1. Introduction
Shale gas is surrounded by hard clay rocks, and it is extracted by a method known as hydraulic fracturing [1, 2, 3, 4, 5, 6, 7]. This method includes injecting a large amount of water containing chemicals into clay structures at high pressure [8, 9, 10]. The development of new technologies to extract gas from unconventional resources in shale structures has increased the potential for gas supply, brought about many economic changes in markets, and changed demand patterns.
Until 1821, shale gas had been produced in small quantities from naturally fractured shales [11, 12, 13, 14, 15]. After the development and optimization of drilling technologies, in 1998, shale gas was produced commercially by Mitchell Energy Company from Barnett formation in Fort Worth Basin using water fracture technology [16, 17].
Successes in the application of horizontal drilling, hydraulic fracturing, and microseismic technologies have been confirmed by some governments [18, 19].
Two thirds of shale gas reservoirs are located in remote areas. Thus due to the difficulty in transferring natural gas to global markets as well as the low price of gas, new technologies have been developed for the conversion of natural gas to materials with higher added value and easier transportation. In addition, a huge amount of natural gas is released along with oil extraction. The cost of storage and transport of natural or associated gas is very large. In most areas, a huge amount of gas is flared, which leads to various environmental consequences. Because of these problems, natural gas conversion to materials with higher added value has become very attractive in the oil and gas industry. In comparison with coal and petroleum, natural gas is considered the largest source of energy. If there are efficient technologies for its conversion, gas resources will be more optimally exploited [20]. If scientists are able to apply economical and efficient methods for converting methane into compounds with higher added value, the large amount of gas trapped in gaseous shales will create many opportunities for investment [21]. Based on general statistics, the global market of shale gas has the potential to grow almost 5.3% (equal to $ 9.19 billion) for commercial purposes during 2014 to 2020 [22].
At present, there are many opportunities and challenges for the conversion of shale gas to more valuable products because of massive exploration of shale gas. The main four valuable products include methanol, liquid fuels, ethylene, and propylene. Each of these products has its own conventional production methods, preservation technologies, derivatives, and uses. Al-Douri et al. studied different methods of converting shale gas into compounds with added value [23]. If new technologies develop and investments expand, the gas conversion will significantly reduce gas flare. One of the problems with gas conversion has been the low price differential between feed and chemical products. But recently, the factors influencing the importance of gas conversion have changed. The increase in the volume of flare gases is undeniable. Liquid fuels have also gained a lot of credit due to them being sulfur-free [24, 25]. Gradassi et al. studied the potential of industrial profitability of available natural gas conversion processes. They presented a basic research to calculate operating costs and initial investment for these processes. Their selected processes all required high initial investment costs. However, the payback period of the gas to methanol (GTM) conversion process has been less than that of other processes [26].
In another research, the direct conversion of shale gas to benzene has been investigated from economic, environmental, and energy-saving points of view. A sensitivity analysis of the operation condition indicates that the highest return on investment (ROI) is achieved when operating conditions of the reactor and the flash drum are (P = 0.3 bar, T = 800 °C) and (P = 10 bar, T = 20 °C), respectively. In addition, heat integration of the process reduces the CO2 emission by 16%, which has a significant impact on environmental requirements and economic parameters [27].
The high cost of natural gas transmission from remote reserves to consumer markets has prevented the full exploitation of these reserves. Liquefied natural gas (LNG) can be transported by pipelines or ships. Nonetheless, the distribution of this liquefied gas to consumers via pipelines requires compression up to approximately 80 atm, and sometimes the pipelines to the destination may not be available. Therefore, the conversion of methane to more valuable products can make huge changes in the gas industry. Havran et al. realized that converting methane and carbon dioxide into more valuable chemicals is one of the new challenges facing the oil and gas industry. They described direct conversion methods of these two gases, including synthesis gas production, direct acetic acid production, photovoltaic conversion, and the dielectric barrier discharge method. They concluded that up until now, dry reforming has been the most successful method of carbon dioxide and methane conversion, which faces obstacles such as excess carbon deposition and catalyst inactivity [28].
1.1. Production of synthesis gas
Synthesis gas is a combination of carbon monoxide and hydrogen. In other words, synthesis gas is a kind of intermediate compound in the production of valuable chemicals. Companies have developed several processes to produce synthesis gas because this product is mostly utilized in the petrochemical industry.
Synthesis gas production is the most significant part of the natural gas conversion process to other materials with added value. Usually, a considerable amount of initial investment is devoted to this part [29].
There are three main methods of producing synthesis gas from natural gas:
Steam reforming reaction:
| (1) |
| (2) |
| (3) |
The combined use of synthesis gas production methods provides an opportunity to take advantage of each process and reduces the drawbacks of each process. For instance, autothermal reforming is a combination of steam reforming and partial oxidation reforming in which controlling the reactor temperature is easier. Furthermore, the ratio H2/CO can be adjusted by different applications and various operating conditions [30]. In the late 1950s, Topsoe started using the ATR process to industrially produce synthesis gas in ammonia and methanol production units [31]. If low-cost oxygen is available, the ATR process will be considered a proper method for synthesis gas production [32]. Hao et al. simulated the gas to liquid (GTL) process by using Aspen Plus. They used a Gibbs reactor for the autothermal process and utilized a kinetic reactor for the Fischer–Tropsch process in this software [33].
1.2. Shale gas conversion to liquid
Natural gas has been recognized as the cleanest and the most available fossil fuel in the world. It is essential to convert this gas into a liquid that has less volume than gas. For this purpose, there are two main solutions: the liquefaction of natural gas and the chemical conversion of natural gas to liquid (GTL). The GTL process involves the chemical conversion of gas to long-chain hydrocarbons, which include a wide range of transmissible and liquid fuels. Shale gas conversion takes place in the presence of catalysts such as cobalt and/or iron through Fischer–Tropsch synthesis.
In recent years, due to the great potential for the production of high-quality liquid fuels, many technical and economic studies have been conducted on gas to liquid conversions. The final products of this process are mostly equivalent to petroleum products produced in distillation towers of crude oil refineries, which are in the range of C10 to C20 compounds. They are also called middle distillate products. Other products of Fischer–Tropsch synthesis include different types of liquid fuels such as naphtha, lube oil, and wax.
The naphtha obtained from the GTL process is a suitable feed for the ethylene production unit due to its smaller cetane number than the refinery products. Due to the lack of sulfur, naphtha and kerosene obtained from GTL have a high smoke point. Saturated hydrocarbons generated from this process such as waxes and lubricants have high added value due to their high quality.
Using the Fischer–Tropsch process in GTL technology has various environmental advantages such as producing fuels with a smaller amount of sulfur compounds and NOx and less aromatics.
Another advantage of GTL is producing enormous products as fuel with high cetane numbers (70–80). These products can be mixed with refinery products, especially diesel fuel [34]. In a case study for evaluating the GTL process, 1.16 billion Standard Cubic Feet per Day (SCFD) of natural gas was used to produce 118,000 barrels per day (bbl/d) of liquid products. Depending on the gas price, the ROI has been different. By reducing the price of gas or increasing the selling price of liquid products, the profitability of this process has increased [35, 36].
