1. Introduction
Over 200 different minerals have been reported in the literature to host uranium, but only 20 of them are of economic importance (from which uraninite, coffinite, brannerite and orthobrannerite) [1]. Uranium is generally finely disseminated within the ore, in a tetravalent or hexavalent form, meaning that concentration steps to remove the gangue material is not possible since it would result in loss of uranium in tailings. The ore is then usually completely leached after crushing or grinding. In the leaching process, sulfuric acid is mainly used, and the dissolution equations from U(IV) and U(VI) are respectively listed in Equations (1) and (2).
| (1) |
| (2) |
| (3) |
| (4) |
Bio-oxidation of iron uses chemolithotrophic microorganisms (various Leptospirilli and Thiobacilli), which take their energy exclusively from the oxidation of ferrous iron by oxygen, using dissolved CO2 as the unique source of carbon, and need for their growth a high level of acidity in solution with pH values below 3. These microorganisms participate in the natural biogeochemical cycles related to sulfide metal deposits. Acidophilic bioleaching, i.e., catalysis of sulfur and ferrous iron oxidation by microorganisms, is a recognized industrial scale process used for the dissolution of Au, Cu, Ni or Co from concentrates, ores, and mining waste (see BIOX or KCC operations [3, 4]). Through the oxidation of sulfides into sulfates and ferrous into ferric iron, microorganisms enhance metal dissolution. In the presence of a low sulfide content or in the absence of sulfides, bioleaching can still be used, the main action of the microorganisms being the bio-oxidation of ferrous iron (Equation (4)). Bioleaching presents the advantages of in situ generation of the leaching agent (ferric iron and protons), mild operating conditions in terms of pH (around 1–2) and temperature (20–50 °C), and low operating costs [5]. The main drawback is a longer residence time compared to chemical leaching [6]. The potential of bioleaching for the recovery of uranium from ores was recognized since the 1950s [7]. However, uranium was reported to inhibit microorganisms growth and activity, the toxic threshold concentration depending on the species and strains. This toxicity can be addressed using an indirect leaching approach in which the production of the acidic ferric iron solution and the leaching of the ore are separated in two-stage processes [7]. This indirect approach is quite unusual for conventional bioleaching operations, where the minerals to leach often serve as a solid support for microorganisms. This means that the use of the indirect approach requires overcoming several scientific bottlenecks such as O2 supply, attachment of the microorganisms on a solid support, and management of the precipitates.
This work aims to investigate the indirect bio-oxidation of ferrous iron into ferric iron on two case studies, the KATCO mine in Kazakhstan and the Somaïr mine in Niger, with the objective of increasing uranium recovery yields and kinetics. This paper summarizes the different steps undertaken to study the process and to perform its scale-up on the two case studies. It also provides insights into the main lessons learned in bringing a biotechnology process from laboratory to industrial scale.
2. Presentation of the case studies
The two case studies are mining sites predominantly owned by Orano Mining: the KATCO mining site (Kazakhstan), owned at 51% by Orano Mining and 49% by Kazatomprom; and the Somaïr mining site (Niger), owned at 63.34% by Orano Mining and 36.66% by SOPAMIN (SOciété du PAtrimoine des MINes du Niger). Table 1 provides some general information about these two mining sites.
Case studies description (data from Orano Mining)
| KATCO | Somaïr | |
|---|---|---|
| Average content of U | 0.2 to 0.5 kg U/t ore | 1.8 kg U/t ore |
| Annual production capacity | 4000 t/year | 2000 t/year |
| Starting production year | 2006 | 1971 |
| Total uranium production | 46,000 t | >75,000 t |
| % of annual world production | 7% | 3.5% |
2.1. KATCO case study (Kazakhstan)
KATCO is the world’s largest in situ uranium mine, accounting for 15% of Kazakhstan’s annual uranium production and 7% of world production. At this site, a leaching solution containing dilute sulfuric acid is injected through wells into the uranium deposit. Uranium is dissolved as the leaching solution passes through the deposit and is pumped to the surface. Uranium is then extracted from the leachate with ion exchange resins, followed by steps of refining, concentration, and precipitation leading to the production of the so-called yellowcake. The raffinate is re-injected into the wells after acidification, continuing the closed circuit process.
An iron-oxidizing bioprocess was investigated in order to increase uranium recovery. To allow the adequate integration of this process into the current flowsheet (Figure 1), the following requirements were considered: a bio-oxidation kinetics of at least 1 g⋅L−1⋅h−1, since the ferrous iron concentration in the leaching solution reaches around 1 g⋅L−1 after U recovery and the Hydraulic Residence Time (HRT) of the current flowsheet is 1 h, no addition of nutrients to enhance microbial growth, and no temperature regulation. The latter requirement is all the more important that the temperature at the KATCO mining site varies from −5 °C to 20 °C (data from Suzak city, 60 km away from the KATCO site).
Uranium leaching flowsheet for the KATCO case study including the investigated bio-oxidation processes.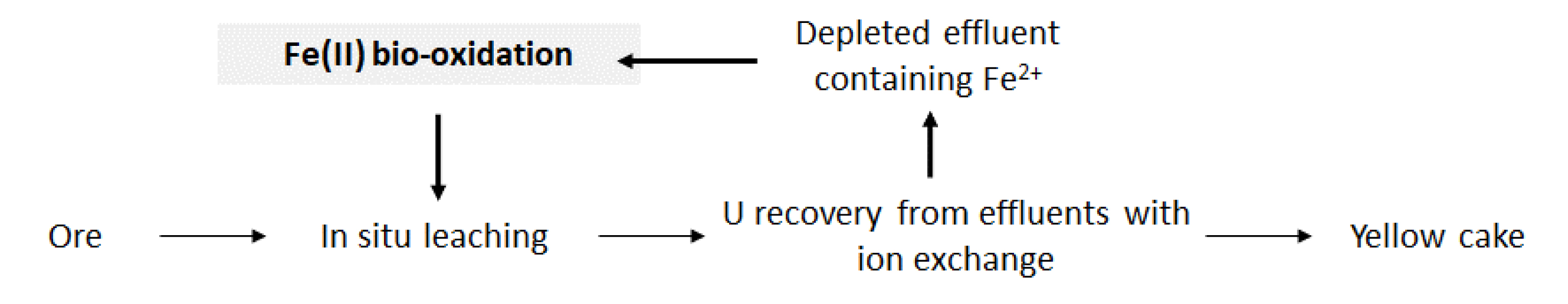
2.2. Somaïr case study (Niger)
In Somaïr, the deposit is a horizontal sedimentary deposit at a depth of around 50 to 70 m. Extraction is performed from an open pit mine. The ore is then treated either by heap leaching or pugging-and-curing leaching, depending on its uranium grade (Figure 2). The static treatment (acid heap leaching) is operated on the low- or very low-grade ore (<1 kg U/t ore). It consists in crushing the ore, agglomerating it with sulfuric acid and then stacking it in sealed areas to be dripped with an acid solution for several months. By percolating the ore, a leachate enriched in uranium is obtained. For the higher grade ore, a dynamic treatment (pugging and curing) is performed. In dynamic treatment, the ore is crushed and ground before undergoing a sulfuric acid and nitrate leaching for several hours. Uranium leachate is then recovered. After both leaching steps, solvent extraction is performed, followed by purification steps and precipitation. A concentrate of sodium uranate called yellowcake containing 75 wt% of uranium is produced.
Uranium leaching flowsheet for the Somaïr case study including the investigated bio-oxidation processes.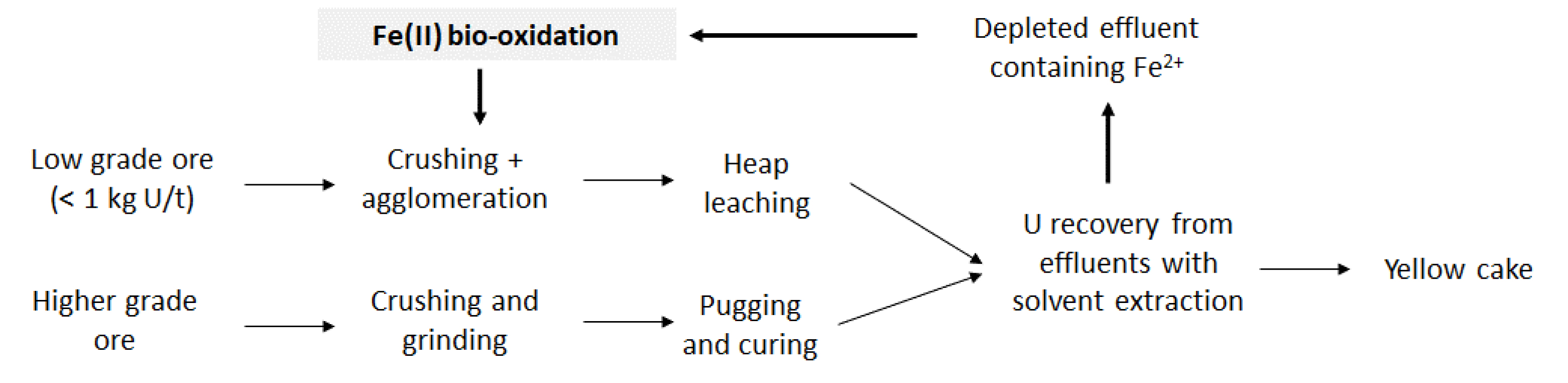
In order to increase uranium recovery, MnO2 is currently used as the oxidizing reagent for heap leaching on-site. The use of iron-oxidizing bacteria was investigated with the objective to reduce the consumption of MnO2 (Figure 2). In particular, it was estimated that implementing bio-oxidation would allow to save more than 43% of MnO2, leading then to a reduction in the related operating costs of more than 547 k€/year [8]. As for the KATCO case study, the implementation of bio-oxidation should be performed without any nutrients addition nor temperature regulation, the temperature at the Somaïr mining site varying from 5 °C to 45 °C. Regarding the processing conditions, the ferrous iron concentration is much higher in Somaïr than in KATCO, reaching 10 to 20 g⋅L−1, and the HRT is also higher with a value of 24 h. A bio-oxidation kinetics of 0.5 g⋅L−1⋅h−1 should thus be reached. Another important parameter to consider is the use of solvent extraction to recover U which may lead to traces of organic solvents in the depleted effluent containing Fe2+ as these traces may inhibit microbial activities [9].
