1. Introduction
Biodiesel is a promising candidate as an alternative clean fuel due to its distinctive properties such as non-toxicity, low-sulfur content, easy biodegradability, renewable and low harmful gas emissions [1, 2]. In recent years, production processes and technologies for biodiesel have been relatively mature and promising [3]. However, it has some disadvantages that hinder its large-scale production, such as high product cost [4], and low reaction rate [5].
Many catalytic systems have been reported for the transesterification reaction of rapeseed oil and methanol, such as acid catalysts, alkali and enzymes [6, 7, 8]. In homogeneous acid or alkali catalyzed biodiesel productionss, the catalyst is characterized by high catalytic activity and catalytic rate, but the post-treatment is complicated [9]. Transesterification reactions using enzyme catalysts have been established to simplify the separation process of catalysts from products. However, enzyme catalysts are susceptible to poisoning and exhibit low selectivity [10]. Among them, heterogeneous acid base catalysts have attracted much attention because they can overcome the problems posed by other catalysts [11, 12]. CaO is a commonly used basic catalyst for transesterification reactions, which is attributed to its cost-effectiveness, high activity, superior basicity, and easy availability of raw materials [13, 14]. Liu et al. [15] described the transesterification process for the conversion of soybean oil to biodiesel using CaO as a solid catalyst. The reaction was carried out using a 1:12 molar ratio of soybean oil to methanol, a catalyst concentration of 8 wt% (in terms of oil weight) at a temperature of 65 °C within a reaction time of 3 h, and a 95% yield of biodiesel. Tang et al. [16] reported that nano-CaO with different particle sizes and crystallinity was prepared by sol–gel method, which exhibited better catalytic performance than commercial CaO for biodiesel production under the same reaction conditions. The best reaction was achieved at 1:1:8 molar ratio of oil to DMC to methanol, 5 wt% nano-CaO catalyst (calcined at 800 °C), and, 65 °C reaction temperature, and the fatty acid methyl ester yield exceeding 4 h was 92%, which is 2 h shorter than that of commercial CaO. Degfie et al. [17] prepared nano-CaO via thermal decomposition and used it as a catalyst to valorize waste cooking oil to biodiesel. It was revealed that the highest conversion of 96% was obtained at optimized reaction conditions of 50 °C, methanol/oil ratio of 8, and catalyst dosage of 1 wt% with a reaction time of 90 min. Another notable source of CaO for biodiesel synthesis could be derived from waste shells. Eggshells and aquatic shells have been widely reported as renewable and inexpensive CaO sources to replace rapidly depleting chemicals. Santos et al. [18] developed micro-structured CaO from chicken eggshells by calcination at 850 °C for 3 h and then used it for biodiesel synthesis from soybean oil. They reported a yield of 84.53% at a catalyst loading of 5 wt%, which is lower than some other studies that used chicken, oyster, duck, or ostrich shells. Apart from this, doped and loaded versions of other modified CaO-based catalyst materials have also been reported. Maryam et al. [19] reported the doping of CaO derived from calcined eggshells with Na–K using a wet impregnation method to improve the catalytic activity at low reaction temperatures and to shorten the reaction time. The effect of different Na/K molar ratios was investigated on biodiesel yield from canola oil, and the highest FAME yield of 97.6% was obtained under optimal reaction conditions. Moradi et al. [20] described the use of CaO/SiO2 as a heterogeneous catalyst for biodiesel production from corn oil. The transesterification reaction was carried out in the presence of corn oil, methanol (methanol to oil molar ratio of 16:1) and 6 wt% of catalyst (in terms of oil) at 60 °C for 8 h. The catalyst loading of 70%, calcination temperature of 650 °C and acid to water ratio of 0.5 were optimal values, and the purity and conversion of biodiesel produced were 98.5 and 85.6%, respectively. MacLeod et al. [21] evaluated four alkali-doped metal oxide catalysts, LiNO3/CaO, NaNO3/CaO, KNO3/CaO, and LiNO3/MgO, for biodiesel production from rapeseed oil. The results show that there is a correlation between base strength and catalyst performance, and that the alkali-doped metal oxide catalysts are promising catalysts having short reaction times.
Here we investigated a series of supported solid base catalysts that were prepared by impregnating CaO with different chloride salts, as well as their catalytic applications in the transesterification of rapeseed oil for biodiesel production. The influence of various process parameters, such as. kinds of active components, reaction temperature, catalyst dosage, and reaction time, were investigated in detail.
2. Experimental
2.1. Materials
Refined rapeseed oil was purchased from JianXing Agriculture Technology Group Co., Ltd. (Xi’an, Shaanxi) Calcium oxide was purchased from Sinopharm Chemical Reagent Co., Ltd. Kalium chloratum, methanol, cyclohexane, were purchased from TianLi Chemical Reagent Ltd. (Tianjin) Methyl heptadecanoate was purchased from Sigma. All chemicals were of analytical reagent grade were used without pretreatment.
2.2. Catalyst preparation
The supported solid base catalysts were prepared using a well-known immersion method based on the supporter CaO. The CaO support was pretreated into a muffle furnace for calcination at 600 °C for 6 h according to the previous results as suggested by Mohammed’s [22], and then impregnated with the prepared solutions of different chloride salts at room temperature. After 12 h, the resulting pre-catalyst was immediately dried in an oven at 110 °C for 5 h and then calcined under a specified time and temperature conditions.