1.3. Shale gas conversion to methanol
Methanol is one of the products obtained in the conversion of gas to chemicals. Methanol is an intermediate product for producing other chemical products such as acetic acid, formaldehyde, dimethyl terephthalate, methyl tert-butyl ether, etc. Regarding the potential of using shale gas for methanol production, Laura M. Julian-Duran et al. provided technical, economic, and environmental analyses of the conversion process of shale gas to methanol. They investigated various synthetic gas production methods such as partial oxidation, steam reforming, autothermal reforming, and combination reforming. The results show that partial oxidation and autothermal reforming provide more financial profit for the methanol production process. From an environmental point of view, combination reforming is more acceptable; carbon emission is much lower in this alternative [37]. The first synthesis of methanol was carried out by BASF Company chemists in Luna, Germany, in 1923 [38]. Kung Harold believed that shale gas conversion to methanol using traditional and common methods was more economical than gas transportation by LNG ships [39]. Minbu Young and Fengi analyzed the profitability of shale gas economically and environmentally. They concluded that methanol production on a small scale was more economical than the common processes of shale gas conversion and enormous ranges of methanol production [40]. Ross et al. considered autothermal reforming as the suitable and probable method for high-capacity methanol production [41]. The role of methane in modern industries, specifically in methanol production, has been studied. Methanol is an essential feed for the synthesis of valuable products in chemical industries. Furthermore, the direct production of methanol from methane with effective oxidation of hydrogen–carbon bonds is one of the most attractive topics in the petrochemical industry [42]. In a comprehensive study, methanol production using natural gas obtained from the Brent shale has been simulated by Aspen Plus software. The results of techno-economic analysis indicate that methanol production in a wide range of selling prices of methanol and shale gas has desirable economic parameters. For example, for the selling prices of methanol and shale gas, $2 gal and $3.5 MMBtu, respectively, the ROI value was 31%. In addition, it became clear that the ROI would increase by approximately 2.5% after energy integration. Moreover, natural gas pre-treatment processes depend on the gas composition, which can change the feedstock price of the methanol production unit [43].
The demand of methanol to olefin (MTO) conversion has extremely increased. Jasper and Halwagi have compared the MTO and methanol to propylene (MTP) processes in a comprehensive technical–economic analysis. They compared the use of natural gas for methanol production with the purchase of methanol. If natural gas is used as feedstock to produce methanol, more acceptable economic results will be obtained [44].
1.4. Shale gas conversion to ethylene
At present, ethylene is one of the most important and useful chemicals in the world. It has many advantages for use in the petrochemical industry. The global demand for ethylene has increased. Reportedly, 140 million tons of ethylene were produced in 2010. The importance of ethylene is due to binary bonds in its molecular structure, which increases reactivity and the ability to form chemical compounds [45]. Ethylene can be industrially converted into intermediate products. Ethylene is mainly used for conversion to light or heavy polyethylene, which is applied in industries such as construction, communication, packing, and other industrial plants [46]. Olefins such as ethylene and propylene are the most important feedstock for petrochemical units [47].
Chang He and Fengqi You investigated new methods for producing ethylene from natural gas and presented economic parameters such as net present value for each of them. They concluded that their proposed methods such as steam cracking and propane dehydrogenation have a positive effect on process efficiency and fixed investment cost in comparison with common methods of gas conversion to ethylene [48, 49]. Zolfaghari et al. examined different methods for flare gas recovery (gas conversion to ethylene, gas to liquid, and power generation). They concluded that the flare gas conversion to ethylene has the highest annual benefit, while the ROI is lower than that of other methods [49].
Ethylene can be produced by thermal cracking of ethane and propylene that are obtained from natural gas. However, new methods such as direct methane conversion to ethylene by using catalyst reactors have been widely used. In this method, natural gas components are used to produce ethylene without initial separation [45].
Common ethylene industrial production processes include thermal cracking of ethane, naphtha and natural gas. The oxidative coupling of methane (OCM) and the methanol conversion to olefins are among these processes. In the OCM process, methane is converted directly into ethylene in a catalytic reactor, while in the MTO process, Methane is first synthesized and then the synthesis gas is fed to methanol production reactors. Eventually, methanol is converted in a catalytic reactor into ethylene and other by-products. The economic analysis of the aforementioned methods for a certain amount of shale gas feedstock has shown that the MTO process has a higher ROI than OCM [50].
Natural gas conversion to olefins (GTO) has been introduced as one of the affordable and practical methods for chemical production. The two main products of this process are ethylene and propylene. In this process, natural gas is converted into methanol through the UOP/Hydro MTO process, and then light olefins are produced from methanol. Three methods, including the use of naphtha crackers, ethane crackers, and GTO, to produce 500,000 MM MTPA ethylene have been compared. The ROI values for the naphtha crackers, ethane cracker, and GTO are 8, 27, and 22, respectively. However, the GTO process is economical in areas where low-cost natural gas is available and has a significantly higher ROI than that of traditional methods such as naphtha cracking [51].
Regarding the above description of various recovery processes, the main purpose of this research is to present and evaluate the conventional processes of converting shale gas into valuable chemicals from a technical point of view and to compare their economic parameters in order to select the optimal and appropriate method for converting a certain volume and composition of the shale gas. Finally, the optimal process for the shale gas conversion is selected by comparing the amount of products obtained from each process, a technical review of each process’s flow diagram, the equipment used per process unit and its costs, and comparison of parameters such as profit, ROI, total investment cost, etc.
2. Methods and principles
2.1. Modeling method and economic evaluation of processes
Aspen HYSYS software is one of the best simulators in chemical engineering. Aspen HYSYS v10 is used for the process simulation of shale gas conversion technologies. Then, the technical information obtained from each process is imported to Aspen Capital Cost Estimator software, and economic analysis is accomplished in great detail. Aspen Capital Cost Estimator is the appropriate software for preparing detailed reports on designing processes and economic evaluation related to a project.
The composition of feed shale gas
| Component | Mole percent |
|---|---|
| Methane | 79.9 |
| Ethane | 11.61 |
| Propane | 3.98 |
| Nitrogen | 0.09 |
| CO2 | 0.73 |
| Butane | 2.12 |
| C5+ | 1.22 |
| H2O | 0.23 |
| H2S | 0.12 |
The composition of input shale gas feed applied in each process is retrieved from the essay of Jian Gang and colleagues (Table 1) [52]. It is noteworthy that for comparing three processes of shale gas conversion correctly, the feed flow rate of each process has been considered equal to 7945 kgmol/h.
2.2. Process description
2.2.1. GTL process
The GTL process is one of the best methods for shale gas conversion to various hydrocarbons due to economic profitability and converting associated gases into eco-friendly fuels. Fischer–Tropsch synthesis products include linear and branched hydrocarbon compounds and other oxidized compounds. The main products of this synthesis are linear paraffin and alpha-olefin. In fact, Fischer–Tropsch synthesis is a catalytic process that converts synthesis gas into a combination of hydrocarbons (liquid fuels). The Fischer–Tropsch reaction can be considered as the hydrogenation of carbon monoxide, which is as follows:
| (4) |
Furthermore, other reactions occur in the Fischer–Tropsch reactor. Table 2 summarizes some of the possible reactions in the reactor [53].