3. Main steps of the study
The study of the potential of an iron-oxidizing bioprocess for improving uranium recovery and of its on-site implementation started in 2013. This study was performed in close collaboration between Orano Mining and the BRGM (French Geological Survey), which has a great expertise in bioleaching processes [4]. Figure 3 presents the main steps of this study. Firstly, theoretical studies were performed on both case studies in order to get valuable information on the process such as the theoretical increase in uranium recovery, the formation of precipitates, and the influence of the ambient temperature on the evolution of the temperature within the bioprocess. Experimental works were then performed using the KCC consortium, mainly composed of the genera Leptospirillum, Acidithiobacillus and Sulfobacillus, which are either iron or sulfur oxidizers or both. This consortium was chosen as it previously demonstrated high efficiency for sulfides bioleaching and ferrous iron bio-oxidation [10]. These first experimental works were performed with a focus on the KATCO case study as it presents a higher potential for the bio-oxidation process (details are given in the following paragraphs). In 2018, due to an Orano Mining internal decision, it was decided to perform the implementation of this innovative process on the Somaïr case study and to no longer work on the KATCO case study. All these steps are detailed in the following paragraphs.
Schedule of the study related to bio-oxidation of ferrous iron to ferric iron to improve uranium recovery.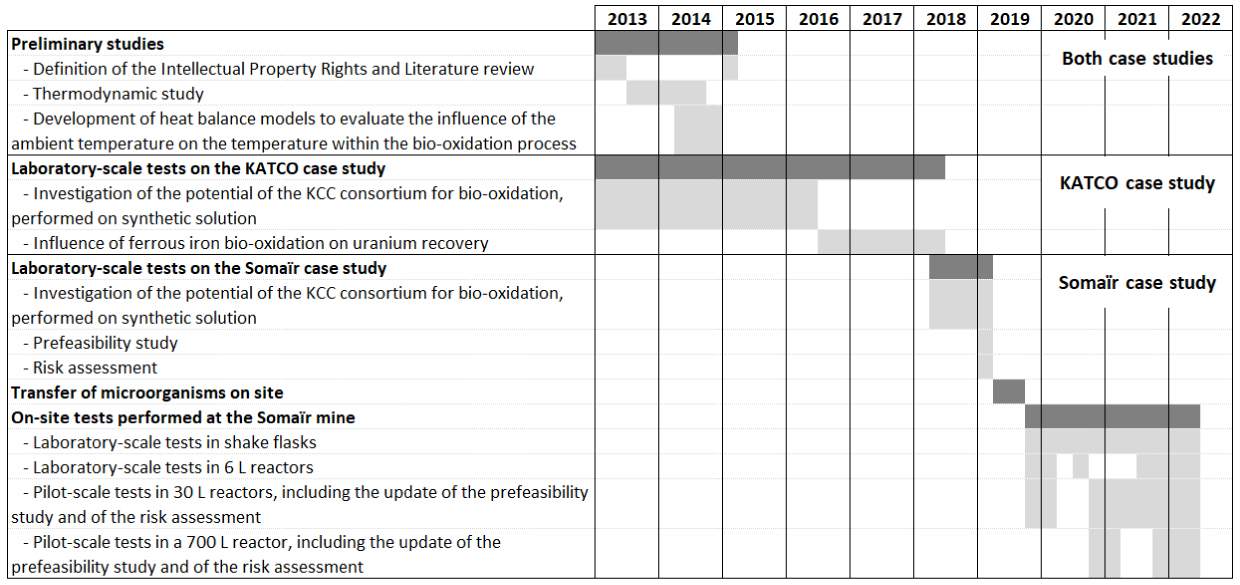
3.1. Preliminary studies
The first objective of the preliminary studies was to define the intellectual property rights, by reviewing the commercial operations and the associated patents. The Total Patent database was used with the following key words: “Iron oxidation AND ferrous AND bio”, “Iron III production AND bio”, “Ferrous iron oxidizing bacteria”, “Uranium leaching AND bio AND oxidation”. Several patents were found on the use of ferrous iron bio-oxidation, such as US4139456A 1979-02-13 [11] or US6043022A 2000-03-28 [12], but no patents were found on its application for uranium recovery. Moreover, no commercial operation related to such process was identified.
Another objective of the preliminary studies was to perform thermodynamic calculations in order to get valuable information, even if theoretical, on the bio-oxidation process. These calculations aim at (i) describing the various reaction equilibria affecting the dissolution of uranium when an acidic ferric iron solution is used, (ii) obtaining a quantitative description of the compositions of the solutions, in terms of dissolved species in particular, and (iii) predicting the formation of precipitates during the bio-oxidation of ferrous iron to ferric iron. Simulations were performed with the PhreeqC 7.3 software, which uses the llnl database developed for uranium speciation complexation equilibria. Simulation results confirmed the increase in U(IV) recovery by using acidic and oxidizing solutions and indicated that precipitates of iron hydroxides or jarosite (K, Na) may occur. These precipitates will have to be taken into account for the design of the pilot and the industrial processes since they can lead to clogging and then require the definition of a dedicated cleaning strategy.
The last objective of the preliminary studies was to evaluate the influence of the ambient temperature on the evolution of the temperature within the bioprocess and to check if it is compatible with microbial activity. For the KATCO case study, the heat balance modeling was carried out following the methodology used by Talati and Stenstrom [13] and considering that the bio-oxidation is performed in a pond of 3000 m3 since this pond was used for the recovery of leachates. The equipment chosen for performing the bio-oxidation was a floating agitation system recently developed by Milton Roy Mixing and Air Liquide for wastewater treatment applications. This system allows both mixing and suspending solids in the solution as well as injecting gases into the solution. It was chosen as it was proven that it allows decreasing the costs of bioleaching processes when implemented in stirred tank reactors [14]. Results showed only small variations of the temperature in the pond regardless of the atmospheric conditions (from less than −5 °C in January to 20 °C in July), the pond temperature varying between 24.2 °C and 24.8 °C (Figure 4), and these temperatures are compatible with the growth and activity of mesophilic or moderate thermophilic microorganisms. This is due to the short HRT in the pond, which was 1 h. Indeed, a longer HRT in the pond would have led to a higher amplitude in the temperature evolution, as showed in Figure 4. This is because main thermal losses are linked to the aeration of the pond and surface convection.
Air and pond temperatures simulated for the KATCO case study.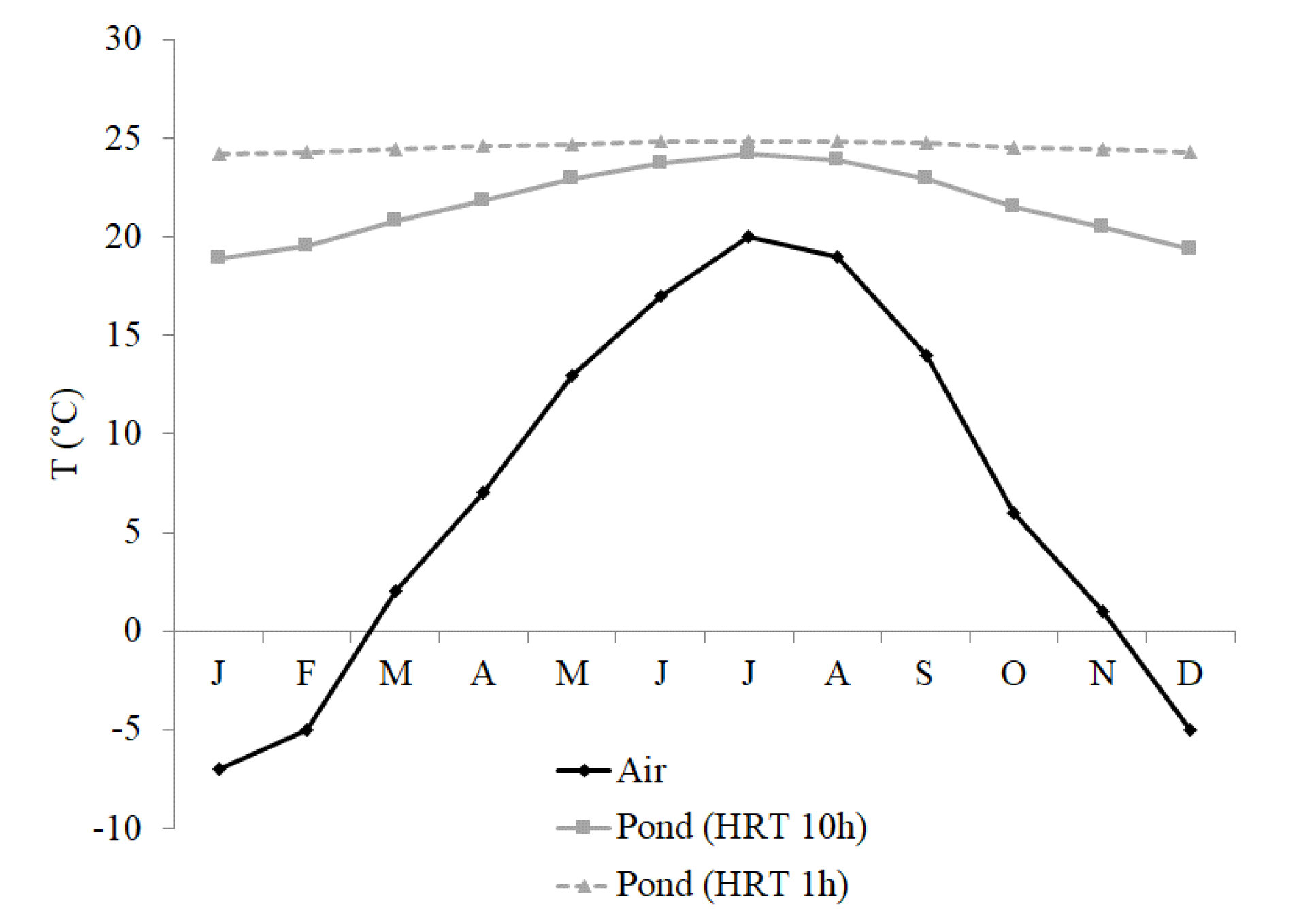
A heat balance model was also developed and implemented for the case study of Somaïr. Similarly as for the KATCO case study, results showed that the temperature of the pond is very close to the temperature of the inlet effluent even if the HRT is 24 h. This is because the main parameter controlling the temperature of the pond considered in the Somaïr case study is the temperature of the inlet effluent with only a minor influence of the aeration of the pond.