2.3. Catalytic testing
Amount of rapeseed oil and methanol were added in a three-necked round-bottomed flask equipped with a reflux condenser and a thermometer, and the catalyst was added under stirring. Then the mixture was heated to refluxing temperature of methanol (65 °C) under stirring. The catalytic performance was evaluated by analyzing the sample taken out from the reaction mixture every 1 h. All samples were treated by centrifugation for the separation of catalysts, and the excess methanol in the samples was distilled off under vacuum.
2.4. Characterization of catalysts
The particle sizes and shapes of the prepared catalysts were examined using SEM (JSM-6390A, HI2TACHI). Adsorption isotherms of nitrogen on the prepared catalysts were obtained using an automated volumetric adsorption apparatus (ASAP2010, Micromeritics). Samples were evacuated at 300 °C for 2 h before exposure to nitrogen gas at 77 K. The BET surface areas were calculated using the Brunauer–Emmett–Teller equation. Temperature programmed desorption (TPD) of carbon dioxide was carried out using ChemiSorb2750 instrument (Micromeritics). The temperature of the sample was increased to 850 °C at a ramping rate of 10 °C/min and the desorbed carbon dioxide was detected using a thermal conductivity detector.
2.5. Pretreatment of biodiesel
If vegetable oil contains free fatty acid (FFA), it will react with homogenous base catalysts to form soap and water [23]. NaOH was used to remove free fatty acid from raw rapeseed oil. After multiple washings with water, then cyclohexane was used to remove remain water and the refined rapeseed oil was obtained.
The transesterification reaction was carried out in a 100 mL flask with a three-necked, round-bottomed, and thermometer. Rapeseed oil and methanol were first mixed under magnetic stirring and then heated to the reflux temperature of methanol in a water bath. The reaction occurs after adding the amount of catalyst into the mixture. After the desired time, the catalyst was separated by centrifugation and unreacted methanol was separated from the supernatant by vacuum distillation. Then the purified samples were collected for analysis.
2.6. Analyses
Samples, 1 μL, were analyzed by gas chromatography equipped with HP-INNOWAX (30 m × 0.15 mm) column with a flame-ionization detector and N2 as carrier gas. The column was maintained at 100 °C for 1 min, then increased at 20 °C/min to 240 °C and maintained for 1 min. Detector and injection temperature were 280 °C and 320 °C, respectively. The yield of biodiesel was calculated by the flowing equation given by Leung and Guo [24].
3. Results and discussion
3.1. Transesterification of rapeseed oil to biodiesel
3.1.1. The catalytic performance of different catalysts
According to previous studies, it was observed that the introduction of Cl not only increased the number of base sites, but also significantly changed its base strength [25]. In our study, biodiesel was prepared by the catalysis of CaO supported with different active components, namely KCl, AlCl3, NH4Cl, FeCl3, and LiCl. The results are shown in Figure 1. As shown, all supported catalysts catalyzed the transesterification reaction of rapeseed oil. Among them, KCl/CaO showed the best catalytic activity under the same reaction conditions. The optimal performance was obtained when KCl/CaO was used as a catalyst, with 98.4% of yield to biodiesel, which was 15.1% higher than commercial CaO. However, Reyero et al. [26] studied the heterogenization of biodiesel synthesis over CaO catalysts and found that Ca-glyceroxide possessed appreciable basicity and could increase the yield of biodiesel to about 83.4% within 2 h reaction time. The results suggest that active components have an important influence on the catalysis of CaO. The performance of the catalyst was significantly improved with the addition of KCl, which could appropriately modify the pore structure and inner surface area of CaO. The specific reason for this needs to be further studied and will be explained in detail later. Based on the results, KCl/CaO was used for further experiments.