A number of possible reactions in F–T reactor
| Reaction | (kg/mol) |
|---|---|
| CO + 2H2 → –CH2– + H2O | −165.0 |
| 2CO + H2 → –CH2– + CO2 | −204.7 |
| CO + H2O → H2 + CO2 | −39.8 |
| 3CO + H2 → –CH2– + 2CO2 | −244.5 |
| CO2 +3 H2 → –CH2– + 2H2O | −125.2 |
Process flow diagram of GTL unit.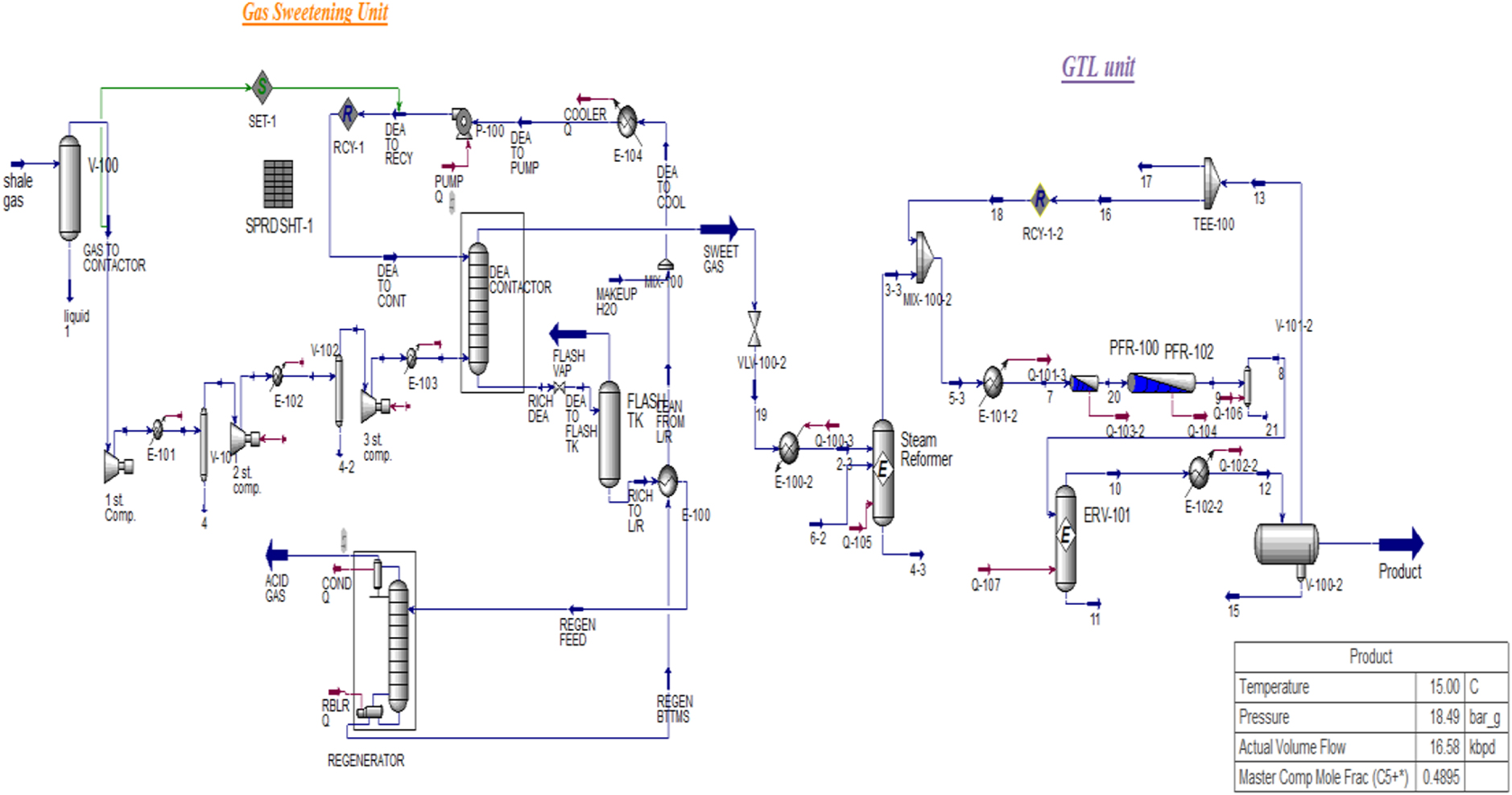
Figure 1 shows the simulation of the Fischer–Tropsch process. This process includes three main stages: synthesis gas production, treatment and purification, and Fischer–Tropsch synthesis and optimization of products using common refining processes. Prior to synthesis gas production, the acid gas removal process takes place through the amine solution in the absorption and desorption columns. Since Fischer–Tropsch catalysts are sensitive to H2S, the pre-treatment process is crucial to remove acidic gases from the feed. The next stage takes place in a steam reformer in which synthesis gas is produced by steam reforming. After cooling, the synthesis gas moves to Fischer–Tropsch reactors. Eventually, synthesis products are extracted in a separator; heavier products are separated. The kinetic parameters and other details related to the reaction mechanism have been studied in previous research [54].
2.2.2. Methanol production process
Figure 2 demonstrates that the methanol production process from shale gas consists of four main stages: desulfurization of natural gas, steam reforming, methanol gas synthesis, and methanol purification. Since natural gas has sulfur impurities, hydrogenation of acid gases is essential. Thereby, part of the purging gases of the synthesis unit that contain a high amount of hydrogen is mixed with feed stream in the hydrogenation reactor. Then, the gas outlet from the hydrogenation reactor enters two-stage desulfurization catalytic reactors. In the first reactor, the sulfur components of gas are converted into H2S by the cobalt–molybdenum catalyst, and in the second reactor, which includes the ZnO catalyst, the H2S gas produced in the previous reactor is absorbed by zinc oxide. For increasing desulfurization efficiency, the inlet temperature is set as 350–400 °C. The purified gas is saturated after the temperature drops to 95 °C.
Block diagram of GTM unit.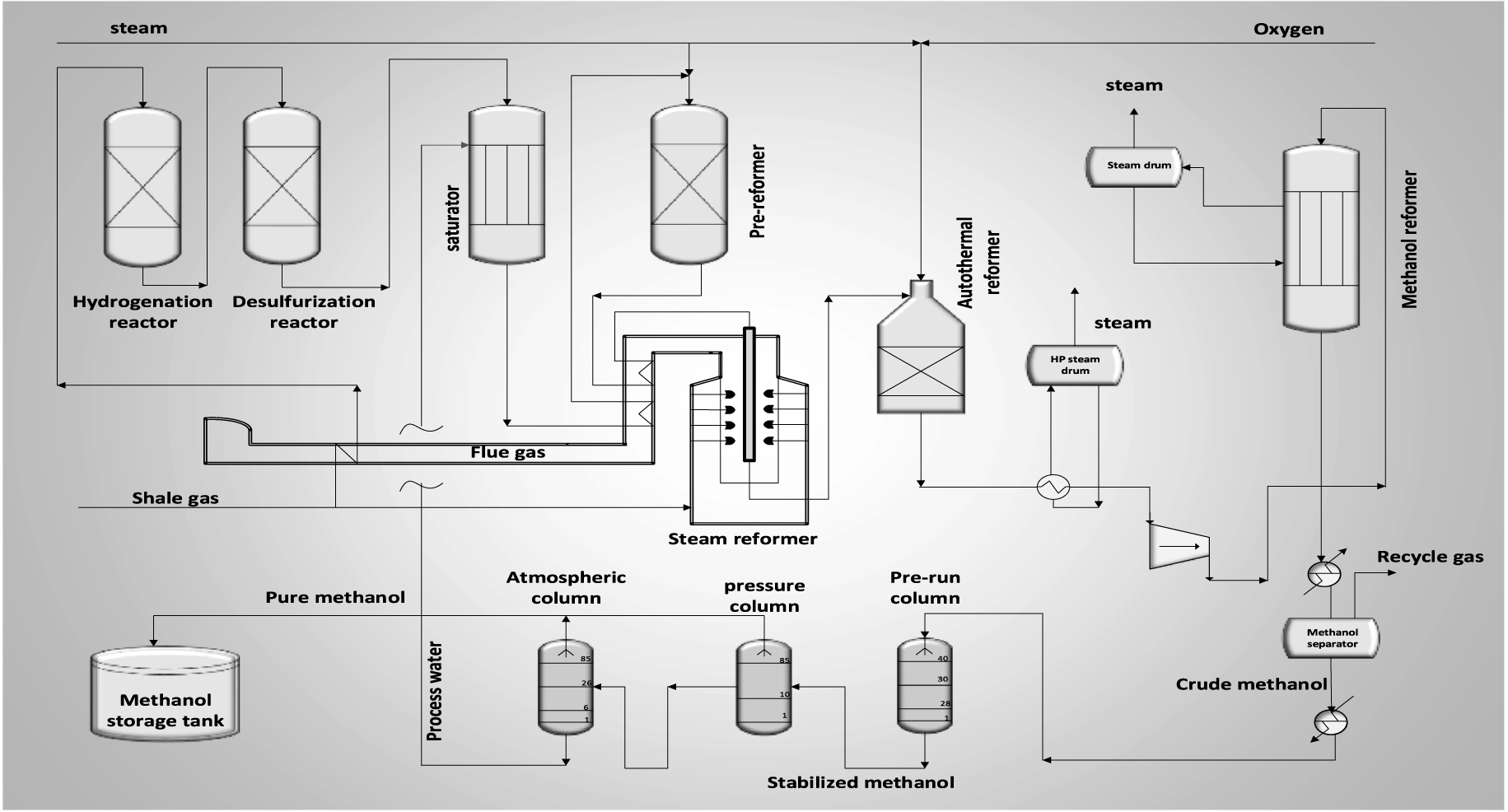
The saturated feed gas is moved to a pre-reformer after several heating stages, and heavier hydrocarbons are converted into lighter ones through the following reactions.