3.2. Laboratory-scale tests at the BRGM and at Orano Mining’s Innovation Center for Extractive Metallurgy (CIME)
3.2.1. Laboratory-scale tests on the KATCO case study
3.2.1.1. Objectives
The first experimental works were performed on the case study of KATCO since it presents a higher potential for the bio-oxidation process (considering the mineralogy of the deposit) and some operating conditions are more favorable for such a process compared to the case study of Somaïr. This is in particular the case of the lower Fe concentration, which reduces the process piloting issues associated with the formation of Fe-precipitates, and of the use of ion exchange resins instead of solvent extraction for uranium recovery, therefore avoiding the presence of traces of organic solvent which could inhibit the microorganisms.
The laboratory-scale tests were performed with a threefold objective:
- To assess the feasibility of reaching and maintaining bio-oxidation rates with a well-known microbial consortium in continuous mode at a temperature of 20 °C.
- To determine the influence of the HRT on bio-oxidation rates.
- To demonstrate the increase of uranium recovery yield and rate in the presence of biogenic ferric iron.
3.2.1.2. Methods
Experiments were performed in two steps. First, 2 L stirred tank reactor (STR) experiments were performed at the BRGM laboratory on a synthetic solution with a composition in major elements similar to the KATCO effluent (Fe, Ca, Mg, Al, and Cl).
As mentioned before, the KCC consortium was chosen for these tests. It is important to mention that similar acidophilic microorganisms (same genera and species) were previously found in Kazakhstan [15, 16, 17]. One test was also performed in abiotic conditions in order to assess the influence of the biomass on the bio-oxidation of the ferrous iron. A solid support made with coal particles was introduced into the STR in a basket (Figure 5) in order to allow the biomass to create biofilms. Indeed, attached bacteria are often more efficient than planktonic cells due to higher resilience of biofilms towards potential inhibitions [18]. As O2 and CO2 supply are key parameters for the bio-oxidation of ferrous iron (Equation (4)), STRs were equipped with baffles and a Rushton turbine to ensure high mass transfers. The temperature was regulated to 25 °C as the preliminary study showed that this would be the operation temperature in KATCO. The reactor was first launched in batch mode to allow the fixation of the microorganisms on the solid support and then operated in continuous mode by progressively reducing the HRT. HRT is related to the liquid phase in all the works performed in this study since the solid support was kept within the reactor in order to allow the biomass to grow.
Schematic diagram of the experimental devices used to assess bio-oxidation kinetics with KCC consortium, (a) STR and (b) bubble column.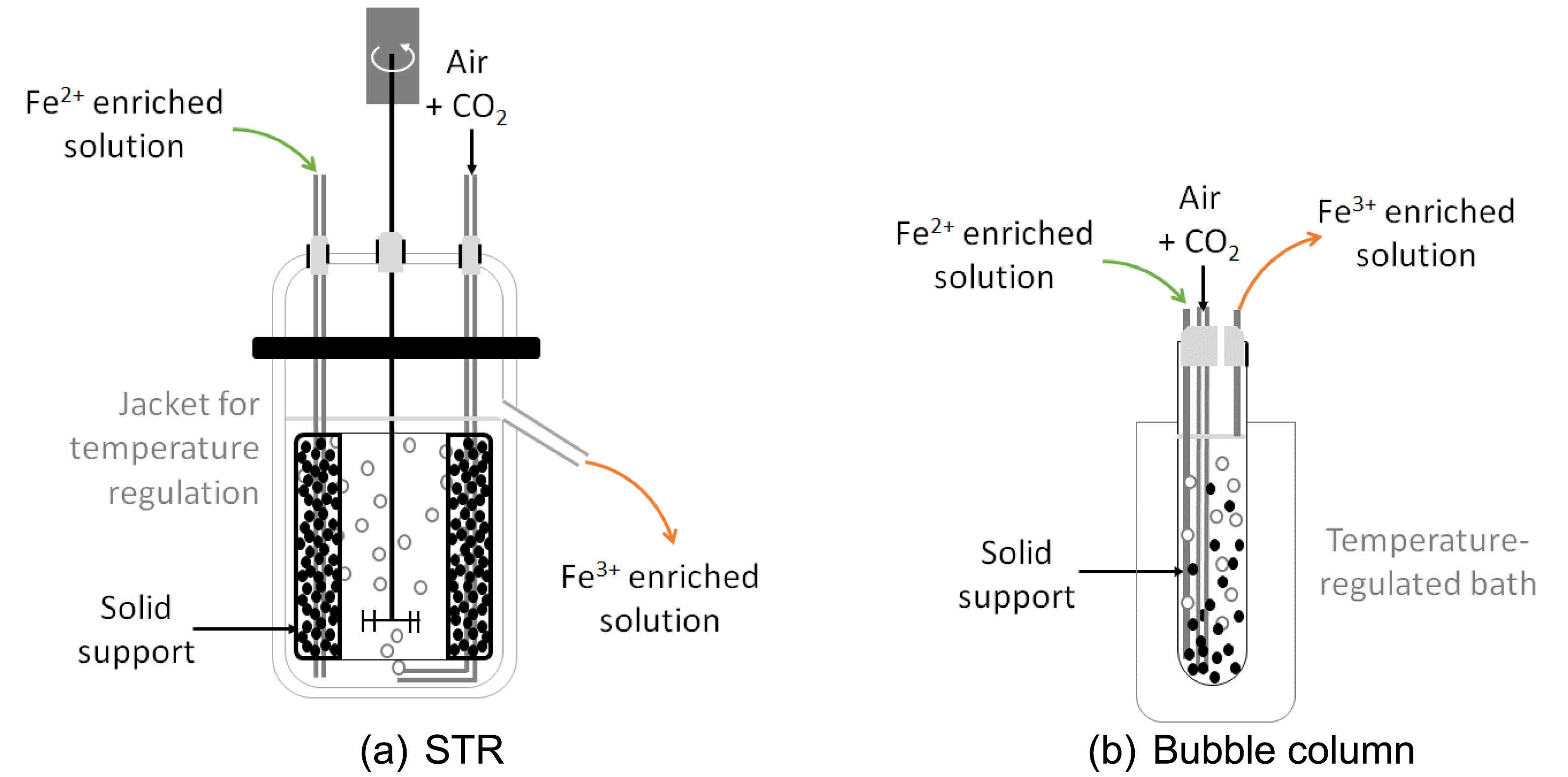
Secondly, tests coupling the ferrous iron bio-oxidation and the KATCO ore leaching were performed. A bubble column, considered as fluidized bed reactor, was considered for the bio-oxidation reactor. This choice was motivated by the small volume of the bubble column which allows continuous coupling with the uranium leaching test since its input flow rate was 100 mL⋅h−1 (Figure 5). The bubble column was operated at 20 °C with coal as solid support. The reactor was first launched in batch mode to allow fixation of the microorganisms on the solid support and then operated in continuous mode by progressively reducing the HRT. Bio-oxidation in bubble columns was optimized at the BRGM laboratory and then operated in close loop by Orano Mining in their laboratory. Tests were performed with a real effluent from KATCO. Uranium leaching was performed in columns, each column containing about ten kg of ore each. Fe3+-enriched solution was injected at a flow rate of 100 mL⋅h−1 allowing an ascending volumetric flow rate per unit area of 4 L⋅h−1⋅m−2, close to the one used at the KATCO mine. Effluents were acidified to pH 1.6 with dilute sulfuric acid, before passing through anionic resins to recover uranium. Uranium-depleted solutions were then treated in the bio-oxidation column for ferrous iron oxidation.
Each reactor was monitored daily for pH, redox potential and temperature. Sampling was performed daily in the Fe3+-enriched solution in order to analyze the total Fe concentrations—by Atomic Absorption Spectroscopy (AAS)—and the Fe2+ concentrations—by titration with Ce(IV) sulfate 0.001 M. Bacterial cells were also counted occasionally in the Fe3+-enriched solution. In order to determine the dynamics of microbial populations, DNA extraction followed by CE-SSCP (capillary electrophoresis with single-strand conformation polymorphism) was performed at the end of each test. Results related to the biomass are not given here due to confidentiality issues.