Comparison of biodiesel yield over different catalysts.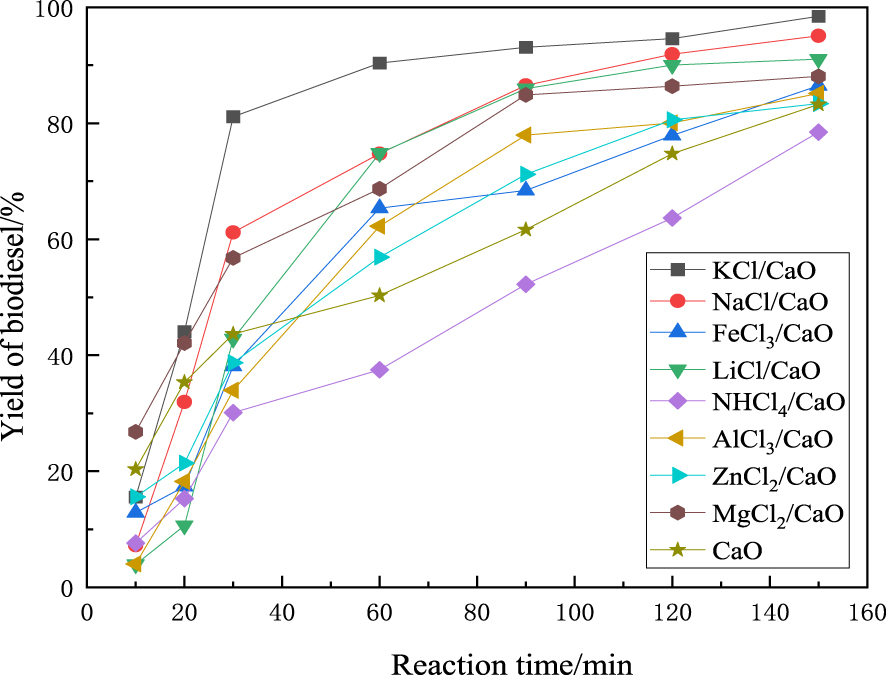
3.1.2. The influence of molar ratio
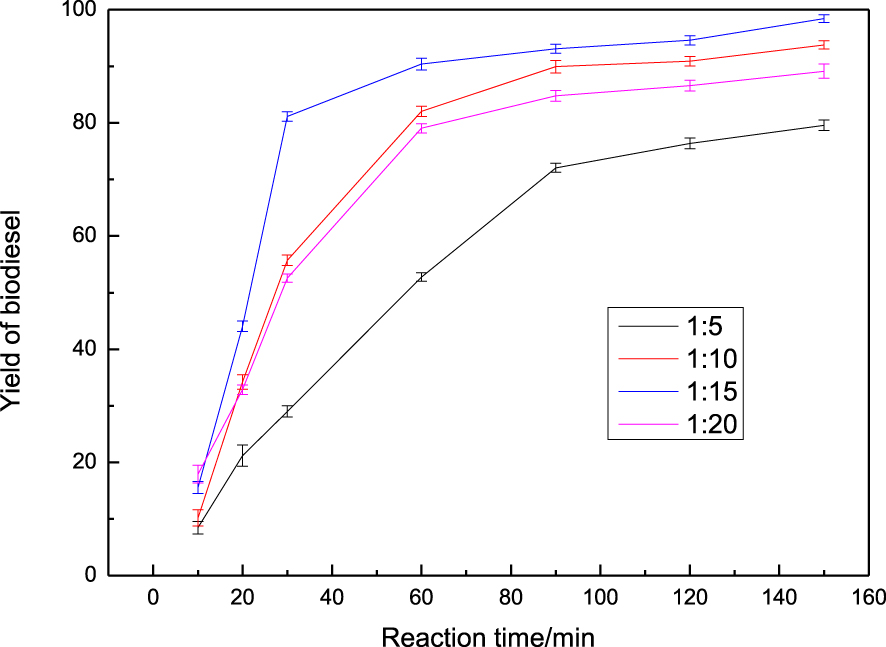
One of the most important parameters affecting the transesterification process is the molar ratio of the reactants [27]. Theoretically, the biodiesel yield can reach a maximum when the molar ratio of methanol to rapeseed oil at 3:1. However, more methanol was introduced to keep the equation shifted to biodiesel production. The effect of the molar ratio of methanol to rapeseed oil was examined and the results are shown in Figure 2. The biodiesel yield tended to increase with increasing amount of methanol, and reached a maximum of 98.4% when the molar ratio of methanol to oil was 15:1. In contrast to Huang’s study [28], the biodiesel yield reached only 97.8% at the same molar ratio of methanol to oil. It was also observed that when the molar ratio of oil to methanol was increased to 20:1, the yield of biodiesel decreased remarkably due to dilution effect. Hence, the optimum operating ratio for conversion will be 15:1.
3.1.3. The influence of calcination temperature
Calcination temperature was found to be of great importance in the preparation of catalysts with high catalytic activity [29]. The effect of calcination temperature on biodiesel yield is depicted in Figure 3. Increasing the calcination temperature leads to an increase in biodiesel yield due to the appearance of active sites derived from the decomposition of chloride salts at higher temperatures. The most favorable yield of biodiesel at 2.5 h was 98.3% when the reaction was carried out by calcination of the catalyst at 600 °C. However, when the catalyst was calcined above 600 °C, the excessively high temperature led to CaO sintering, resulting in a decrease in the biodiesel yield. These results indicate that the catalyst activities strongly depend on the calcination temperature [30] and that a suitable calcination temperature is beneficial to the performance of the in terms of flavor and appearance. The optimal calcination temperature was set at 600 °C. In a similar study, Chen et al. [31] reported a biodiesel yield of about 90% using CaO/MgO/Fe3O4 calcined at 600 °C as a catalyst.
