1. Introduction
Cyclodextrins (CDs) are cyclic oligosaccharides obtained from the enzymatic degradation of starch [1]. Their three-dimensional structure forms a truncated-cone-shape due to the chair conformation of glucose residues. The external surface of the torus is hydrophilic and the central cavity is hydrophobic. This allows them to encapsulate guests and consequently form stable inclusion complexes via noncovalent interactions. The three native CDs, α-CD, β-CD and γ-CD have six, seven and eight D-glucose units, respectively. They consequently possess different cavity sizes allowing the encapsulation of a wide range of guest molecules of various structures. The attractive features of CDs, low price, high availability, ease of production, large-scale synthesis and safe nature, make them the most investigated macrocycles [2, 3]. They have been widely used in medicine, food, environment, cosmetics, chemical analysis, catalysis, and other fields. For more than 130 years, the mechanism of formation of an inclusion complex between CD and a guest has been explored and explained mainly in aqueous solution [4] and to a less extent in some organic solvents [5, 6, 7]. Lately, a new generation of green solvents, deep eutectic solvents (DESs), has gained interest as an alternative to water for applications that require drier conditions and for organic solvents that are being phased out in favor of more environmentally friendly alternatives. They are gaining interest in different fields such as gas absorption, solvation, extraction, formulation, synthesis, electrochemistry and so on [8, 9, 10]. A DES is a mixture of pure compounds for which the eutectic point temperature is below that of an ideal liquid mixture [11]. DESs are classified into five different types (I–V) based on the nature of compounds used for their preparation [12]. The nature of the constituents and the addition of a third component (i.e. water) can modulate the functionalities and physicochemical properties of the solvent [13, 14]. Recently, CDs were used to provide supramolecular properties to these sustainable solvents [15, 16, 17]. This could be achieved either by dissolving CDs in previously prepared DES (ternary systems) or using CDs as a partner of the DES constitution (binary systems) [17]. In both cases, the development of these new supramolecular mixtures requires the study and understanding of the CD/guest inclusion mechanism in these non-conventional media. However, to the best of our knowledge, no study has been conducted to investigate the thermodynamics of the inclusion mechanism of CD in DES/water mixtures. The present paper investigates, for the first time, the thermodynamic parameters of the CD inclusion complex in different DES/water mixtures. β-CD/adamantanol was chosen as a model inclusion complex due to the high recognition ability of β-CD towards this guest (Figure 1). Eight choline chloride (ChCl)-based DES described in the literature were prepared [18] (Figure 1). Then, the obtained liquid solvents were mixed with different amounts of water to form various DES/water mixtures. The competitive and solvation contributions of these mixtures were then investigated. The results were compared with those obtained in water, individual components of DESs and ethanol/water mixtures.
Chemical structure of (a) β-CD, (b) adamantanol and the (c) DESs components.
2. Experimental section
2.1. Materials
Choline chloride (ChCl, 98%) was purchased from Sigma-Aldrich, China. Urea (99%) was purchased from Sigma-Aldrich, USA. Propane-1,3-diol, glycerol, D-(−)-sorbitol, D-(−)-fructose and D-(+)-glucose anhydrous were provided by VWR Chemicals, Belgium. Xylitol and (±)-butane-1,3-diol were provided by Alfa Aesar, Germany. β-CD was provided by Wacker-Chemie, Lyon, France. 1-Adamantanol was purchased from Acros, Belgium. ChCl was dried at 60 °C for at least 2 weeks before use. All other compounds were used as received. Distilled water was used throughout this work.
2.2. Preparation of deep eutectic solvents
DESs were prepared using the heating method by mixing ChCl as the HBA and different HBDs (urea, glycerol, xylitol, sorbitol, butane-1,3-diol, propane-1,3-diol, glucose and fructose) (Figure 1) under stirring until the formation of a clear and homogenous liquid (Table 1). HBA: HBD molar ratios and temperature used for DES preparation are presented in Table 1. The water content of the prepared DESs were determined using the Karl Fischer titration method (Mettler Toledo DL31) and listed in Table 1.