Bio-oxidation rates and yields in the STR experiments (left) and bubble column (right) with a KATCO synthetic effluent and for various operating conditions (from [19]).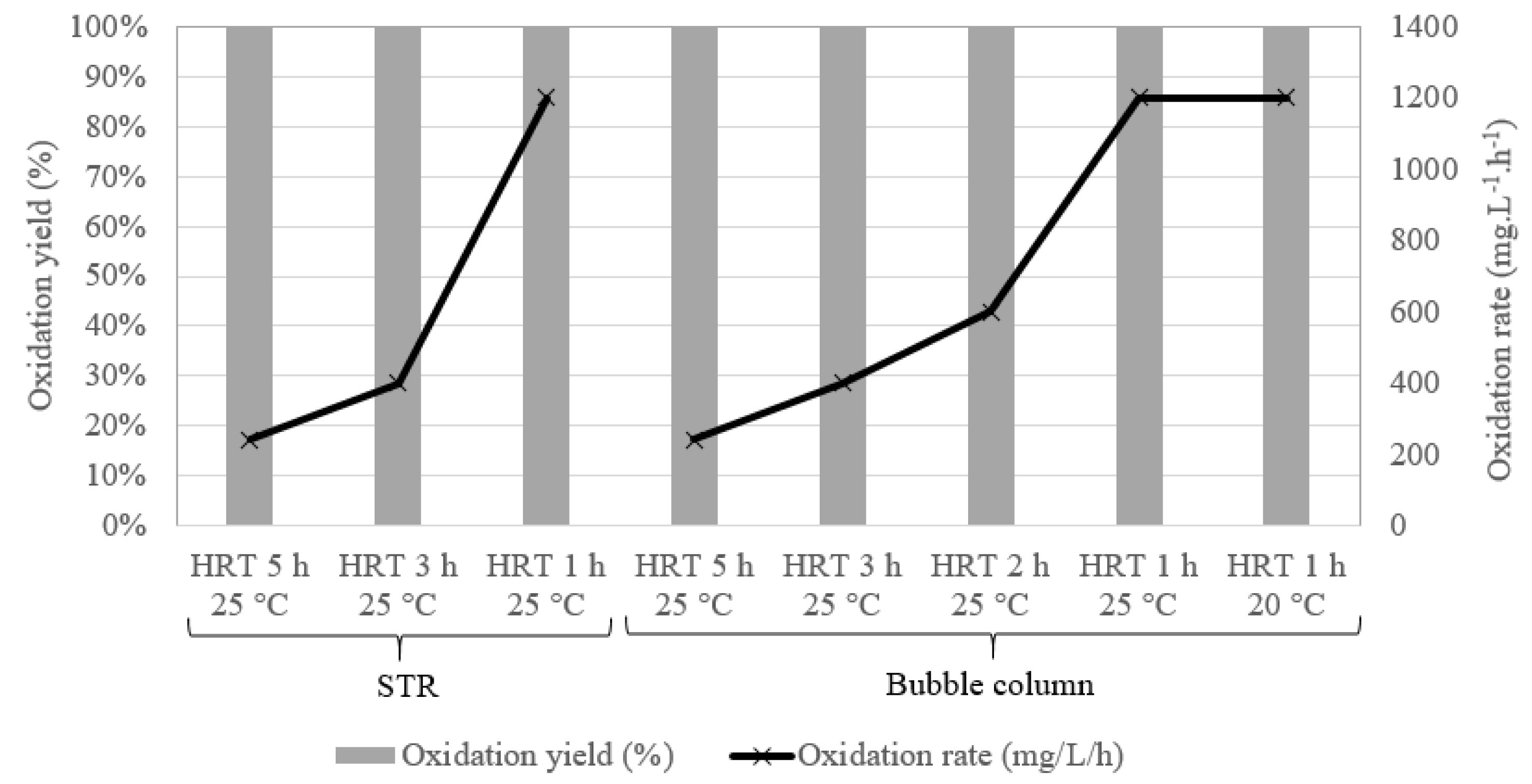
3.2.1.3. Results
STR experiments performed in abiotic conditions, i.e., without microorganisms, led to an oxidation rate of about 0.1 g⋅L−1⋅h−1, showing that the air oxidation of ferrous iron does not allow to reach industrial requirements (here set at 1 g⋅L−1⋅h−1). When using the KCC consortium, an oxidation rate of about 1.1 g⋅L−1⋅h−1 was obtained at a HRT of 1 h, confirming the potential of the biomass to perform the bio-oxidation of ferrous iron into ferric iron. It should be mentioned that this performance was obtained at 25 °C, whereas the optimal temperature for the KCC consortium is 40–42 °C [10], confirming the robustness of this consortium (Figure 6, left). Similar results were obtained with the bubble column (Figure 6, right). It is then interesting to highlight that it was possible to adapt the biomass for oxidizing 100% of Fe2+ at a HRT of 1 h and at 20 °C, as shown in Figure 6. Moreover, no washout of the biomass was observed, confirming that the use of a solid support enables the biomass to be maintained in the reactor even if the HRT was lower than its doubling time since, for example, the doubling time of L. ferriphilum is around 3.1 h [20, 21, 22]. During these tests, the clogging of the biomass support by a biofilm growth and/or by precipitates remained moderate.
A high reproducibility was observed between the results obtained with the STR and with the bubble column, confirming the robustness of the process. Moreover, the bio-oxidation rate showed only small variations when the oxidation yield reached its maximum value. For example, at a HRT of 3 h in the STR, the relative standard deviation of the bio-oxidation rate was 1.2% during 6 days, which corresponds to 48 HRT and 6 measurements of the kinetics. At a HRT of 1 h in the bubble column, the bio-oxidation rate varied by 2.4% during 14 days, which corresponds to 336 HRT and 10 measurements of the kinetics.
When the bio-oxidation of ferrous iron was coupled with uranium leaching, an increase in uranium recovery was observed compared to acid leaching only (Figure 7).
Uranium dissolution yields and acid consumption as a function of L/S ratio (from [19]).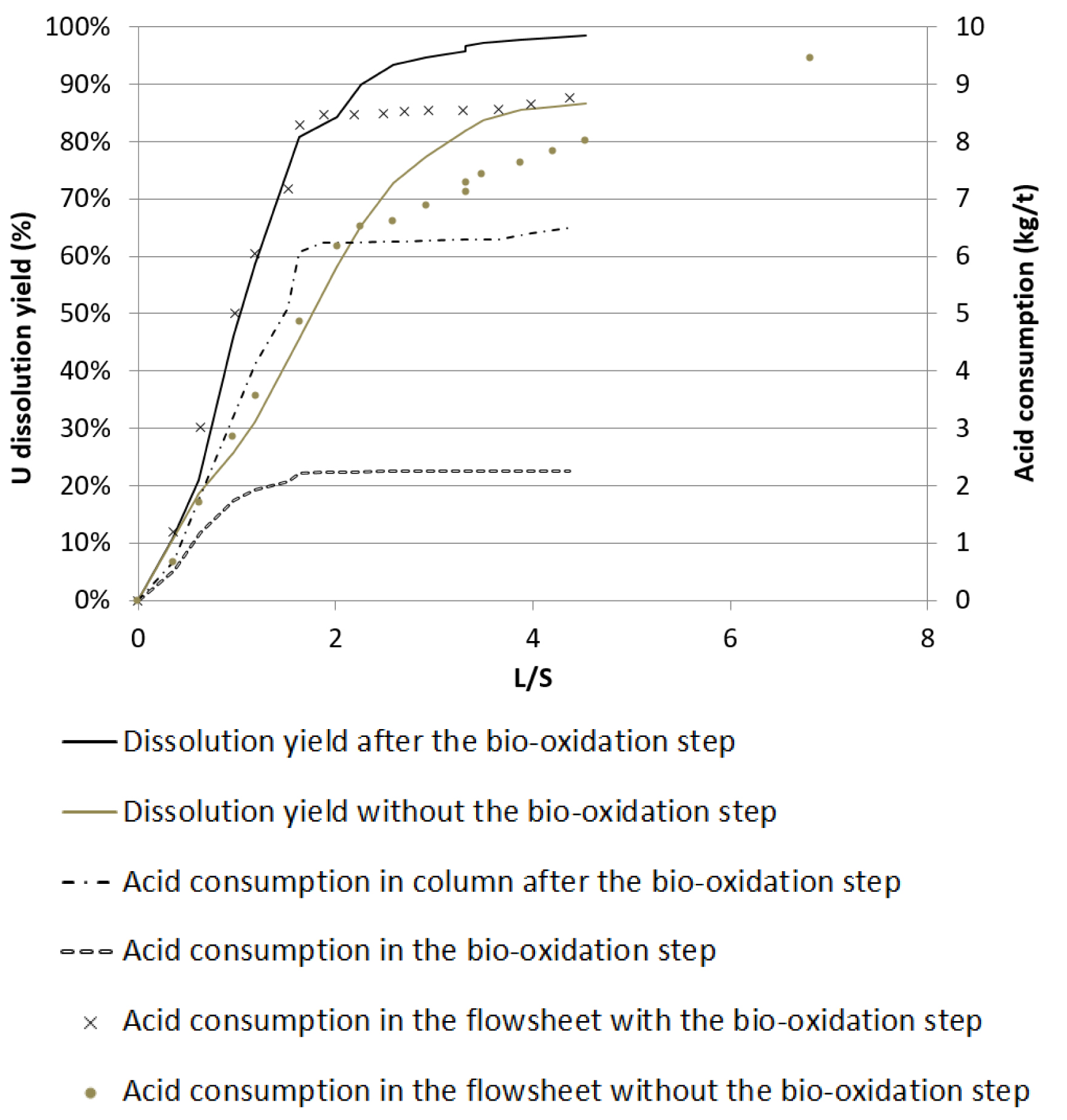
This was accompanied by a decrease in acid consumption of 0.6 kg/t ore, which represents a reduction of 6.4% in the total acid consumption. These tests were performed on a real effluent from the KATCO case study and therefore showed that the presence of traces of radioelements did not significantly affect the bio-oxidation performances.
3.2.2. Laboratory-scale tests on the Somaïr case study
3.2.2.1. Objectives
As mentioned previously, a change in the case study occurred in 2018 due to an Orano Mining internal decision and the Somaïr case study was chosen for the on-site implementation of the bio-oxidation of ferrous iron. Since the composition of the Somaïr effluent markedly differs from the KATCO one, in particular regarding the Fe2+ concentration with values up to 20 g⋅L−1, complementary laboratory-scale tests in bubble columns were performed in Orano Mining’s Innovation Center for Extractive Metallurgy (CIME). The objectives of these tests were to assess the influence of the change in effluent composition on the bio-oxidation performances and to collect reliable data to design the pilot-scale experiments.
3.2.2.2. Methods
Tests were performed in a 5 L STR similar to the one described in Section 3.2.1.2 with a similar monitoring. A synthetic solution with a composition similar to that of the Somaïr effluent was used, in particular regarding the content in iron, nutrients (K, Mg, P, ) and potential inhibitor elements (Al, , Cl−). Continuous tests were performed at 30 °C, the mean temperature of the effluent, and the pH was regulated at 1.2. The reactor was first launched in batch mode to allow the fixation of the microorganisms on the solid support and then operated in continuous mode by progressively reducing the HRT.
3.2.2.3. Results
Some of the results obtained during the experiments are given in Figure 8. As showed in Figure 8, it is possible to reach and maintain the bio-oxidation rate targeted for the Somaïr case study, which is around 500 mg⋅L−1⋅h−1. This bio-oxidation rate was obtained for a bio-oxidation yield lower than 100% and did not change regardless of the decrease in the HRT, which seems to indicate that the maximum oxidative performances of the biomass have been reached. Moreover, the solid support concentration seems to be a limiting factor since it was not possible to reach a bio-oxidation of 500 mg⋅L−1⋅h−1 with a solid support concentration of 40 g⋅L−1. Figure 8 also shows a decrease in the oxidation rate after several days of operation. This could be explained by the formation of iron precipitates, which clogged the solid support leading to a reduction in the bio-oxidation performances, since the biomass was fixed on the solid support. This assumption was supported by the observation of a very large quantity of red–orange precipitates when the reactor was stopped and opened. This precipitate is most probably mainly composed of jarosite, which is the predominant precipitate detected in bioleaching systems, even at low pH [20]. A similar effect of the iron precipitates when coal particles are used as solid support for bio-oxidation was reported by Hubau et al. [20]. In particular, they demonstrated that jarosite formation clogged the solid support pores, inducing very low mass transfers and a reduced microbial activity. This clogging effect was somehow mitigated after some time when using a bubble column due to the attrition occurring within the fluidized bed which opened the coal pores, leading to a new increase in the bio-oxidation rate [20]. These phenomena, observed at microscopic scale, create high variations in kinetics at macroscopic scale and should then be considered carefully when designing a bioreactor. In particular, it seems that other solid supports could be more appropriate for the bio-oxidation of Fe2+-rich effluents, such as woven nylon [23].