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
Process flow diagram of GTM unit.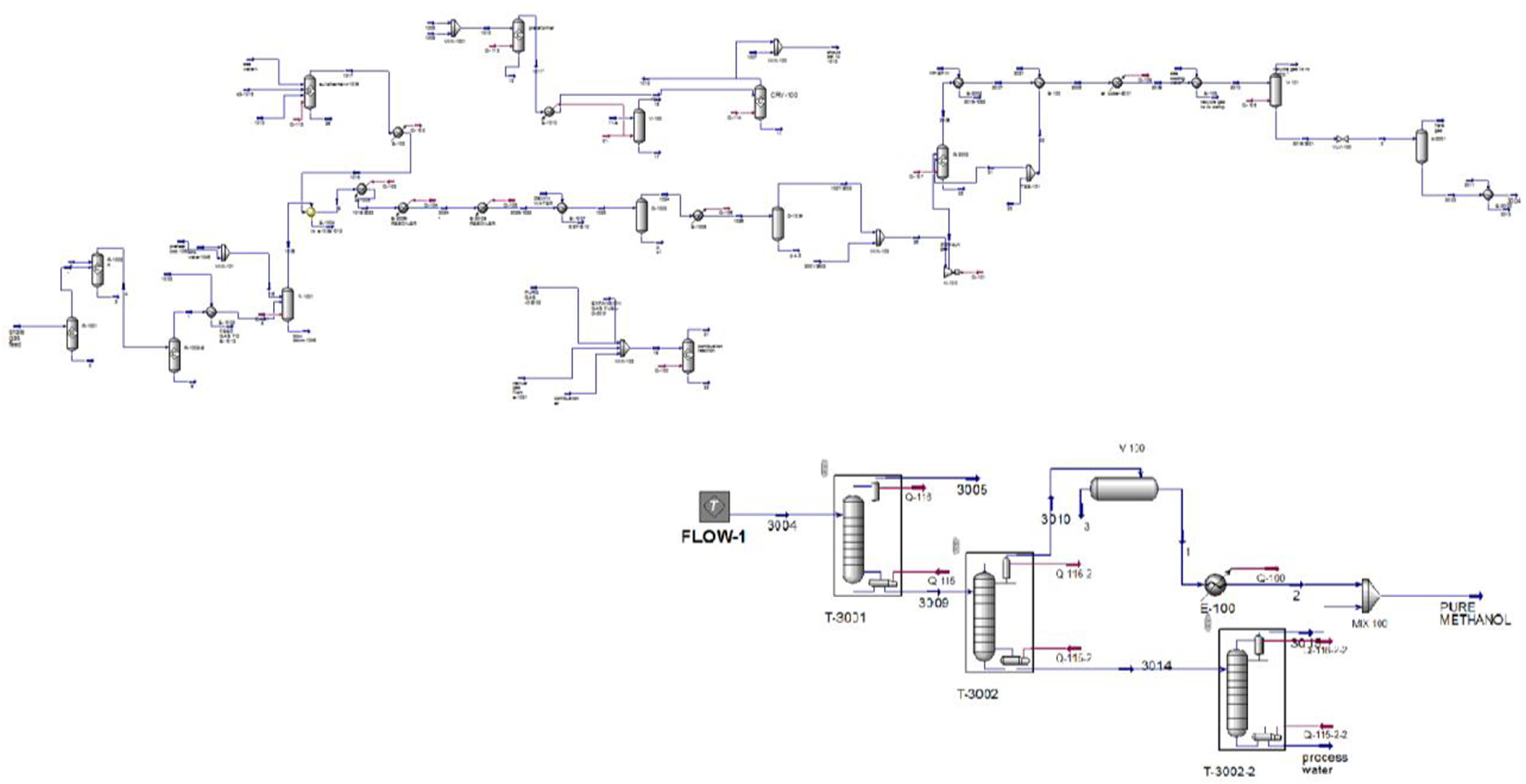
The methanol produced is associated with some dissolved gases, water, and impurities with various boiling points. These impurities are separated in a distillation unit. Finally, pure methanol and process water are obtained. Methanol distillation is implemented in three stages, including gas separation, by-product separation with a low boiling point, and separation of by-products with various boiling points. The gas separation stage occurs in a stabilizing column in which methanol pressure is reduced to approximately 2–3 bar. The dissolved gas is released. Then, crude methanol with water and dissolved gases is transmitted to the first distillation tower. The first distillation tower consists of 85 trays, and the input flow enters the 10th tray. Volatile impurities and inert gases are separated from methanol in this column. The output methanol stream from the bottom of the column, which is almost degasified, enters the second distillation column at 94 °C 2.4 bar. In the second column, methanol, water, and other heavy components are separated and the process water is removed from the bottom of the column. The output methanol vapor enters an air cooler from the top of this column at 69 °C and then passes through a heat exchanger. Consequently, its temperature decreases to 40 °C, and then it is transmitted to a reflux drum. A part of the condensed methanol is returned to the column by reflux pumps and the rest of the pure methanol is transmitted to storage tanks. Figure 3 illustrates the methanol production unit simulation in Aspen HYSYS software.