Effect of calcination temperature on yield of biodiesel.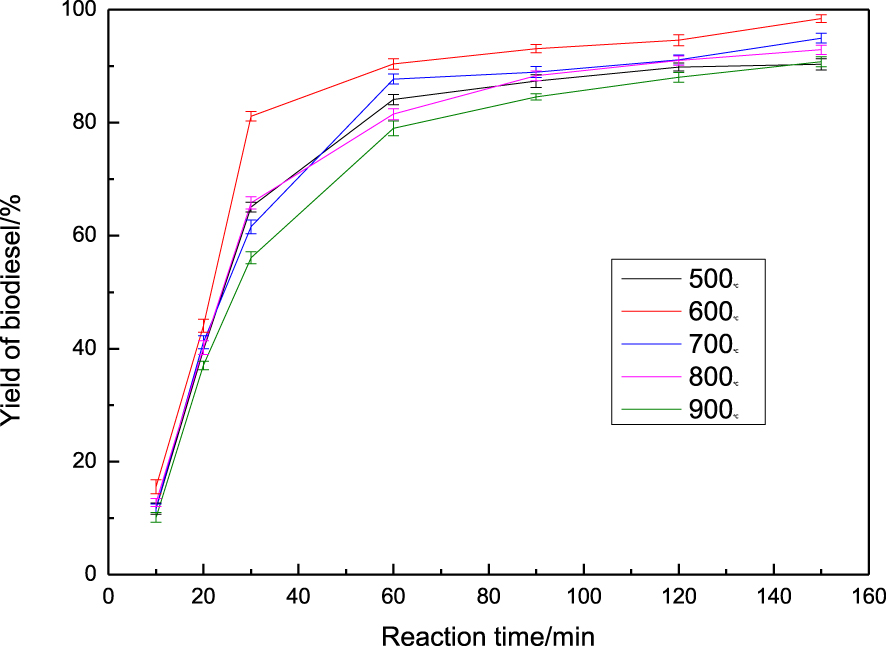
3.1.4. The influence of loading amount of KCl
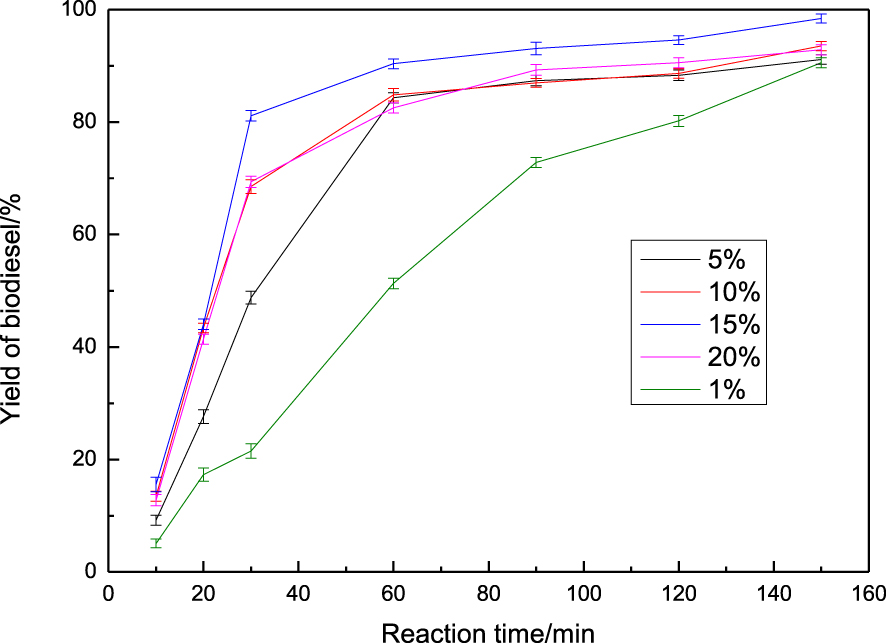
Considering that the highest catalytic performance was exhibited over KCl/CaO, the loading amount of KCl was further considered for evaluating the reaction parameters. As illustrated in Figure 4, the catalytic activity was increased by increasing the amount of KCl from 1% to 15%. While increasing the amount of KCl further, the yield of biodiesel did not increase but decreased significantly due to the excess of KCl covering the active sites on the CaO surface. Therefore, the optimum amount of KCl in this reaction is 15% by weight of CaO. In contrast to Tang’s study [25], it was found that KCl/CaO impregnated in a 15% KCl solution for 12 h and calcined at 600 °C gave the best performance with the highest yield of 96.4%, where there was an appropriate dispersion of CaO particles with strong basic sites.
3.1.5. The influence of catalyst amount
The influence of catalyst amount on the catalytic performance of rapeseed oil transesterification to biodiesel was investigated from 1% to 15%, and the results are shown in Figure 5. From the results, it can be seen that the yield of biodiesel gradually increased with an increase in catalyst weight, and reached the highest value of 98.4% at 10% catalyst usage. Oliveira et al. [32] reported that the obtained catalysts were tested at the following reaction parameters: 20:1 methanol:oil molar ratio; 10% of catalyst amount; 60 °C temperature for 5 h, with the highest yield (92.2%) to be obtained. Whereas the yield of biodiesel decreased to some extent as the catalyst amount was increased to 15%. The results clearly indicate that a sufficient number of basic sites are required to catalyze the completion of the reaction [33]. This decreasing trend was attributed to the formation of soap in the presence of large amounts of catalyst, which increased the viscosity of the reactants and lowered the yield of esters [34].