Bio-oxidation yield and rate during laboratory-scale tests with the synthetic effluent from the Somaïr case study.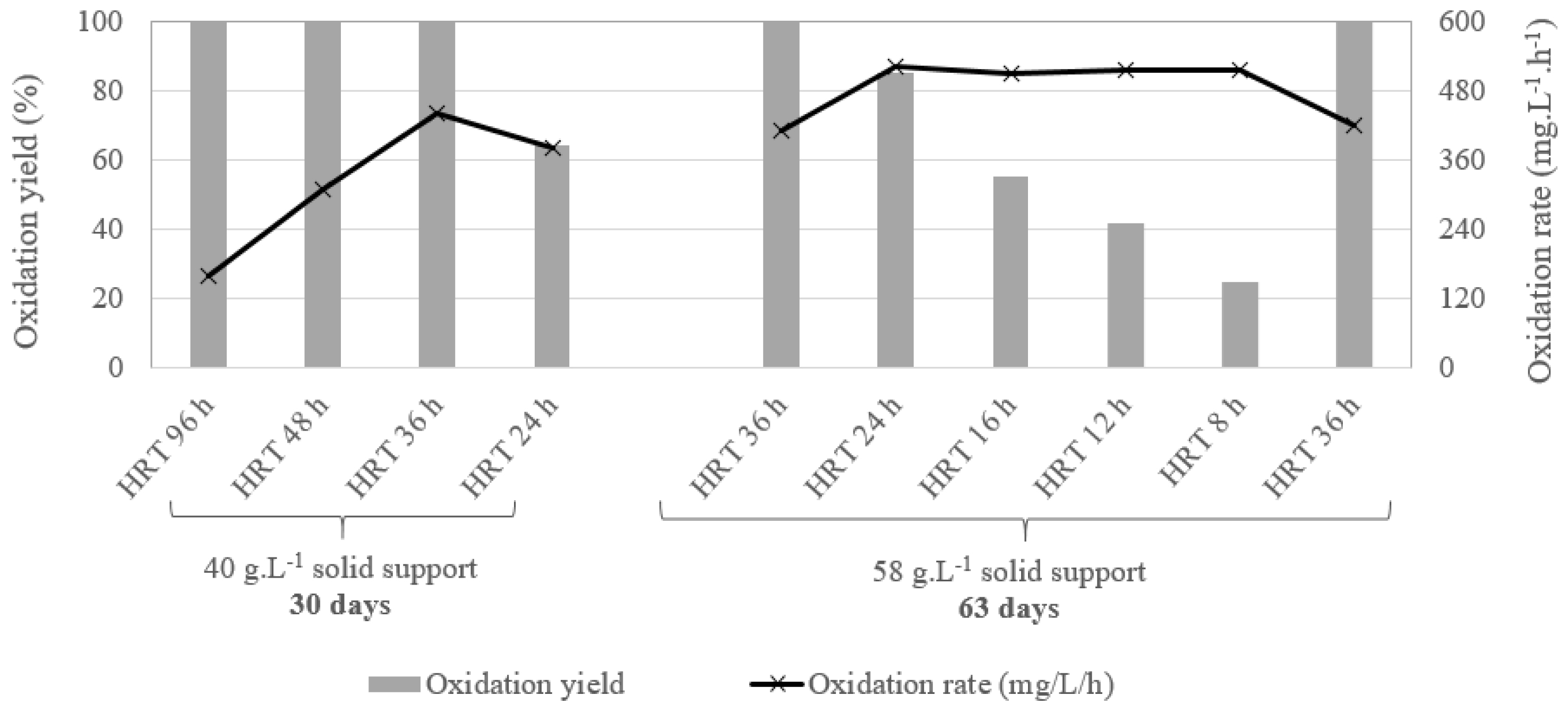
The results obtained with these works performed at laboratory scale on a synthetic effluent from the Somaïr case study confirmed the potential of the bio-oxidation to improve uranium recovery. A prefeasibility study and a design to cost analysis were performed based on these first results, including in particular the comparison of the two designs for the bioreactor which are the STR and the bubble column, the latter being similar to a fluidized reactor, with no mechanical agitation. First estimations of the capital expenditures (CAPEX) and operational expenditures (OPEX) as well as a risk assessment were performed. In particular, results showed that CAPEX and OPEX are reduced by more than 30% and 40%, respectively, when using a fluidized reactor. The main drawback identified for such reactor is related to mass transfers, meaning that works will have to be performed on its design to optimize them. A planning for on-site implementation and up-scaling of the process was then defined.
3.3. On-site tests performed at the Somaïr mine
First of all, the transfer of microorganisms on-site followed Nagoya protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the Convention on Biological Diversity. This protocol, adopted in 2010 and ratified by 137 parties, deals with the use of genetic resources, in order to share fairly and equitably the benefits and thus to contribute to the conservation and sustainable use of biodiversity.
Moreover, a dedicated training of the mining operators was conducted allowing them to efficiently monitor and operate bioprocesses. This training also included information related to the risks associated to handling the bio-oxidation solutions that would be produced, the main one being the acidity of the solutions. It should be mentioned that the mine operators already had several acid effluents to manage on-site and had therefore defined the appropriate safety measures. In addition, a laboratory equipped for bioprocesses development and monitoring was implemented on-site (Figure 9). In particular, it included a microscope for cell counting with Thoma plates. These were a prerequisite for the transfer to the Somaïr mine of the process development, operation and up-scaling works.
Photo of the laboratory developed at the Somaïr mine (@Orano Mining).
A method for the continuous monitoring of the main operating parameters was established on-site and was used at each scale. The reactors were equipped with pH, redox potential, dissolved O2 and temperature probes with continuous records (time step around 2h30). Total Fe concentrations were determined daily through AAS, while an empirical correlation was used to evaluate Fe2+ concentrations from redox potential values [24]. In order to determine the biomass concentration, Thoma cell counting was performed. Regarding the biomass attached to the solid, a detachment protocol based on the use of ultrasounds was established on-site (Thoma cell counting after detachment) but its execution remained limited due to difficulties in sampling the solid support.
3.3.1. Laboratory-scale tests in shake flasks
3.3.1.1. Objectives
The main objectives of the tests in shake flasks performed on-site were to adapt the microbial consortium to the industrial effluents and to define how the bio-oxidation process could be integrated in the current flowsheet. In particular, two integration options were investigated:
- In the first one (Figure 10), bio-oxidation is performed on the leachate from the heap leaching (called “production effluents”). In this case, uranium-enriched leachate is recirculated in the heap after its bio-oxidation in order to concentrate uranium in the leachate before its recovery in the solvent extraction unit. The main drawback of this option is the potential toxicity of uranium towards bio-oxidation microorganisms.
- In the second one (Figure 11), bio-oxidation is conducted on the raffinate, i.e., the uranium-depleted effluent after the solvent extraction unit. The advantage of this option is that radioelements concentration in the raffinate is very low. However, the raffinate may contain traces of organic solvents due to its treatment in the solvent extraction unit.
First bio-oxidation processing option for the Somaïr case study.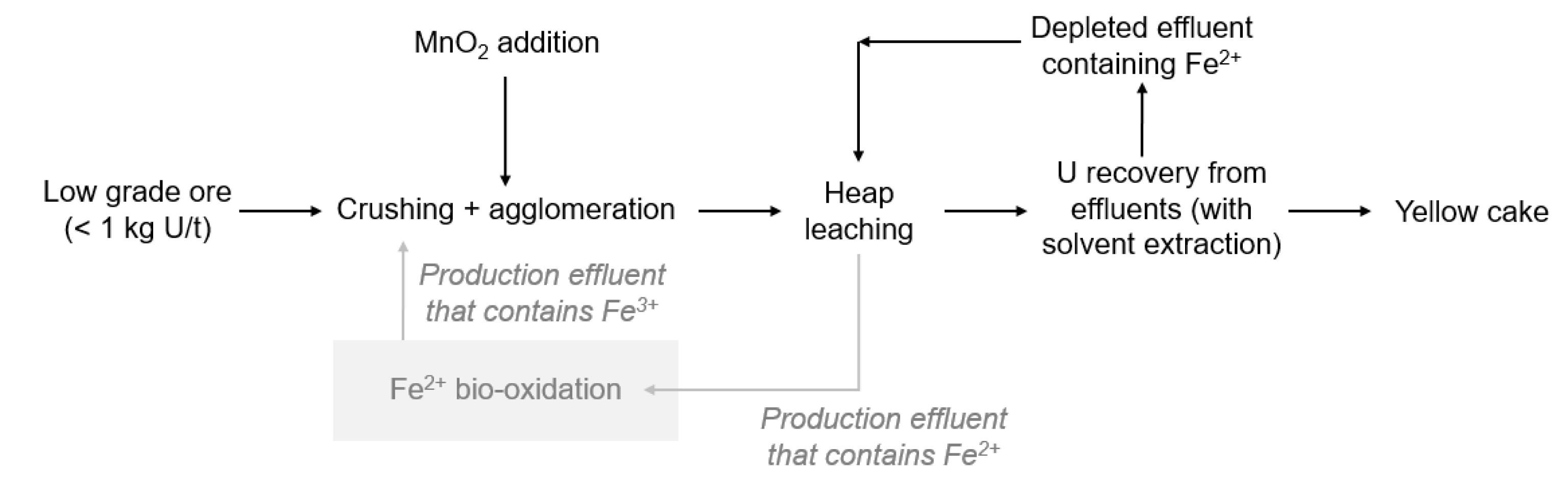
Second bio-oxidation processing option for the Somaïr case study.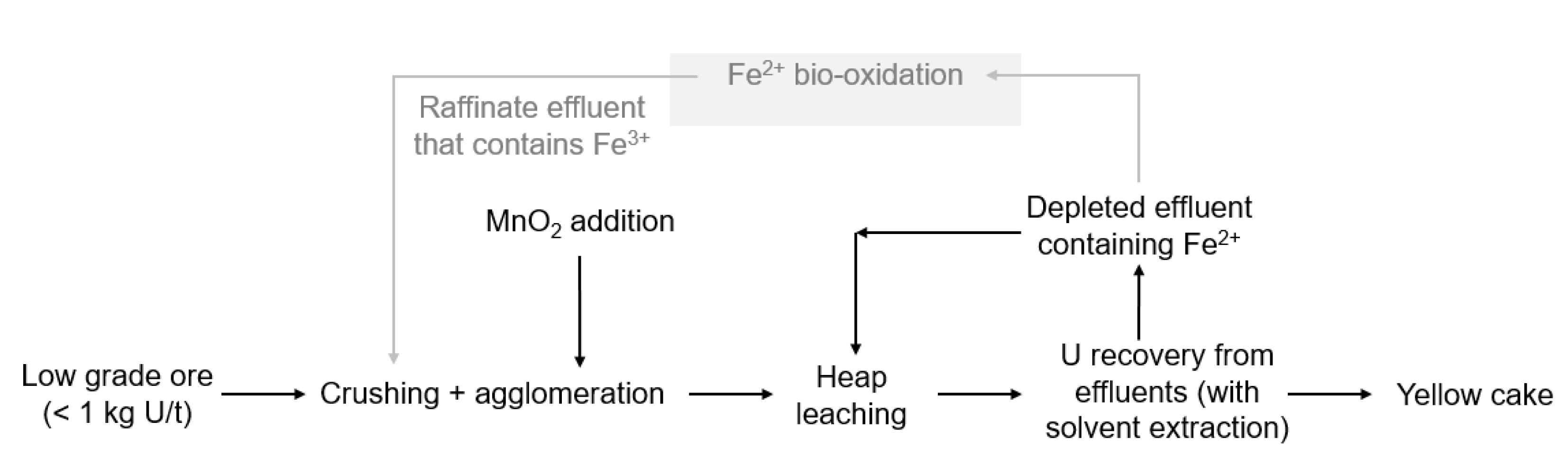
This step also aimed to check that all the equipment planned to be used in the pilot and industrial operations do not present any toxicity towards the microorganisms. Last but not least, this step aimed to produce the biomass that will be used to inoculate the 6 L bioreactors.