2.2.3. Ethylene production process
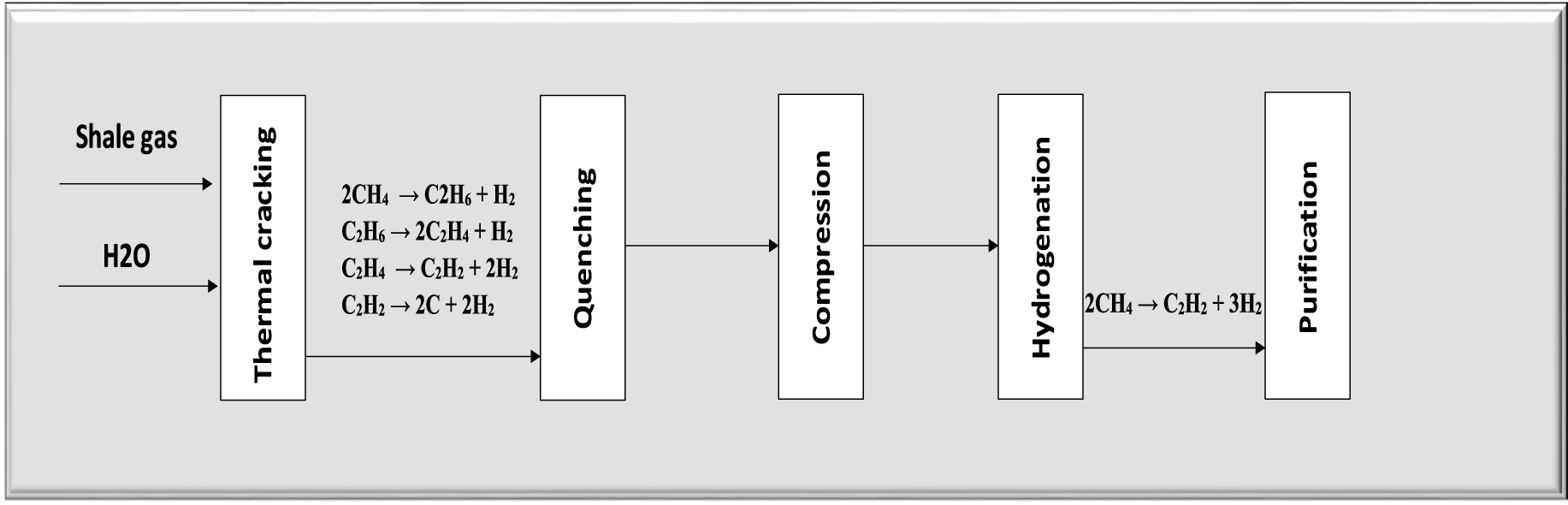
The direct conversion of shale gas to ethylene (GTE) has been simulated in this research (Figure 4). In this process, sweet shale gas mixture is transmitted to a thermal cracker. In this container, methane and other hydrocarbons existing in the gas are converted into hydrogen and acetylene.
Process flow diagram of GTE unit.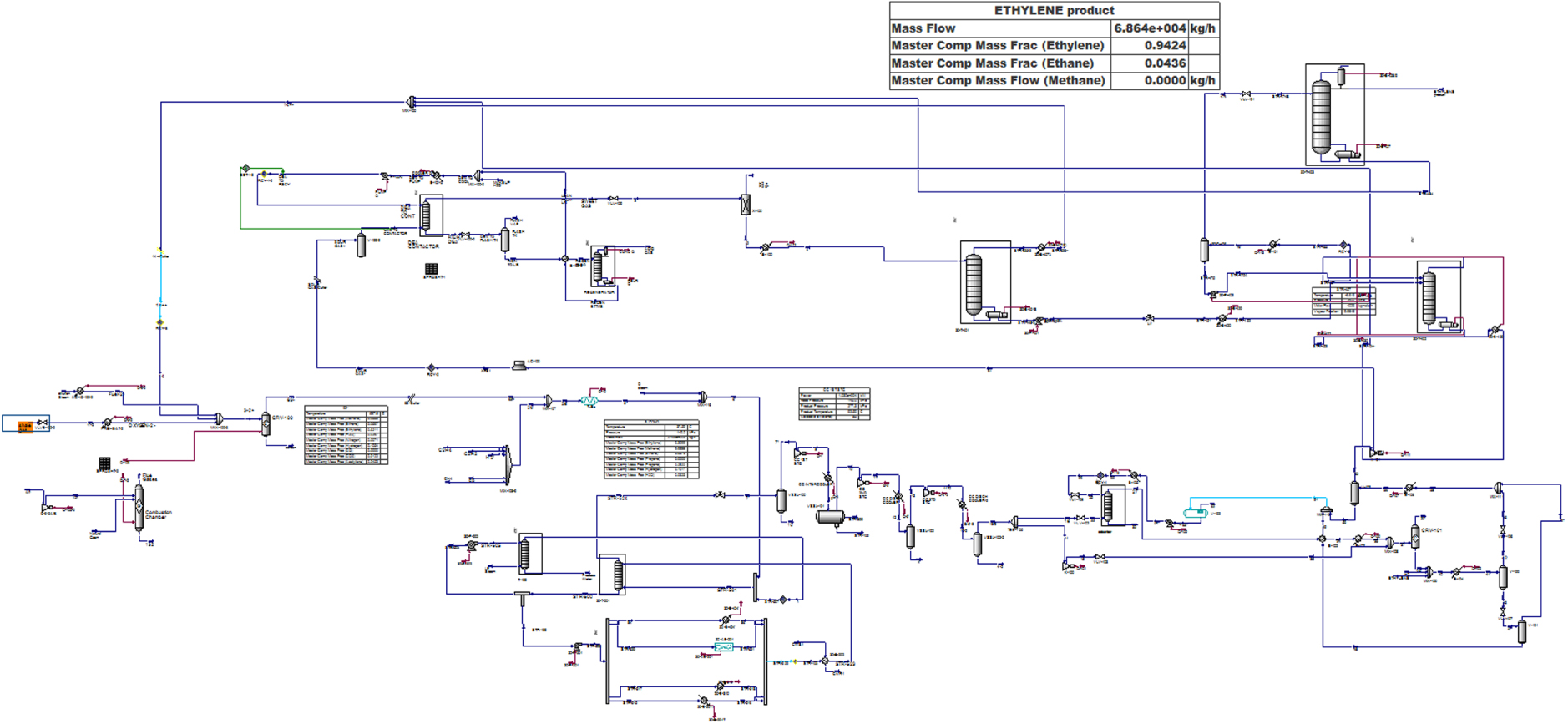
The kinetics of this mechanism are very complicated. Figure 5 shows the main reactions that occur in the cracker.
3. Discussion and results
3.1. Technical results and simulation outputs of GTL production unit
The synthesis fuels produced in the GTL process do not contain any aromatic and sulfuric compounds. GTL synthesis fuels can be considered green and clean fuels. Before simulating the Fischer–Tropsch synthesis process, a gas sweetening unit is simulated. The composition of sweet gas is presented in Table 3.
Table 4 indicates the simulation results of GTL, GTM and GTE processes. In the GTL process, the required fuel (gasoline, gasoil, and diesel) is produced from gas instead of crude oil. According to the fact that this process is considered one of the best solutions for converting shale gas into compounds with high added value.
3.2. Technical results and simulation outputs of methanol production unit
Shale gas produces less greenhouse gas than coal. As explained, the input feed gas passes through one pre-purification process including a hydrogenation reactor and desulfurization before synthesis gas production. The composition of exhaust gas from these reactors is presented in Table 7. The reduction of acidic gas can be observed after this process. After treating the input gas, it is necessary to produce synthesis gas with an appropriate ratio of H2 and CO. The production of synthesis gas is recognized as the most fundamental part of the shale gas conversion to methanol. Simulation results including the composition of the synthesis gas in the output of the autothermal reactor and specification of pure methanol are presented in Tables 6 and 7, respectively.