Molar ratio of preparation, heating temperature and water content (%) of the studied DESs
| DES | Molar ratio | Heating temperature (°C) | Water content (%) |
|---|---|---|---|
| ChCl:urea | 1:2 | 60 | 0.05 |
| ChCl:glycerol | 1:2 | Room temperature | 0.20 |
| ChCl:xylitol | 1:2 | 80 | 0.07 |
| ChCl:sorbitol | 1:1 | 80 | 0.36 |
| ChCl:butane-1,3-diol | 1:2 | 40 | 0.08 |
| ChCl:propane-1,3-diol | 1:1 | 80 | 0.21 |
| ChCl:glucose | 2:1 | 80 | 0.26 |
| ChCl:fructose | 2:1 | 80 | 2.15 |
2.3. Isothermal titration calorimetry (ITC)
ITC experiments were conducted using a VP-ITC, MicroCal Inc., USA. Titration experiments were carried at three different temperatures (15, 25 and 35 °C) in water, 25 wt% (weight percent) of each studied DES component alone in water, DES/water mixtures (25, 50 and 70 wt% DES) and ethanol/water mixtures (25, 50 and 70 wt% ethanol). Solutions of β-CD (5 mM) and adamantanol (0.5 mM) were individually prepared in each studied mixture and loaded respectively in the syringe and the cell of the calorimeter. When the obtained Kf value was less than 3000 M−1, release experiments were also performed in order to increase the accuracy of the determined thermodynamic parameters. For release experiments, the solution of inclusion complex (5 mM of β-CD with 5 mM of adamantanol) was loaded in the syringe and the corresponding solution (the studied mixture) in the cell.
All experiments consisted of an initial injection of 25 μL followed by 10 injections of 25 μL (delivery time of each injection: 50 s; interval between two consecutive injections: 60 s; stirring speed: 1000 rpm). Blank experiments were conducted and the heats of dilution were subtracted from the measured heat of the corresponding titration or release experiment. Formation constants (Kf) and thermodynamic parameters (ΔH, ΔS and ΔG) were determined by global analysis of all isotherms obtained for a given solvent, using an algorithmic treatment as described in [19].
3. Results and discussion
3.1. Influence of DES components on adamantanol complexation in cyclodextrin
First, the effect of the presence of each DES component on the stability of the β-CD/adamantanol inclusion complex was evaluated. ITC titration experiments were carried out in water containing 25 wt% of each DES component at different temperatures (15, 25 and 35 °C). The Kf values determined at 25 °C are listed in Table 2 in comparison to those obtained in water. All thermodynamic parameters (Kf, ΔH, ΔS and ΔG) obtained at 15, 25 and 35 °C are given in Table S1.
Formation constant (Kf) values of β-CD/adamantanol inclusion complex in the absence and the presence of 25 wt% of each DES component at 25 °C
| Medium | Kf (M−1) | |
|---|---|---|
| Water | - | 29,700 ± 400 |
| 25 wt% DES component | ChCl | 23,000 ± 2000 |
| Urea | 29,000 ± 1000 | |
| Glycerol | 16,800 ± 900 | |
| Xylitol | 24,000 ± 1000 | |
| Sorbitol | 34,000 ± 2000 | |
| Butane-1,3-diol | 2000 ± 300 | |
| Propane-1,3-diol | 3800 ± 400 | |
| Glucose | 44,000 ± 2000 | |
| Fructose | 27,000 ± 2000 |
The obtained Kf value of β-CD/adamantanol at 25 °C in water was equal to 29,700 M−1. This high Kf value is due to the strong binding of the adamantyl moiety to the cavity of β-CD [20]. A decrease in the Kf value indicates that the DES component has a competitive effect for the β-CD cavity while an increase in the magnitude of this thermodynamic parameter reveals a stabilizing effect of the studied compound.