The effect of catalyst amount on yield of biodiesel.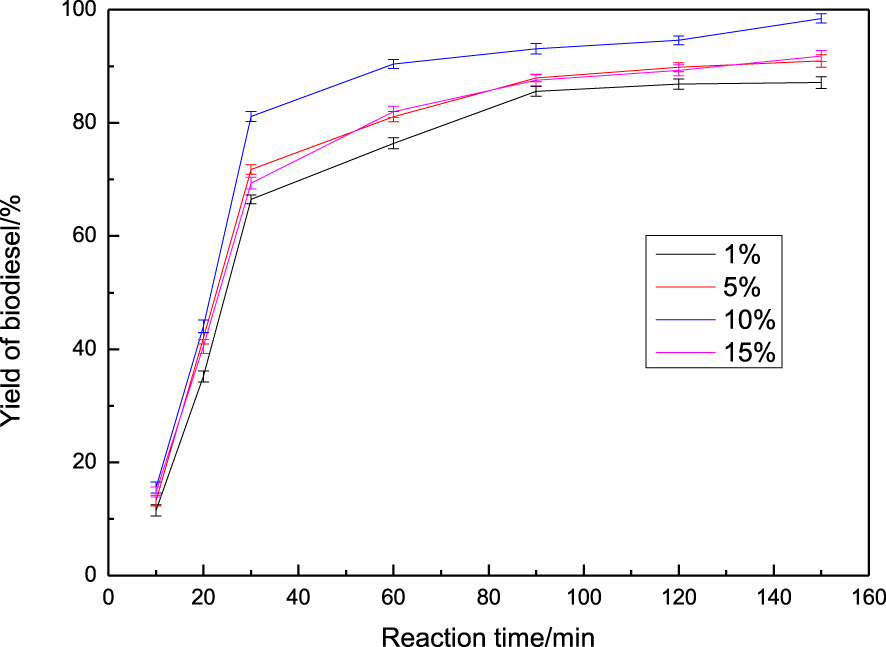
3.1.6. The influence of reaction time
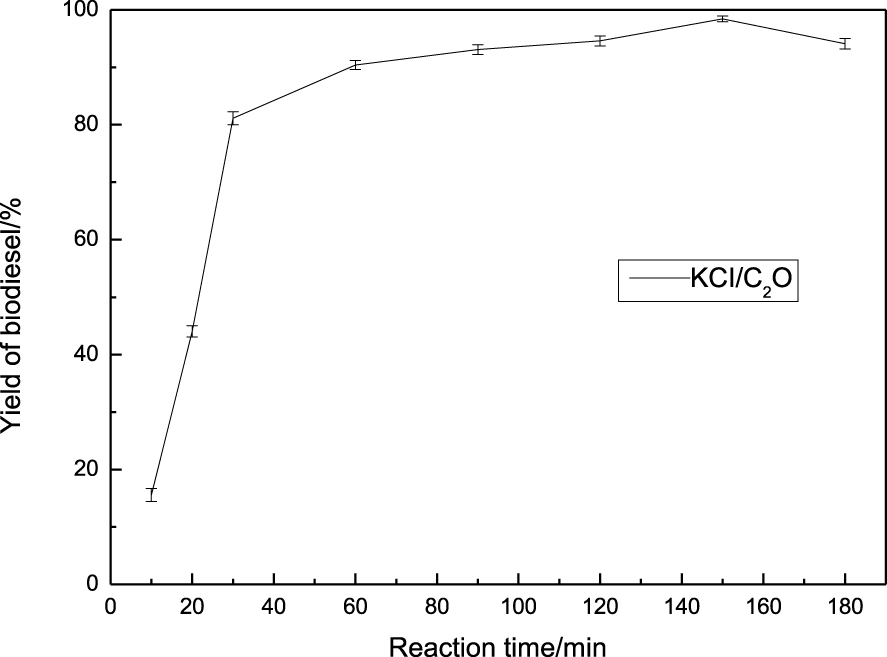
Figure 6 illustrates the influence of reaction time on the yield of biodiesel, which varies in time from 10 to 180 min at reflux temperature. From the results, it can be found that the yield of biodiesel initially increases with increasing reaction time [35], with a maximum yield of 98.3% at the time when the reaction was carried out at reflux temperature for 2.5 h. Whereas, in Wen’s study [36], they prepared the KF/CaO nanocatalyst by impregnation method and used it to convert Chinese tallow seed oil to biodiesel. The optimum reaction conditions were 12:1 alcohol to oil molar ratio, 4 wt% catalyst loading /w of oil, 65 °C reaction temperature, and 2.5 h reaction time resulting in 96.8% biodiesel yield. Further extension of the time to 4 h did not result in an increase in biodiesel yield, but indicated a slight decline in the stage of side reactions that appeared, such as the decomposition of glycerides.