The composition of shale gas after sweetening in GTL process
| Component | Mole fraction |
|---|---|
| Methane | 0.806 |
| Ethane | 0.117 |
| Propane | 0.040 |
| Nitrogen | 0.001 |
| CO2 | 0.000 |
| i-Butane | 0.022 |
| n-Butane | 0.000 |
| i-Pentane | 0.012 |
| n-Pentane | 0.000 |
| H2S | 0.000 |
| H2O | 0.002 |
| n-Hexane | 0.000 |
| CO | 0.000 |
| Hydrogen | 0.000 |
| Ethylene | 0.000 |
| C5+ | 0.000 |
Characterizations and conditions of products from various flare recovery processes
| GTL | GTM | GTE | |
|---|---|---|---|
| Conditions | |||
| Temperature (°C) | 15 | 85.02 | −28.549 |
| Pressure (bar) | 18.49 | 2 | 19.9 |
| Molar flow (kgmol/h) | 991.1 | 4041 | 2427 |
| Mass flow (kg/h) | 6.643e+004 | 1.295e+005 | 6.864e+004 |
| Molar enthalpy (kJ/kgmol) | −3.842e+004 | −2.116e+005 | 3.888e+004 |
| Molar entropy (kJ/kgmol⋅°C) | 167.81 | 102.8 | 141.3 |
| Heat flow (kJ/h) | −3.808e+007 | −8.551e+008 | 9.438e+007 |
| Mole fraction | |||
| Methane | 0.015 | 0.000 | 0.056 |
| Ethane | 0.048 | 0.000 | 0.060 |
| Ethylene | 0.004 | 0.000 | 0.321 |
| Propane | 0.217 | 0.000 | 0.292 |
| i-Butane | 0.080 | 0.000 | 0.000 |
| n-Butane | 0.052 | 0.000 | 0.046 |
| i-Pentane | 0.079 | 0.000 | 0.000 |
| n-Pentane | 0.008 | 0.000 | 0.027 |
| n-Hexane | 0.000 | 0.000 | 0.007 |
| M-Mercaptan | 0.000 | 0.000 | 0.000 |
| E-Mercaptan | 0.000 | 0.000 | 0.000 |
| H2S | 0.000 | 0.000 | 0.000 |
| Nitrogen | 0.001 | 0.000 | 0.001 |
| Hydrogen | 0.004 | 0.000 | 0.105 |
| H2O | 0.000 | 0.001 | 0.027 |
| CO | 0.003 | 0.000 | 0.000 |
| CO2 | 0.000 | 0.000 | 0.012 |
| Methanol | 0.000 | 0.999 | 0.000 |
| C5+ | 0.489 | 0.000 | 0.000 |
3.3. Technical results and simulation outputs of ethylene production unit
Ethylene was produced by thermal cracking of ethane and propylene from shale gas. The composition of the exhaust gas from the thermal cracker is presented in Table 4. This table presents the final product specification of direct ethylene production from methane. Obviously, there is a negligible amount of acid gas due to the purification process. Therefore, highly purified ethylene has been obtained.
3.4. The economic analysis of the processes
The results of the process simulation were imported to the Icarus software to economically evaluate the gas conversion processes. Effective factors of the total costs include all direct and indirect costs, operational costs, feedstock cost, and the market prices of GTL, methanol, and ethylene.
The total investment for the production of 1100 barrels of GTL per day was calculated. In addition, the production costs of 162 m3/h of methanol and 1838 m3/h of ethylene were calculated (Tables 5–8). For comparing these processes, their important economic parameters were calculated according to Figure 6.
Cost summary of GTL process unit project
| GTL project cost summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Account | Key qty | Unit MH | MH | Wage rate | Labor cost | Unit matl | Matl cost | Total cost |
| Equipment | 18 item(s) | 622.30 | 11,201.00 | 43.07 | 482,441 | 3,233,167 | 58,197,000 | 58,679,441 |
| AG pipe | 1935 M | 15.00 | 29,012.00 | 42.57 | 1,235,121 | 2,108 | 4,078,790 | 5,313,911 |
| Concrete | 906 M3 | 9.5 | 8,643 | 33.79 | 292,014 | 288.29 | 261,219 | 553,233 |
| Grout | 3.3 M3 | 163.1 | 540.00 | 32.37 | 17,472 | 4,255 | 14,084 | 31,556 |
| Steel | 21.6 tons | 46.30 | 998.00 | 39.51 | 39,440 | 8,919 | 192,364 | 231,804 |
| Instrumentation | 355 each | 18.00 | 6,404.00 | 42.83 | 274,291 | 2,545 | 903,508 | 1,177,799 |
| UG electrical | 597 M | 0.94 | 559.00 | 37.44 | 20,933 | 18.79 | 11,222 | 32,155 |
| AG electrical | 15771 M | 0.50 | 7,853.00 | 41.46 | 325,579 | 62.76 | 989,715 | 1,315,293 |
| Pipe insulation | 1760 M | 1.80 | 3,221.00 | 32.00 | 103,062 | 72.31 | 127,293 | 230,355 |
| Equip insulation | 935 M2 | 2.90 | 2,715.00 | 31.90 | 86,619 | 61.54 | 57,566 | 144,185 |
| Paint | 8331 M2 | 0.46 | 3,850.00 | 31.48 | 121,204 | 6.00 | 50,022 | 171,227 |
| Direct totals | - | - | 74,996.00 | 2,998,175 | - | - | 67,880,959 | |
| Const equip & indirects | - | - | - | - | - | - | - | 2,522,300 |
| Const mgt, staff, supv | - | - | 10,900.00 | - | - | - | - | 1,263,600 |
| Engineering | - | - | 28,361.00 | - | - | - | - | 3,523,800 |
| Other project costs | - | - | 1,516.00 | - | - | - | - | 4,431,686 |
| Contingency | - | - | - | - | - | - | 11,943,352 | |
| Indirect totals | - | - | 40,777.00 | - | - | - | - | 23,684,738 |
| Project totals | - | - | 115,773.00 | - | 2,998,175 | - | - | 91,565,696 |
Stages of calculating economic parameters.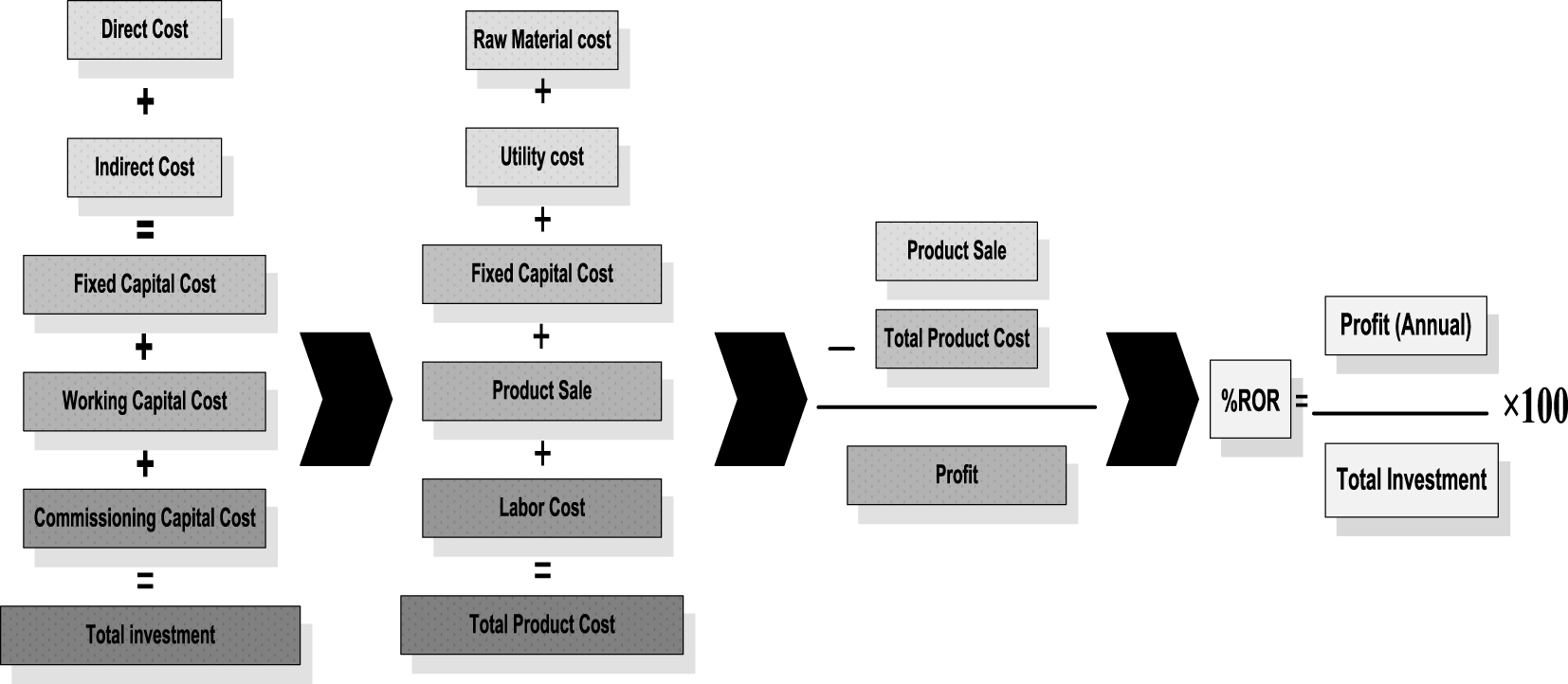
Cost summary of GTM process unit project
| GTL project cost summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Account | Key qty | Unit MH | MH | Wage rate | Labor cost | Unit matl | Matl cost | Total cost |
| Equipment | 30 item(s) | 736.20 | 22,085 | 4,304.00 | 950,615 | 275,889 | 82,766,900 | 83,717,515 |
| AG pipe | 3509M | 46.80 | 164,145 | 42.80 | 7,025,903 | 3,060 | 10,736,340 | 17,762,243 |