As can be seen in Table 2, ChCl, the HBA used for the preparation of the eight DESs in this study, has very low competitive effect on the β-CD/adamantanol inclusion complex, as Kf value was slightly affected by its presence in solution. The results collected for the HBD showed diverse effects for the eight compounds. Some presented a strong (butane-1,3-diol, propane-1,3-diol) or weak (glycerol) competitive effect, while others showed either no effect (urea, xylitol, fructose) or a weak stabilizing effect (sorbitol, glucose). Additionally, results showed that the presence of each of the studied DES components at 25 wt% maintained the enthalpy driven mechanism of the inclusion complexation of adamantanol in β-CD (Table S1).
3.2. Influence of DES on adamantanol complexation in cyclodextrin
Titration and release experiments were performed in different DES/water and ethanol/water mixtures (25, 50 and 70 wt% solvent) at different temperatures (15, 25 and 35 °C). It should be emphasized that ITC studies were not carried out above 70 wt% due to the excessive viscosity of the mixtures, which prevented reliable recording of heat release. An example of the results of titration and release experiments for β-CD/adamantanol inclusion complex in ChCl:butane-1,3-diol (70 wt%) at 25 °C is represented in Figure 2. The obtained results were treated simultaneously using a global analysis and compared with those found in water. The Kf values of β-CD/adamantanol inclusion complex determined at 25 °C in the different mixtures are listed in Table 3. All thermodynamic parameters obtained at 15, 25 and 35 °C are represented in Tables S2, S3 and S4, respectively.
Typical isothermal titration calorimetry data obtained for the binding interaction of β-CD and adamantanol in ChCl:butane-1,3-diol DES/water 70/30 wt% at 25 °C. (a) Release thermogram, (b) titration thermogram (c) corresponding isotherms presenting the integrated heat data; the dashed curves correspond to the theoretical curves.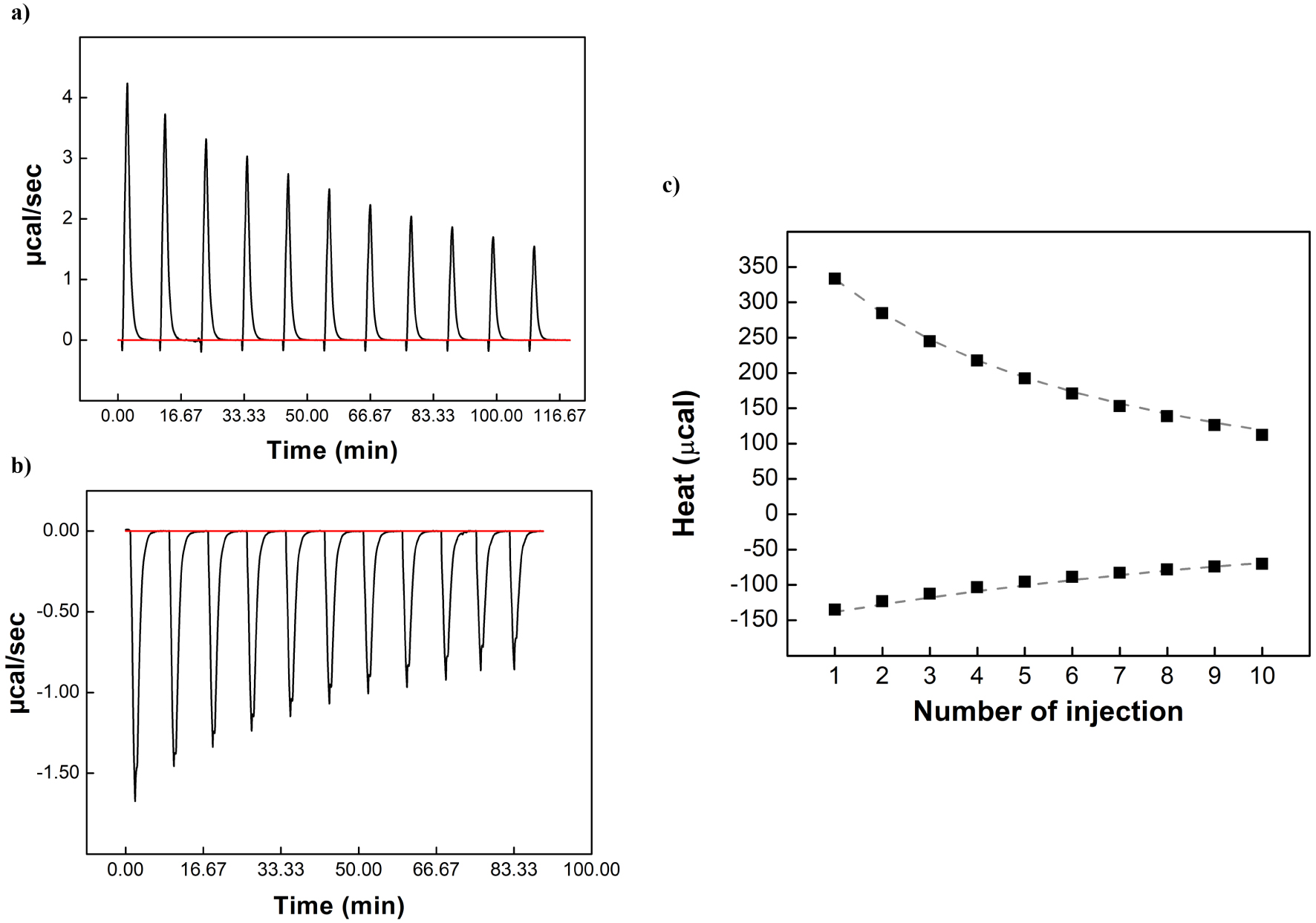