3.3.1.2. Methods
Subcultures were performed in 1 L bottles with a working volume of 250 mL, placed on a shaking table. The temperature was not regulated and could therefore vary from 17 °C to 43 °C in a 24-h period (evolution of temperature in the laboratory). In order to ensure a good adaptation of the microorganisms to the industrial effluents (both from the production effluent and the raffinate), they were first diluted at 50 vol% with the synthetic effluent and their content was gradually increased up to 100 vol%. The protocol used to subculture and scale up the process consisted in inoculating 10% of both solid and liquid phase. pH and redox potentials were measured on a daily basis.
3.3.1.3. Results
Results showed that the bio-oxidation rate strongly depended on the effluent composition, with values varying from 10 mg⋅L−1⋅h−1 to 120 mg⋅L−1⋅h−1. These results have to be considered carefully, since the tests were performed in batch mode with no control of some of the operating conditions, such as temperature and aeration, and with a monitoring carried out only once or twice a day, whereas Fe2+ was sometimes completely oxidized in few hours. It was therefore not possible to use the obtained values of the bio-oxidation rate to predict the performances of a continuous operation. However, these results gave interesting insights into the adaptation of the microorganisms to the two industrial effluents. In particular, it was observed that the use of uranium-enriched effluent led to far lower bio-oxidation rates compared to the ones obtained with the uranium-depleted effluent. Bio-oxidation should therefore be performed on the uranium-depleted effluent, which corresponds to the processing option presented in Figure 11 even if the effluent may contain traces of solvent. The influence of traces of solvent on bio-oxidation performances was investigated and results showed a detrimental effect, consistent with the study from Mazuelos et al. [9]. In order to limit this issue, it was recommended to add a decantation step before the bio-oxidation process and to pump the effluent from the bottom of the decantation system since the solvent will stay at the surface. Another solution could be to add a treatment step with activated charcoal since it is usually used to remove organic solvents but this treatment is quite expensive and complex to operate (in particular for the regeneration of the activated charcoal) at a remote industrial site.
3.3.2. Laboratory-scale tests in 6 L reactors
3.3.2.1. Objectives
The laboratory-scale tests performed in 6 L reactors had three objectives. The first one was to assess the ability of the microorganisms to oxidize industrial effluents at the targeted rate (500 mg⋅L−1⋅h−1) and to test the influence of the operating conditions on the performances. The second one was to investigate the efficiency of woven polypropylene as solid support for biomass. This choice was motivated by previous works showing that bio-oxidation performances with woven polypropylene are not as affected by the formation of precipitates as when using coal particles [23]. The last objective was to produce enough biomass to inoculate larger-scale bioreactors.
3.3.2.2. Methods
Similarly to the laboratory-scale tests performed at the BRGM and at Orano Mining’s CIME, two types of reactors were used: (i) a 5 L STR, similar to the one described in Section 3.2.1.2. Figure 5 was used from September 2019 to March 2020 and (ii) a 6 L bubble column was operated from April 2020 to March 2022. In both reactors, the input effluent was injected at the bottom of the reactor and overflowed from the top. Air was also injected at the bottom through a fritted cylinder. For the supply of CO2, gas bottles were mostly used but Na2CO3 was also considered as it is already used on-site for uranium production, which would then simplify the logistics at this remote site. Some tests were also performed without any enrichment of the air in CO2. In the 5 L STR, the woven polypropylene was placed in the basket and was “crumpled” to reach the targeted solid support concentration. In the bubble column, the solid support was fixed in the middle of the column: woven polypropylene support was either crumpled in a basket or stretched across the height, i.e., in the same direction as the liquid and bubbles flow. The reactors were first launched in batch mode to allow fixation of the microorganisms on the solid support and then operated in continuous mode by progressively reducing the HRT. The tests were first conducted on an effluent made with 50 vol% of industrial effluent and 50 vol% of synthetic effluent, the content of industrial effluent being then increased to 75 vol% and to 100 vol% when the biomass was considered robust enough to be adapted to such effluent. In order to monitor the biomass, DNA extraction and CE-SSCP were performed on the solid phase from time to time. Results related to the biomass are not given here due to confidentiality issues.
3.3.2.3. Results
The first tests were performed in the 5 L STR with a temperature maintained at 40 °C. Results showed that it is possible to reach and maintain a Fe2+ bio-oxidation rate of at least 500 mg⋅L−1⋅h−1, a bio-oxidation rate of about 600 mg⋅L−1⋅h−1 being even reached and maintained in some of the operating conditions investigated (Figure 12). As shown in Figure 12, the HRT was first reduced from 48 h to 18 h, each reduction performed after stabilizing the bio-oxidation yield at 100%. These good bio-oxidation performances obtained with the woven polypropylene showed that this material can be used as solid support for biomass.
Bio-oxidation yields and rates in the 5 L STR operated on the Somaïr site.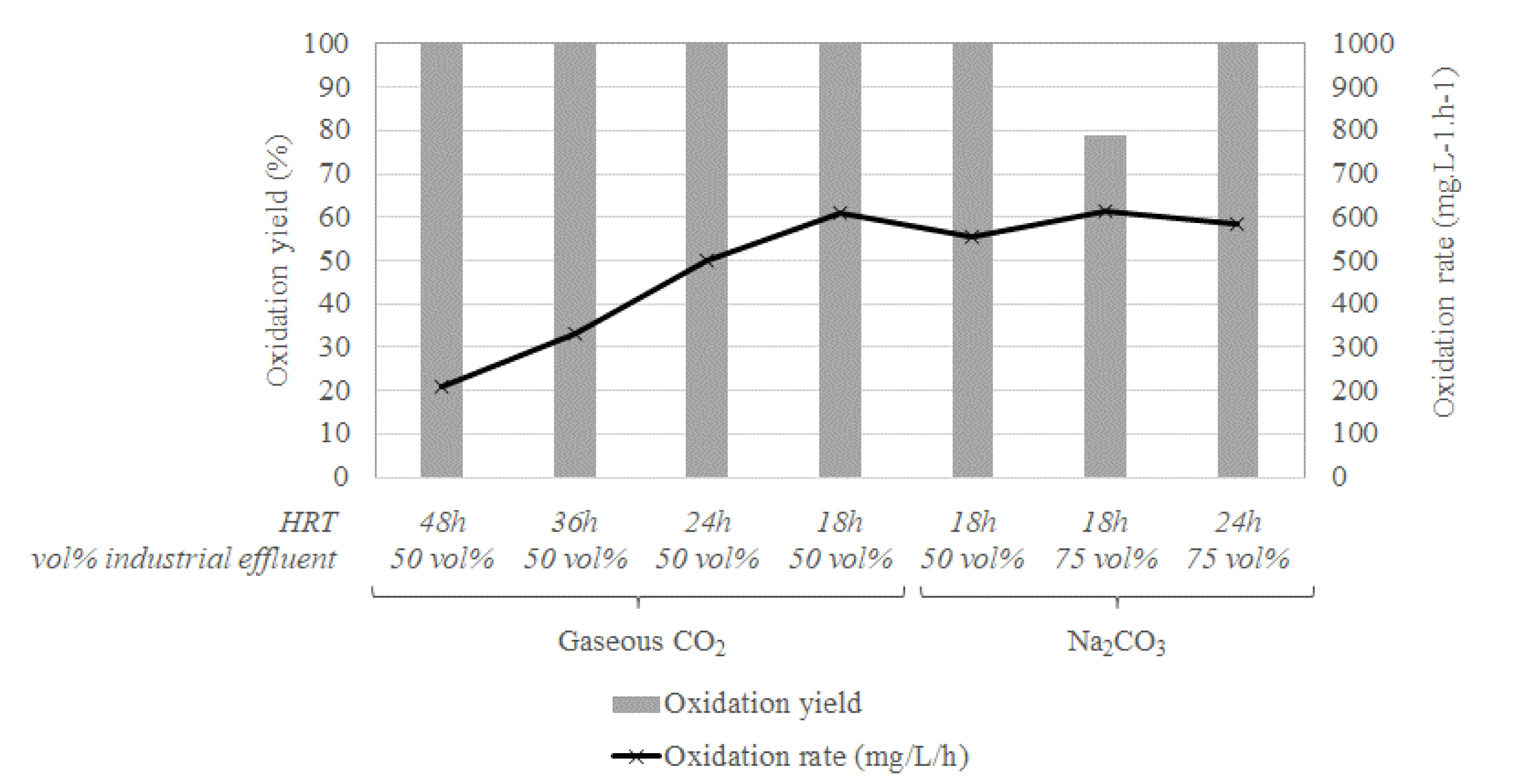
The CO2 supply was changed from gaseous CO2 to Na2CO3 after 70 days of operation without affecting the performances. However, it affected the acid consumption by increasing the pH. H2SO4 addition was finely monitored to estimate the additional cost associated to the use of Na2CO3.