| Concrete | 1912 M3 | 11.00 | 21,068 | 34.09 | 718,150 | 308 | 588,422 | 1,306,572 |
| Grout | 9.2 M3 | 140.80 | 1,301 | 32.37 | 42,114 | 4,271 | 39,468 | 81,582 |
| Steel | 49.1 tons | 45.50 | 2,235 | 39.51 | 88,303 | 8,857 | 435,228 | 523,531 |
| Instrumentation | 486 each | 14.40 | 7,002 | 42.75 | 299,356 | 2,991 | 1,453,428 | 1,752,783 |
| UG electrical | 909 M | 85.00 | 775 | 37.30 | 28,895 | 17 | 15,750 | 44,645 |
| AG electrical | 9631 M | 65.00 | 6,249 | 41.40 | 258,734 | 64 | 618,050 | 876,784 |
| Pipe insulation | 4289 M | 2.30 | 9,872 | 32.00 | 315,903 | 95 | 408,264 | 724,167 |
| Equip insulation | 7387 M2 | 3.20 | 23,749 | 31.90 | 757,612 | 61 | 447,137 | 1,204,749 |
| Paint | 14181 M2 | 0.51 | 7,178 | 31.60 | 226,809 | 6 | 90,137 | 316,947 |
| Direct totals | 265,660 | 10,712,395 | 108,311,518 | |||||
| Const equip & indirects | - | - | - | - | - | - | 8,885,402 | |
| Const mgt, staff, supv | - | - | 37,560 | - | - | - | - | 4,345,001 |
| Engineering | - | - | 42,158 | - | - | - | - | 5,241,401 |
| Other project costs | - | - | 6,322 | - | - | - | - | 8,272,397 |
| Contingency | - | - | - | - | - | - | 20,258,356 | |
| Indirect totals | 86,040 | 47,002,557 | ||||||
| Project totals | - | - | 351,700 | - | 10,712,395 | - | 97,599,123 | 155,314,075 |
Cost summary of GTE process unit project
| GTL project cost summary | ||||||||
|---|---|---|---|---|---|---|---|---|
| Account | Key qty | Unit MH | MH | Wage rate | Labor cost | Unit matl | Matl cost | Total cost |
| Equipment | 50 ITEM(S) | 995.20 | 49,761.00 | 43.16 | 2,147,816.00 | 463,988.00 | 231,994,300 | 234,142,116 |
| AG pipe | 5224 M | 28.70 | 149,957.00 | 42.73 | 6,407,156.00 | 2,290.00 | 11,964,805 | 18,371,961 |
| Concrete | 4323 M3 | 10.10 | 43,672.00 | 34.15 | 1,491,549.00 | 296.97 | 1,283,942 | 2,775,490 |
| Grout | 12.3 M3 | 146.40 | 1,803.00 | 32.37 | 58,350.00 | 4,269.00 | 52,548 | 110,898 |
| Steel | 71.6 tons | 45.90 | 3,287.00 | 39.51 | 129,857.00 | 8,892.00 | 636,252 | 766,109 |
| Instrumentation | 884 each | 17.00 | 15,057.00 | 42.82 | 644,699.00 | 2,432.00 | 2,149,998 | 2,794,697 |
| UG electrical | 1503 M | 0.80 | 1,198.00 | 37.15 | 44,493.00 | 16.35 | 24,583 | 69,076 |
| AG electrical | 34620 M | 0.52 | 17,931.00 | 41.42 | 742,775.00 | 53.82 | 1,863,138 | 2,605,913 |
| Pipe insulation | 5349 M | 2.10 | 11,444.00 | 31.68 | 362,567.00 | 110.45 | 590,838 | 953,405 |
| Equip insulation | 11824 M2 | 2.00 | 23,229.00 | 31.41 | 729,726.00 | 44.98 | 531,840 | 1,261,567 |
| Paint | 20376 M2 | 47.00 | 9,648.00 | 31.51 | 303,978.00 | 6.13 | 124,867 | 428,846 |
| Direct totals | 326,986.00 | 13,062,966.00 | 251,217,111 | 264,280,077 | ||||
| Const equip & indirects | - | - | - | - | - | - | 10,966,303 | |
| Const mgt, staff, supv | - | - | 53,733.00 | - | - | - | - | 6,181,701 |
| Engineering | - | - | 66,115.00 | - | - | - | - | 8,217,002 |
| Other project costs | - | - | 9,800.00 | - | - | - | - | 17,147,746 |
| Contingency | - | - | - | - | - | - | 46,018,920 | |
| Indirect totals | 129,648.00 | 88,531,672 | ||||||
| Project totals | - | - | 456,634.00 | - | 10,712,395.00 | - | 251,217,111 | 352,811,748 |
Summary of economic parameters, advantages and disadvantages of processes for shale gas conversion
| Process | GTL | GTM | GTE |
|---|---|---|---|
| Direct cost (MM$/YR) | 74.67 | 119.14 | 294.01 |
| Indirect cost (MM$/YR) | 26.05 | 51.70 | 97.38 |
| Fixed capital cost (MM$/YR) | 100.72 | 170.85 | 391.39 |
| Working capital cost (MM$/YR) | 37.74 | 63.58 | 146.00 |
| Start-up capital cost (MM$/YR) | 10.07 | 17.08 | 39.14 |
| Total investment (MM$/YR) | 249.26 | 422.36 | 967.92 |
| Raw material cost (MM$/YR) | 157.62 | 157.62 | 157.62 |
| Utility cost (MM$/YR) | 16.79 | 28.47 | 65.23 |
| Labor cost (MM$/YR) | 3.30 | 11.78 | 14.37 |
| Revenue (MM$/YR) | 297.21 | 411.34 | 505.16 |
| Total product cost (MM$/YR) | 204.47 | 244.65 | 313.08 |
| Profit | 92.74 | 166.69 | 192.08 |
| ROR | 37.21 | 40 | 19.84 |
| Advantage | Products without sulfur and aromatic contamination | Best economic parameters (ROR, profit) | Highly used in many industries |
| Suitable for areas water is not available | Highly used in many industries | ||
| Used as feed in other petrochemical units | |||
| Limitations | This process at low gas prices is economical | - | Complex process and equipment |
| Low profitability relative to investment cost | Need a high total investment | ||
| Lowest ROR than other processes | |||
According to Figure 7, the GTM process has the highest ROI. To provide an accurate economic comparison, the feedstock flow is set equal for the three processes. The sales revenue of ethylene and methanol is higher. Additionally, it can be seen that the difference between the RORs of GTM and GTL is not great (Figure 8). As a result, for converting shale gas feed with this capacity, methanol production is a more appropriate process. Since most of the methanol production cost is allocated to feed, the production of methanol in regions with cheaper feed such as shale gas can provide investment opportunity as well as profitability.