Kf values for β-CD/adamantanol inclusion complex determined in the different DES/water and ethanol/water mixtures at 25 °C in comparison to water
| Kf (M−1) | |||
|---|---|---|---|
| Water | 29,700 ± 400 | ||
| Solvent wt% | 25 wt% | 50 wt% | 70 wt% |
| ChCl:urea | 30,000 ± 2000 | 21,000 ± 1000 | 12,100 ± 700 |
| ChCl:glycerol | 21,000 ± 1000 | 10,500 ± 600 | 5600 ± 300 |
| ChCl:xylitol | 28,000 ± 2000 | 17,000 ± 1000 | ND |
| ChCl:sorbitol | 31,000 ± 2000 | 19,000 ± 1000 | ND |
| ChCl:butane-1,3-diol | 4300 ± 500 | 1500 ± 300 | 500 ± 20 |
| ChCl:propane-1,3-diol | 10,100 ± 800 | 4300 ± 400 | 2600 ± 400 |
| ChCl:glucose | 31,000 ± 2000 | 15,000 ± 1000 | ND |
| ChCl:fructose | 26,000 ± 2000 | 12,500 ± 100 | ND |
| Ethanol | 1700 ± 100 | 1800 ± 400 | 100 ± 10 |
ND: Not determined (ITC experiments were not carried out because of the high viscosity of these media).
In the presence of 25 wt% DES, the results (Table 3) indicated that only ChCl:butane-1,3-diol and ChCl:propane-1,3-diol significantly reduced the stability of the inclusion complex. This is coherent with the findings obtained in the presence of 25 wt% of individual DESs components as the lowest Kf values were observed in the presence of butane-1,3-diol and propane-1,3-diol (Table 2). This observation led us to suggest that one can infer the competitive behavior of a specific DES/water mixture (at least for weak DES content) by testing the competitive behavior of the aqueous solutions of each component. As for butane-1,3-diol and propane-1,3-diol, ethanol showed also a strong competitive effect on the complexation of adamantanol in β-CD. This observation is in good agreement with previous studies reporting a decrease in Kf in the presence of ethanol [7, 21, 22].
