohobation (c). Upper images (×250), lower images (×1000).
4 Conclusions
Microwave hydrogravity extraction of U. europaeus L. flowers is highly influenced by the irradiation power and relatively low values (100 W, in a process lasting 70 min) are needed to provide optimal yields and antioxidant capacity, which correlated with the phenolic concentration in the extracts. Steam distillation provided higher yields of phenolic compounds (4.07 g gallic acid/100 g flowers) than MHG (0.12 g gallic acid/100 g flowers) and also higher yields of ABTS radical scavengers (10.72 g Trolox/100 g flowers) in comparison to MHG (0.81 g Trolox/100 g flowers). The reducing power of the distillate was equivalent to 12.5 mg ascorbic acid/100 g flowers, and the value for the MHG extract was 4 mg ascorbic acid/100 g flowers. Extracts obtained with both technologies were characterized by floral, fruity, herbaceous, vegetable and toasty descriptors. MHG allows an efficient water removal from the material and can be proposed for the extraction of compounds with antioxidant and aromatic properties of interest for food and cosmetic applications. Future studies are aimed at the identification of active compounds in extracts.
Acknowledgments
The authors are grateful to Xunta de Galicia (Conecta Peme COSMETINNOVA IN852A 2013/63 and INBIOMED CN2102/13 programs, partially funded by the FEDER Program of the European Union). The authors thank Miguel Estévez for his skillful technical assistance.