The concentration of industrial effluent was increased from 50 vol% to 75 vol% after about 85 days. A decrease in the bio-oxidation yield was observed with no change in the bio-oxidation rate. This can be due to a limited bio-oxidation capacity of the biomass with the amount of solid support used, since an increase in HRT allowed to reach again a bio-oxidation yield of 100%. Similar results were obtained with the 6 L bubble column.
After more than 60 days of operation, no precipitates were observed on the solid support. This confirmed the benefits of using woven polypropylene as solid support instead of coal particles which tend to be clogged quite easily (see Section 3.2.2). Moreover, it was observed that the mode of handling the solid support had a strong effect on the decrease in bio-oxidation performances due to the formation of precipitates: stretching the woven polypropylene across the height allowed to more than double the time of operation before a cleaning is required compared to crumpling the solid support in a central basket.
The potential of the biomass to bio-oxidize the Fe2+ contained in the industrial effluent without any dilution, i.e., with a concentration of 100 vol%, was tested in the 6 L bubble column. A bio-oxidation yield of 100% was obtained for a HRT of 25 h, which corresponds to a bio-oxidation rate of about 350 mg⋅L−1⋅h−1.
The 6 L bubble column reactor was also used to investigate the influence of temperature regulation and CO2 enrichment of air on the bio-oxidation performances. Tests were performed from July 2021 to March 2022 on an industrial effluent concentrated at 50 vol%. Results obtained from December 2021 to March 2022 are given in Figure 13. The relative standard deviation of the bio-oxidation rates during each operating condition was low (less than 5%). These relative standard deviations were in the same order of magnitude than the ones observed during the laboratory-scale tests at the BRGM laboratory (Section 3.2.1) despite the higher difficulty in maintaining stable operating conditions on-site. It is therefore possible to compare the results obtained at different operating conditions. Good performances were obtained at temperatures far lower than 40 °C, which is really promising from an industrial perspective. Regarding the CO2 partial pressure, a bio-oxidation yield of almost 100% was obtained even when the air was not enriched in CO2. These results should be confirmed at a larger scale, especially since in the 6 L bubble column the air flow rate was in large excess to guarantee a good homogenization and mass transfers within the bioreactor, and biomass had time to colonize the solid support before the enrichment in CO2 was turned off.
Bio-oxidation yields and rates in the 6 L bubble column operated on the Somaïr site (from December 2021 to March 2022).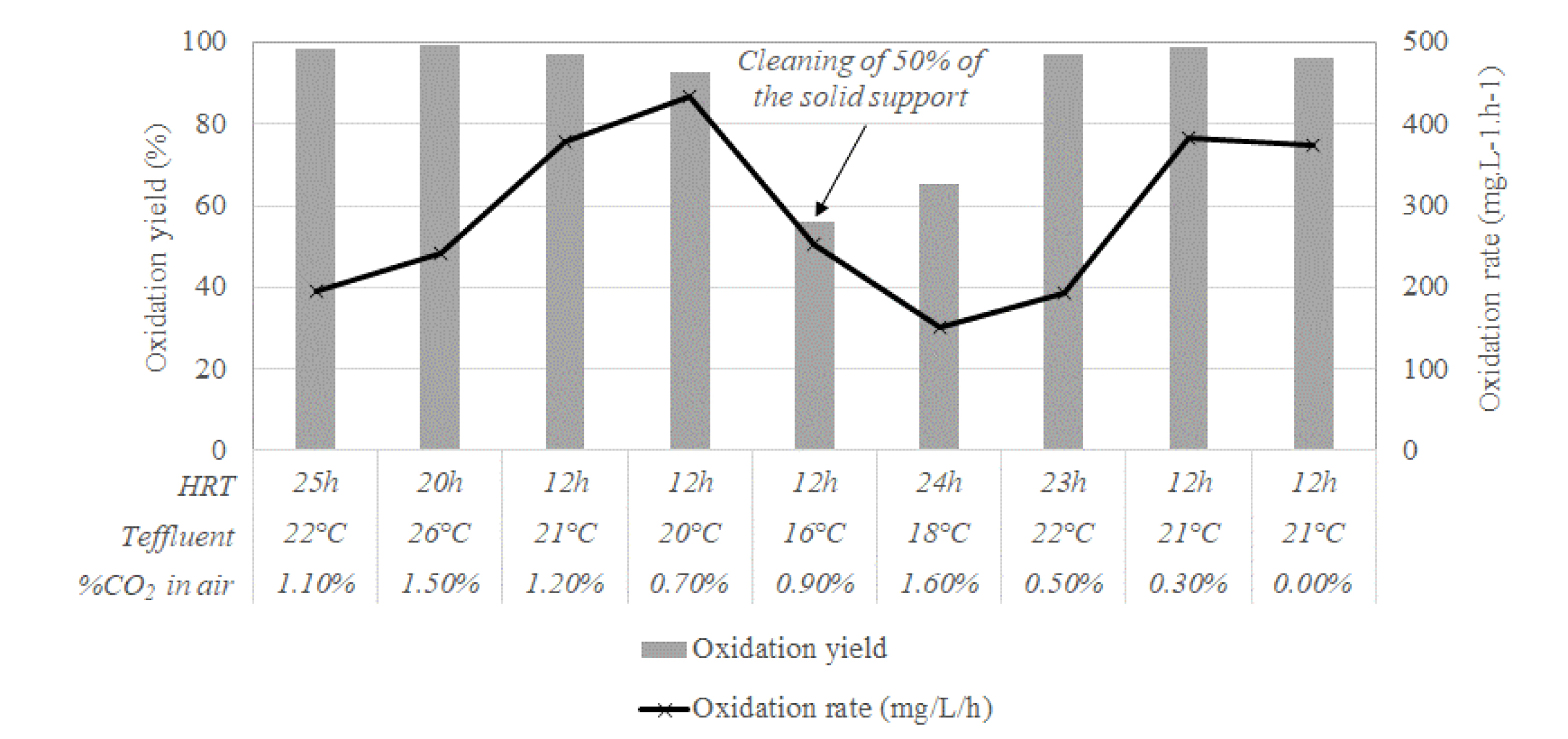
The 6 L bubble column was operated for several months (from April 2020 to November 2022). During this long period of operation, clogging of the solid support by precipitates was observed. Even if the quantity of precipitates was highly reduced compared to when using coal particles, it raised the issue of maintenance. A protocol based on partial cleaning of the solid support on a regular basis was therefore developed and validated.
Based on these results, 9 Key Performances Indicators (KPI) were defined in order to better evaluate and compare the performances of the bio-oxidation process at each step of the scale-up. They are therefore critical milestones to reach for the industrial implementation of this bioprocess. Some examples of KPIs considered here are the following: concentration of industrial effluent, bio-oxidation rate, frequency of maintenance of the solid support and time to reach the optimal performances again after a maintenance operation.
3.3.3. Pilot-scale tests in 30 L reactors
3.3.3.1. Objectives
The pilot-scale tests performed in 30 L reactors aimed to optimize the operating parameters, in particular for the start and the restart of the bioreactor after a maintenance operation. They also aimed to produce enough biomass to inoculate the larger-scale bioreactor.
3.3.3.2. Methods
3 bubble columns, similar to the 6 L bubble column (with woven polypropylene used as solid support and stretched across the height of the columns), were operated from August 2020 to November 2022. Air was initially enriched with gaseous CO2 (1%) before switching to Na2CO3 supply. Temperature was set at 35 °C. The reactors were first launched in batch mode to allow fixation of the microorganisms on the solid support and then operated in continuous mode by progressively reducing the HRT. O2 and CO2 partial pressures were occasionally measured at the top of the reactors. In order to monitor the biomass, DNA extraction and CE-SSCP were performed on the solid phase from time to time.
3.3.3.3. Results
Similarly to the 6 L bubble column tests, the bio-oxidation performances were not affected when the temperature was no longer regulated and when the air was not enriched in CO2. This result was observed when the industrial effluent was concentrated at 100 vol%. However, it was observed that a CO2 enrichment of the air is requested when the operation is being started or during a batch mode operation in order to ensure an efficient microbial growth. It was also observed that a temperature of around 20 °C is a limiting factor in such phases.
The experiments performed with the 30 L reactors also allowed to determine the quantity of solid support required to maintain a biomass concentration allowing to reach the targeted bio-oxidation performances. The initial ratio of woven polypropylene, set to 40 m2⋅m−3, led to a bio-oxidation rate of about 350 mg⋅L−1⋅h−1 when the industrial effluent was used (i.e., concentration of 100 vol%). This ratio was progressively reduced to 9 m2⋅m−3 and results showed that this ratio still allowed reaching and maintaining a bio-oxidation rate of about 350 mg⋅L−1⋅h−1.
Moreover, this testing campaign demonstrated the importance of O2 transfer. In particular, when a clogging of the fritted cylinder was observed, leading then to a reduction of the air flow rate, the bio-oxidation performances were strongly decreased. It was observed that the declogging of the fritted cylinder allowed reaching again the bio-oxidation performances within a short period, confirming the robustness of the biomass. Finally, it should be mentioned that a bio-oxidation rate of about 300–350 mg⋅L−1⋅h−1 was obtained for more than 4 months of continuous operation of the bioreactors.