3.2. Catalyst characterization
3.2.1. FT-IR spectrum of different catalysts
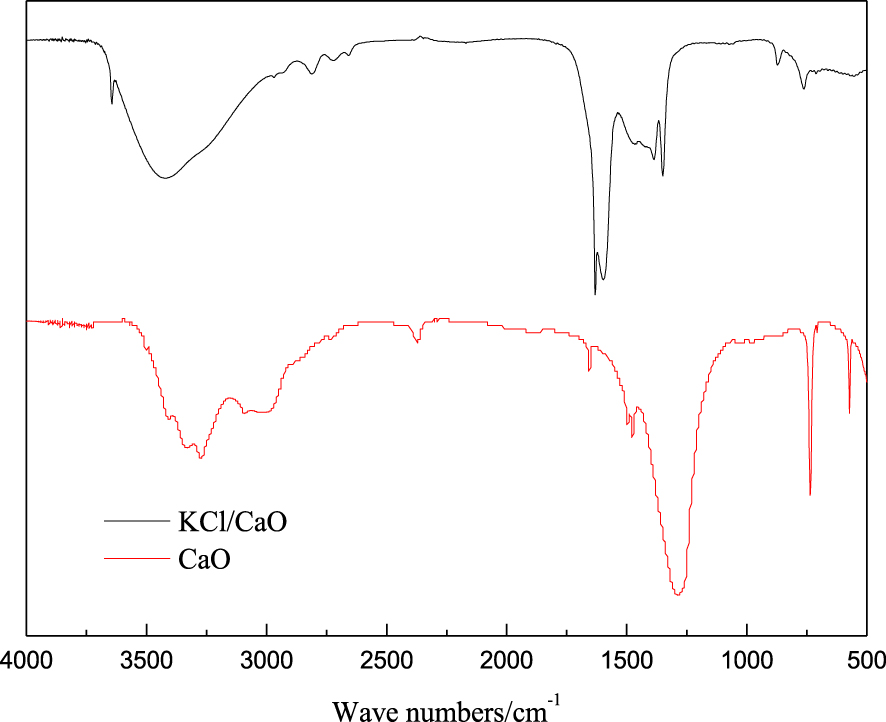
FT-IR patterns of the KCl/CaO catalyst and CaO are shown in Figure 7. The broadband in the 3200–3600 cm−1 region is attributed to the –OH stretching vibrations of the surface hydroxyl groups. The peaks observed at 1625, 1450, and 876 cm−1 could be due to vibrations. The main reason for the formation of –OH and is the absorption of CO2 and water from the air by the catalyst [37]. The presence of KCl obviously reduces the intensity of –OH, which indicates that the formation of KCl/CaO not only enhances the stability of CaO in air, but also makes it well active.
3.2.2. SEM results
The morphologies of CaO before and after support were studied using SEM images. As shown in Figure 8, the surface morphology of CaO particles changed greatly after being supported, and their morphology changed from flocculent particles to spherical granules. The results indicated that the active components could be dispersed on the surface of the catalyst [38]. After loading with KCl, the catalysts were larger in size, with good particle dispersion and exhibited excellent catalytic performance. The EDX analysis of the surface (inserted in b) confirms the composition of the materials. From the results, the amount of KCl over KCl/CaO was found to be lower than the supporting amount, thus indicating the leaching of KCl during the catalyst preparation process.
Scanning electron micrograph of different catalysts.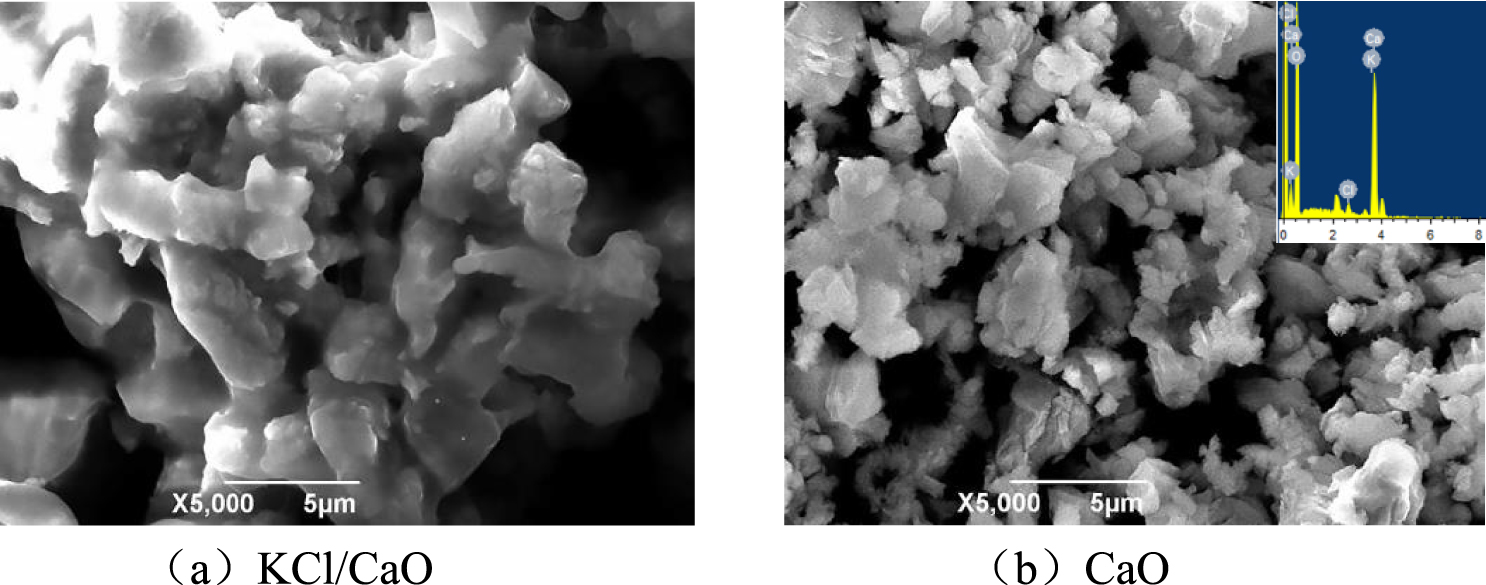
3.2.3. N2 physisorption
The structural properties of the catalysts were determined by N2 adsorption–desorption. As can be seen in Figure 9, the isotherms are typically type II, presenting upward at first and slightly concave upward later in the curve. The curve for CaO seems to overlap, and it may have a cylindrical hole but closed at one end. In contrast, KCl/CaO may have a cylindrical hole open at both ends, or possibly in the shape of an ink bottle. A comparison of the two hysteresis curves of the catalyst suggests that KCl/CaO may exhibit a regular and orderly structure. The unique structure of KCl/CaO may help to improve the catalytic activity.