Concerning the effect of the wt% of DES, results showed that as the wt% of the solvent increased, the stability of the β-CD/adamantanol inclusion complex decreased progressively. This was observed for the eight DESs and not only for those that showed a competitive effect. This indicated that at higher DES content ( >25 wt%), the destabilization of the inclusion complex was not solely induced by the competitive effect of DES components.
Moreover, a negative heat capacity change (ΔCp) was observed in water for β-CD/adamantanol inclusion complex (−95 J⋅K−1). This is typical of macromolecular associations in water and evidenced the significant involvement of the hydrophobic interactions in the binding process [23, 24]. In DES based media (70 wt% DES content), ΔCp values ranged between −51 and −16 J⋅K−1. This increase in ΔCp values confirmed the disruption of the solvatophobic forces in the presence of DES leading to the decrease in the stability of the inclusion complex.
Altogether, these results proved that at high DES wt% content, an additional phenomenon related to the solvation of CD and adamantanol affected the stability of the inclusion complex. When thoroughly adding DES to water, β-CD and adamantanol experiment more favorable interactions with the added surrounding solvent molecules, which in turn disfavor the complex formation. This induces a decrease in the solvatophobic forces, the governing forces for the inclusion complex formation. Among all DESs and for all DES wt% content, the lowest Kf values were observed for ChCl:butane-1,3-diol, consecutively to the highest solvation potency and competitive behavior of butane-1,3-diol. The destabilizing effect of ethanol was even more pronounced, for all solvent contents, in accordance with literature. This is due to the fact that ethanol is able to effectively solvate guest molecules and consequently subtract them from the complexation in the CD cavity [7, 21, 22].
3.3. Influence of DES on the complexation mechanism of adamantanol in cyclodextrin
The thermodynamic parameters of β-CD/adamantanol inclusion complex (ΔH, −TΔS and ΔG) were determined in water, in the different DES/water and ethanol/water mixtures at different temperatures. Results obtained at 15, 25 and 35 °C are presented in Table S2, S3 and S4, respectively. Figure 3 illustrates the results determined at 25 °C in the presence of 25 wt% DES in comparison to water and 25 wt% ethanol.
Evolution of the 
 ΔG,
ΔG,  ΔH and
ΔH and  −TΔS in the different DESs (25 wt%) in comparison to water and ethanol (25 wt%) at 25 °C.
−TΔS in the different DESs (25 wt%) in comparison to water and ethanol (25 wt%) at 25 °C.
The exothermic character (ΔH < 0) of the binding process between β-CD and adamantanol explained the observed decrease in Kf values when increasing temperatures in water and all studied mixtures [25].
In water and for all DES/water mixtures, the entropy effect (−TΔS) of the β-CD/adamantanol complex was fairly close to zero over the studied temperature range. Accordingly, the entropy variation makes a minor contribution to the negative values of standard Gibbs energy (ΔG), to the contrary of the enthalpic term. The observed entropy variation may be due to the compensation between the release of water molecules from the cavity, which has positive contribution to entropy [25, 26] and the β-CD/adamantanol complex formation, which has negative contribution to entropy. Indeed, once adamantanol is encapsulated in the cavity of the CD, it leads to a significant loss in the degree of reagent freedom, which consequently, causes the loss of entropy (ΔS < 0, −TΔS > 0) [27]. Altogether, these observations indicated that the binding event between β-CD and adamantanol in water and all DES/water mixtures was similar and was enthalpy driven (i.e., exothermic binding events with |ΔH| > |−TΔS|) with low entropic assistance [28].
In ethanol/water mixtures, a different thermodynamic signature was detected. An entropy–enthalpy compensation of the binding process appeared at all temperatures and for all ethanol wt%. This could be explained by the fact that when moving from water to a hydroalcoholic medium, the thermodynamic profile of the inclusion complex formation can be influenced by the variation of the solvation of the guest (adamantanol). Ethanol solubilizes adamantanol by displacing water molecules in its hydration shell, affecting consequently both the enthalpy and entropy of the complexation. Enthalpy–entropy compensation was reported in literature when water was progressively replaced by an organic solvent [29, 30]. This can be confirmed by the fact that increasing ethanol wt% prevents the formation of inclusion complex (lowest Kf values) by effectively solvating guest molecules and thus by subtracting them from the complexation. This observed difference in the enthalpic and entropic contributions to Gibbs energy of complexation between DES/water and ethanol/water mixtures underlined the strong difference in solvation mechanisms between the DES and ethanol solutions.