A comparison among product sales, total investment, feed cost, and profit.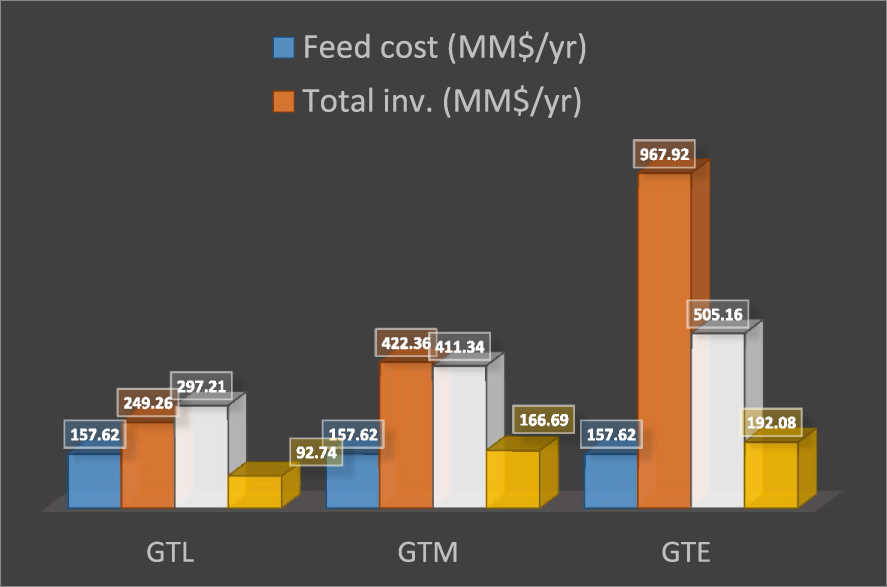
A comparison of rate of return.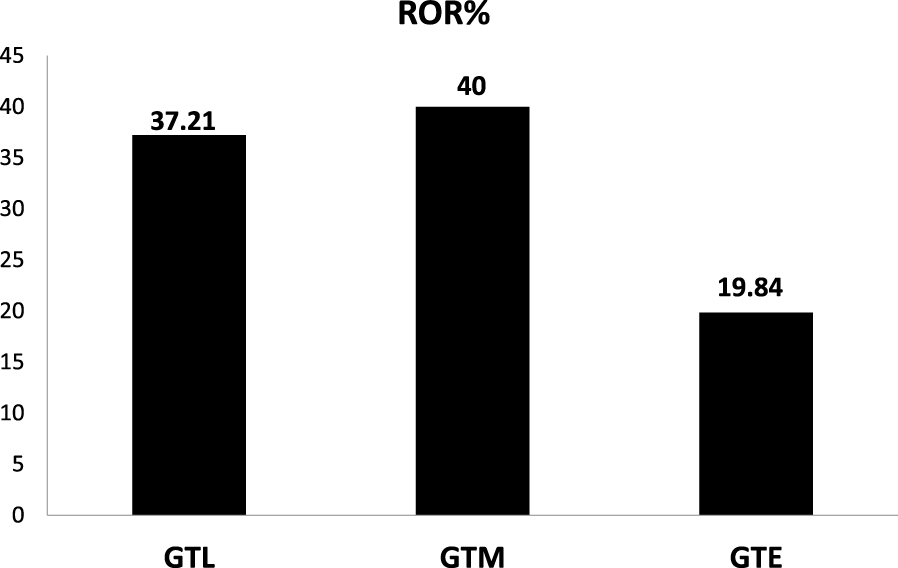
On the other hand, the high demand for diesel production can be met by the GTL method although this method is costly for refineries. If clean fuel production is considered, the GTL method is a good option. It can be observed that ethylene production has the lowest ROI, and its initial investment cost is greater than that of the other two methods. By comparing purchase and installation costs of equipment in each process, it can be realized that these parameters in the GTE process are higher than those in the other two processes due to greater complexity and more expensive equipment, such as cold box and compressors, than those of the ethylene production process. According to the fact that the ethylene production method is more complicated than other methods and by comparing the calculated economic parameters, it can be concluded that GTE is not economical for this capacity of shale gas.
4. Conclusion
As mentioned earlier, in this research, three different methods of shale gas conversion into valuable chemicals were investigated. In addition, these methods were compared from technical and economic points of view. The technical and economic results indicate that shale gas conversion to methanol is more appropriate than other methods. Besides the additional complexities in the ethylene industrial process, the ROI or ROR and the total investment for the GTE process are 19.84% and 967.92 MM$, respectively. Hence the highest total investment cost and the lowest ROR belong to this process (Figures 7 and 8). The initial investment cost required for GTM is 422.355 MM$, while it costs 249.248 MM$ for the GTL process. Despite the higher investment in the methanol production process, the ROR of this process is more favorable than the GTL process. It should be considered that the GTL process is costly and is only economically beneficial if the price of the input feed gas is low. Furthermore, the results indicate that the annual benefits obtained from GTM and GTL processes are 166.69 and 92.74, respectively. This reveals high profitability of the methanol production process even though its investment cost is higher. The production of methanol, which is one of the most useful chemicals in the oil and gas industry, could be a suitable option for shale gas conversion due to a less complex process and better economic parameters than other methods.
Nomenclature
| GTL | gas to liquid |
| GTE | gas to ethylene |
| GTM | gas to methanol |
| F–T | Fischer–Tropsch |
| ROI | return on investment |
| ROR | rate of return |
| supv | supervisory |
| Const mgt | Construction management |
| inv. | investment |



 CC-BY 4.0
CC-BY 4.0