Nitrogen adsorption–desorption curves of different catalysts.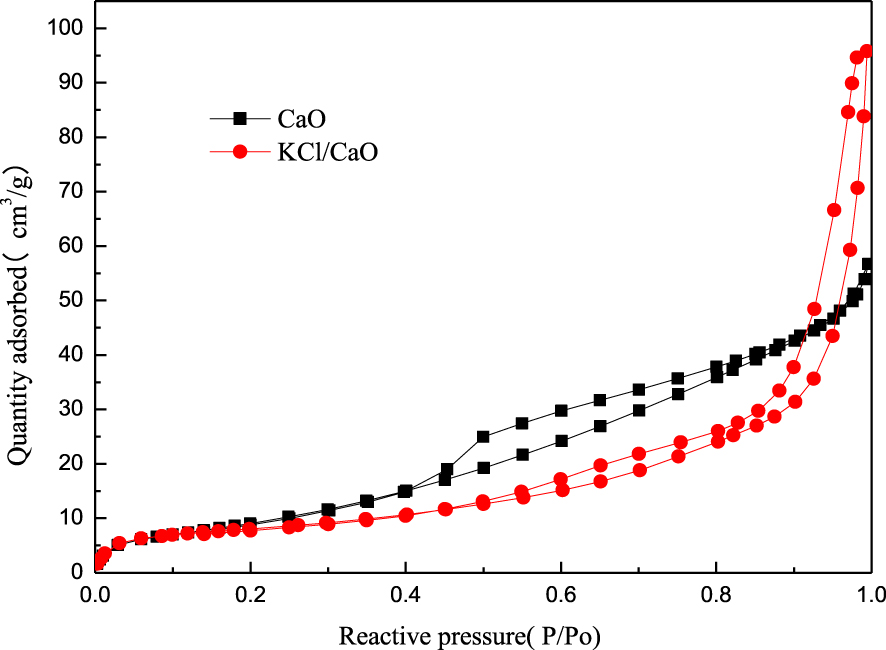
The textural structure and basicity of the samples are summarized in Table 1. It can be found that the surface area and total pore volume of CaO and KCl/CaO gradually decreased from 37.69 to 28.9567 m2/g, respectively. However, the pore size of CaO particles improved greatly from 61.584 to 126.7323 nm upon the addition of KCl. These results indicate that the higher the activity of the catalyst, the larger the average pore diameter of the catalyst. This suggests that the average aperture increases obviously with the addition of KCl, which may be beneficial for the reactive molecules to better access the active sites of the catalysts.
Pore structure properties of different supported catalysts
| BET surface area (m2∕g) | Total pore volume (cm3∕g × 102) | Average pore diameter (nm) | |
|---|---|---|---|
| CaO | 37.6900 | 8.8013 | 61.5840 |
| 15%KCl/CaO | 28.9567 | 9.1744 | 126.7323 |
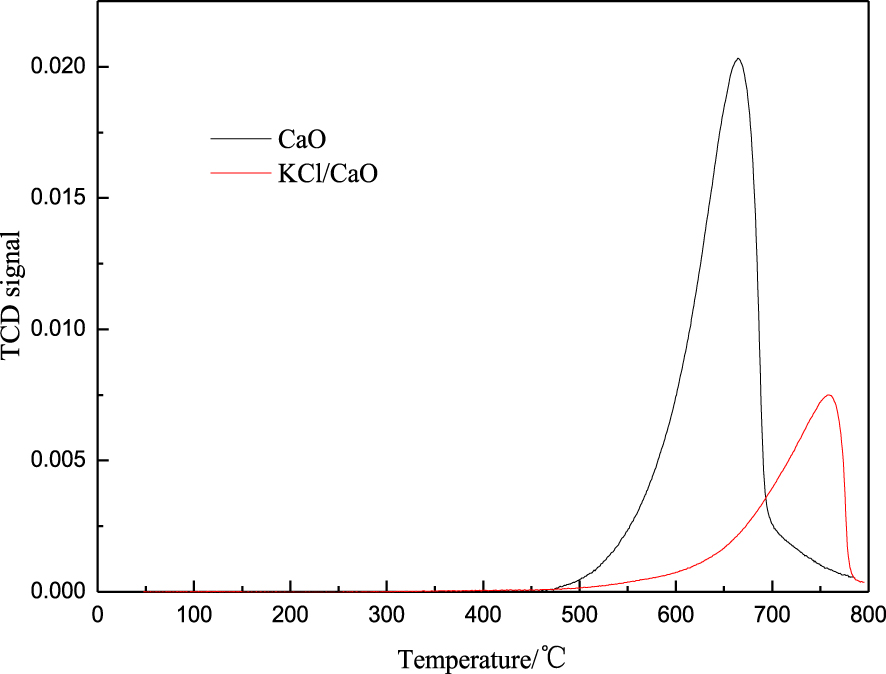
3.2.4. Basic properties
Solid base catalysts have better catalytic activity for the transesterification of rapeseed oil to biodiesel if they have strong basicity and more basic sites [35]. The improvement of catalyst performance should contribute to the formation of methoxyl on the active basic sites of solid catalysts. To find out the basic strength and the number of basic sites, TPD was performed on the calcined catalysts, and the results are shown in Figure 10. No desorption peaks were observed below 200 °C, indicating that both samples are not weakly basic. The desorption peaks appeared in the high temperature range from 600 to 800 °C, attributed to strong basic sites, and the peaks shifted to the high temperature range over KCl/CaO, indicating that the suggested that KCl modified CaO has stronger basic sites, with the CO2 desorption peak appearing at 758 °C. This fact would confirm the presence of strong basic sites in KCl/CaO as well as explain the high catalytic activity observed when it was used as a catalyst for the transesterification of rapeseed oil. Under the same reaction conditions, CaO exhibited a FAME yield of 61.2%, whereas the yield of FAME relative to KCl/CaO was more than 98%.