The evolution of the thermodynamic parameters (ΔH and −TΔS) as function of solvent content wt% (Figure 4) additionally emphasized on the pronounced competition and solvation effects of ethanol, which led respectively to a strong increase then decrease of enthalpic (ΔH up to −55.7 kJ⋅mol−1 at 25 wt% and down to −12.9 kJ⋅mol−1 at 70 wt%) and entropic (−TΔS up to 37.2 kJ⋅mol−1 at 25 wt% and down to 0.8 kj⋅mol−1 at 70 wt%) contributions. On one hand, since the inclusion of ethanol in CDs is generally favored by entropy (i.e., ΔH = −2.4 kJ⋅mol−1; −TΔS = −3.3 kJ⋅mol−1 for α-CD/ethanol [31]) and the inclusion of adamantanol by enthalpy (Figure 3), replacement of ethanol molecules by an adamantanol molecule is characterized by strong enthalpy and entropy variations, thus explaining the thermodynamic signature of competitive phenomena (25 wt%). On the other hand, the fact that both enthalpy and entropy of inclusion were strongly reduced at high ethanol content (70 wt%) tends to demonstrate that adamantanol inclusion in CD cavity is somewhat analogous to its solvation by ethanol.
Evolution of (a) ΔH and (b) −TΔS with increasing the wt% of 
 ChCl:urea,
ChCl:urea,  ChCl:butane-1,3-diol and
ChCl:butane-1,3-diol and  ethanol at 25 °C.
ethanol at 25 °C.
The evolution of both ΔH and −TΔS was far less pronounced for both non-competitive (ChCl:urea) and weakly competitive (ChCl:butane-1,3-diol) DESs, with a moderate increase of enthalpy contribution overbalanced by a negative contribution of entropy, with increasing DES content. Such results showed that adamantanol interacted more favorably with β-CD than with the DES components, but that solvation by DES induced more disorder than inclusion in β-CD.
4. Conclusion
In this study, the thermodynamic parameters of the β-CD/adamantanol inclusion complex in non-conventional green media were investigated for the first time. The studied media included water, DES/water mixtures, aqueous solutions of DES components and ethanol/water mixtures. Eight different DESs were investigated at different wt% content (25, 50 and 75 wt%). According to ITC results, the type and wt% of DES affected differently the inclusion complex stability. A destabilizing effect was observed for all DESs in media with a high concentration of co-solvent (>50 wt%). However, DESs were much less destabilizing than ethanol. In addition, DESs did not disturb the complexation mechanism as observed in the presence of ethanol. Also, the thermodynamic signature of the complexation in DES/water mixtures was similar to that in water and was enthalpy driven with low entropic assistance, in contrast to ethanol/water mixtures. Taken together, the results proved that replacing organic solvents with DESs could be advantageous in maintaining the complexation and solvation capacity of CDs in a drier environment. Nevertheless, further investigations and refinements with other guest species, e.g. with lower binding affinity to CDs, should be explored to confirm whether they could lead to the same thermodynamic behavior of the complexation in the presence of DESs. In addition, other DES systems, belonging to the five DES classes, should be investigated in a broader comparative study.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
The authors would like to acknowledge the National Council for Scientific Research of Lebanon (CNRS-L) and Université du Littoral Côte d’Opale (ULCO) for granting a doctoral fellowship to LN. This work is a contribution to the CPER (Contrat de Plan Etat-Région) research project IRenE (Innovation et Recherche en Environnement) and is supported by the French Ministère de l’Enseignement Supérieur, the region Hauts-de-France and the European Regional Development Fund.




 CC-BY 4.0
CC-BY 4.0