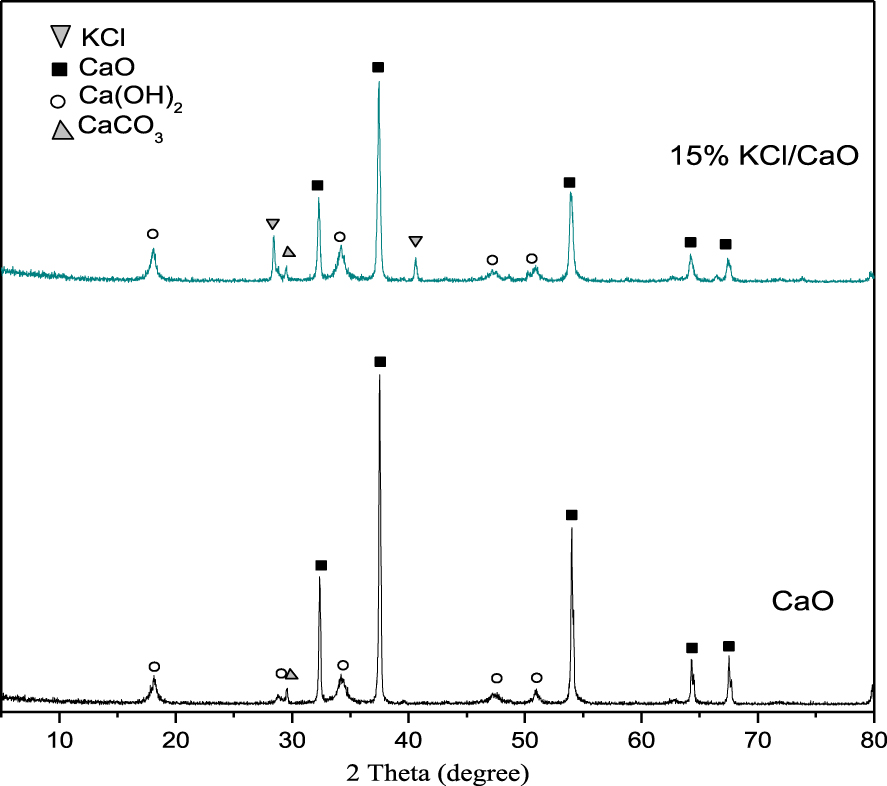
3.2.5. XRD results
The XRD patterns associated with KCl/CaO and CaO are shown in Figure 11. The characteristic XRD lines of CaO appeared with relatively low intensity, while a significant KCl crystallographic diffraction intensity was observed after modification with KCl, indicating that the presence of KCl limits the sintering of CaO particles. Since CaO is known to have a very high basicity, it is considered to be the main basic site responsible for transesterification. The more dispersed the CaO particles are, the higher the amount of FAME yield. Furthermore, weak peaks of CaCO3 could be detected, indicating the presence of amorphous carbonates and a slower carbonation process of CaO compared to rapid hydration. The results are in agreement with the data reported by Granados [39].
4. Conclusions
The transesterification reaction of rapeseed oil with methanol was carried out using modified CaO with various chloride salts. Improvements were made by adding alkali metals to eggshell-derived CaO, and these catalysts typically require almost longer reaction times and high temperature, which can result in spending more time, energy, and cost. The influence of metal types has been investigated and the greater activity of KCl/CaO relative to other modified CaO can be explained by the presence of CaO with a higher concentration of super-basic sites not present in CaO. The novel solid basic catalysts showed high activities for biodiesel production with yields exceeding 98%. The structural properties and performance of these catalysts in biodiesel production from rapeseed oil were investigated and the reaction conditions were optimized to see if biodiesel could be produced under milder conditions (i.e., at a lower price) in the presence of these catalysts. Finally, the stability of the catalysts with the highest yields was also examined. CaO was found to possess the advantages of high basicity, high pore volume and good dispersion.
Conflicts of interest
The authors confirm that this article content has no conflict of interest.
Acknowledgments
This work was supported financially by National Science Foundation of China (21763030) and Youth Innovation Team of Shaanxi University. We also thank the Center of Advanced Analysis and Testing at Xi’an Shiyou University for their work.



 CC-BY 4.0
CC-BY 4.0