3.3.4. Pilot-scale tests in a 700 L reactor
3.3.4.1. Objectives
The 700 L bioreactor was operated in order to continue the scale-up of the bioprocess and in particular to define the operating protocol to inoculate a bioreactor with a scale factor higher than the usual one. Indeed, upscaling in bioprocesses is usually performed with a scale factor of 10 but here it was planned to go from 30 L to 700 L, i.e., a scale factor of 23. This step also aimed to define additional KPIs and to get more reliable data for the design of the industrial system.
3.3.4.2. Methods
A tank of 700 L was designed in which the industrial effluent was injected at the bottom of the reactor and overflowed from the top. An air diffuser tube bound in a spiral at the bottom of the reactor was used to inject air. Na2CO3 was used for the CO2 supply, with a CO2 content in the air of 0.4–0.6%. No regulation of temperature was used. Woven polypropylene was used as solid support and a dedicated system was designed in order to stretch it across the height of the tank. The volumetric ratio of solid support was the same as for the 30 L bioreactor in order to keep the same concentration of biomass. KLa (Volumetric Mass Transfer Coefficient) was determined for different air flow rates in order to ensure sufficient O2 mass transfer by comparison to O2 uptake rate and stoichiometry of the reaction.
This reactor was operated between April 2022 and November 2022. It was first launched in batch mode to allow fixation of the microorganisms on the solid support and then operated in continuous mode by progressively reducing the HRT. O2 and CO2 partial pressures were occasionally measured at the top of the reactor, as well as O2 uptake rate (through dissolved O2 probe).
3.3.4.3. Results
The batch phase and the first continuous steps went quite fine even if the inoculation ratio was low (3.5% for the solid support). In particular, a bio-oxidation yield of 80% was obtained with a HRT of 48 h, which corresponds to a bio-oxidation rate of 160 mg⋅L−1⋅h−1. The effluent was then changed to another one produced on the mining site (details not communicated here due to confidentiality issues), which led to a strong decrease in bio-oxidation performances. This may be caused by the presence of elements which inhibit the microorganisms. However, it was not possible to validate this assumption since Orano Mining decided to stop the study.
Even if it was not possible to reach good bio-oxidation performances with this 700 L bioreactor, its piloting still allowed defining a protocol to observe the colonization of the solid support by the biomass, as showed in Figure 14. This protocol and the feedback of its use could be used in another case study as they bring insightful information on the colonization of the solid support by the biomass, which is a critical parameter for a successful scale-up of a bioprocess.
Evolution of the colonization of one piece of the solid support (woven polypropylene) by the biomass (©Orano Mining).
4. Discussion
These works carried out at different scales highlighted that the main critical parameters for the implementation of the process of bio-oxidation of ferrous iron to ferric iron are related to biomass and to mass transfers. Specific works were then performed both at the mining site and at the BRGM and CIME laboratories.
First of all, it is critical to understand the mechanisms related to biofilm formation and growth in order to reach good performances of the bioprocess. Due to limited access to characterization equipment at the Somaïr mine, the in-depth characterization works were performed at BRGM, on a 150 mL bubble column operated in continuous mode fed with a model solution (ferrous sulfate in a nutrient medium). The aim was to better understand the biofilm formation and its influence on the bio-oxidation rate of the bioreactor. In particular, cryogenic scanning electron microscopy (Cryo-SEM) was used to observe the attachment of cells onto the solid support without prior preparation steps (the methodology is described in [20]). A comparison of biofilms between new and old woven polypropylenes (used as solid support for 1.5 month and 1.75 year respectively) was performed (Figure 15). These works showed that even though the woven polypropylene had large holes (around 400 μm, as used on-site), biofilms completely clogged the solid support, probably affecting the mass transfer. Thin sections were also observed with a scanning transmission electron microscopy (STEM) (the methodology was adapted from [25]). It enabled to see that the microorganisms did not seem to be directly in contact with the polypropylene, a layer of extracellular polymeric substances secreted by the bacteria being in-between. It was also observed that precipitates were included in the biofilms and that microorganisms did not seem to be located in the same areas than precipitates, as if their inclusion in the biofilm was not simultaneous. Monitoring of the biofilm growth was performed on-site with the development of specific methodologies. In particular, a methodology was developed in order to estimate the amount of biomass attached to the solid support, this methodology including a dedicated protocol for recovering the attached cells followed by counting in Thoma cells. A methodology was also developed to estimate the growth of the biofilms on the woven polypropylene used in the 700 L reactor. This methodology is based on taking pictures of several given pieces of the solid support, at the same distance and with the same light on a regular basis (see Figure 14).
Cryo-SEM (A and B) and STEM (C and D) photographs of biofilms on woven polypropylene support (new support A and C; old support B and D).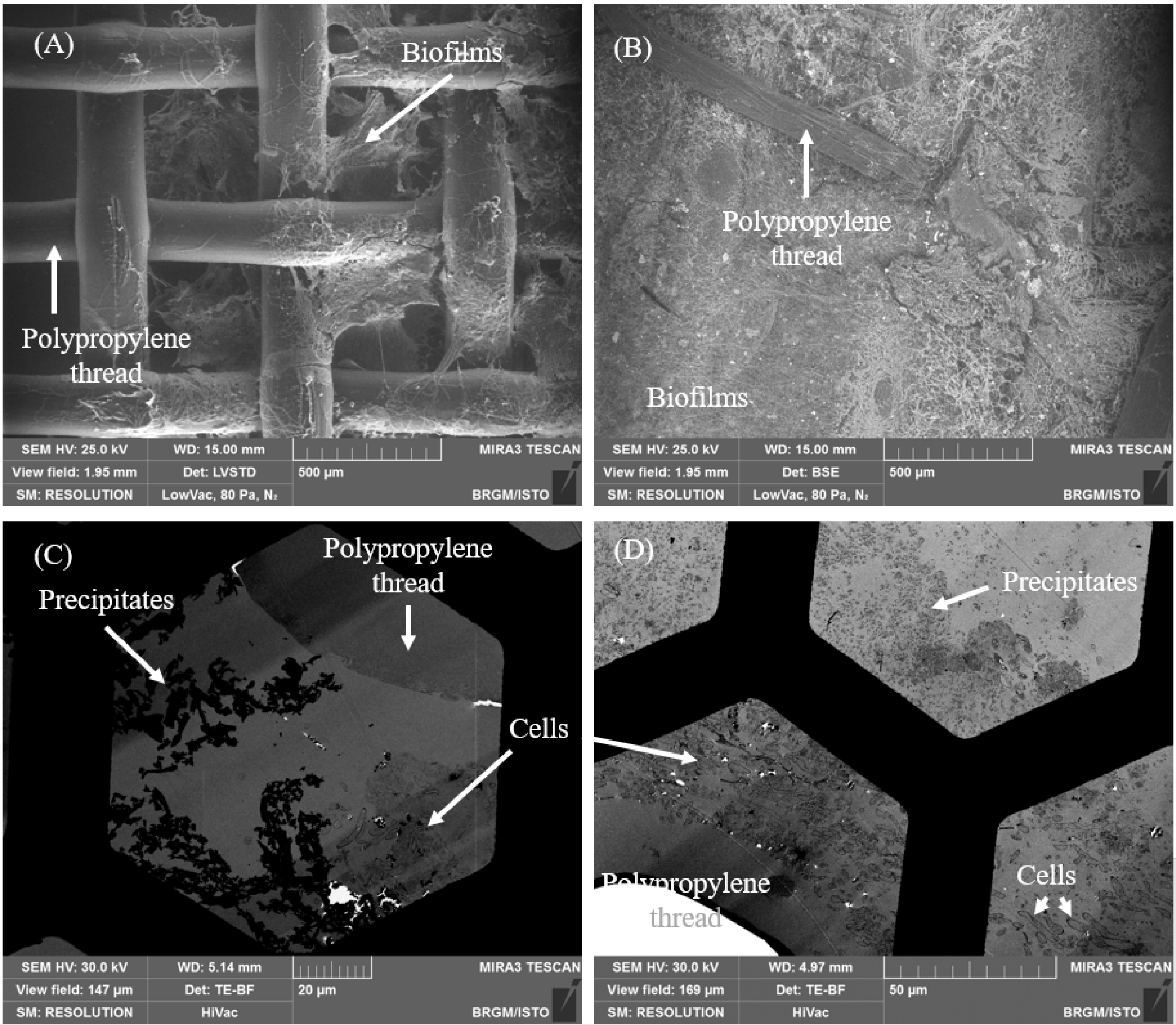
Another important feature of the biomass is related to its inhibition by elements possibly found in the industrial effluents. Indeed, even if some potential inhibitory elements were identified before performing the tests, such as uranium in the production effluent (Figure 10) or traces of organic solvents in the raffinate (Figure 11), others elements may have an inhibitory effect on the metabolic activity of the bacteria. An ecotoxicity approach was then developed on-site. In this approach, detailed analysis of the effluents was combined to laboratory-scale tests in shake flasks. Such approach was implemented on a regular basis on the industrial effluent used for the tests since some variations in its chemical composition can be found.
Another key parameter of the performances of this bio-oxidation process is the formation of precipitates, as they play a significant role in the mass transfers. In this study, precipitates were produced even though pH was most of the time regulated to 1.2. These precipitates were not characterized due to the difficulty in collecting and analysing them, since they are associated to radioelements. However, some information could be obtained from Hubau et al. [20] who performed bio-oxidation tests in a similar context with SEM-EDS (Scanning Electron Microscope-Energy Dispersive Spectroscopy) characterization of the precipitates [20]. They showed that precipitates were a mixture of jarosite and ammoniojarosite, which are often reported in bioleaching systems. These precipitates came from ferric iron and sulfates together with cationic nutrients (). Understanding the precipitation mechanisms is crucial to decrease their production allowing then improving mass transfers and optimizing maintenance of the bioreactors.
Last but not least, it is essential to optimize gas–liquid mass transfers all along the upscaling of the process. Gas–liquid mass transfers were studied at each step of the on-site tests (except for the laboratory-scale tests in shake flasks) by analyzing gas composition in the outlet flow of the bioreactor, by measuring O2 uptake rate during bio-oxidation and by estimating kLa. It should be mentioned that there is a need to develop monitoring instruments dedicated to acidophilic bioleaching environments in order to optimize gas–liquid mass transfers. For example, it would have been very useful to measure the dissolved CO2 content. However, the related probes available on the market are, to our knowledge, not adapted to this application.
5. Main lessons learned
Even if the study was stopped before industrial implementation of the investigated bioprocess, several lessons were learned from the tests performed at laboratory- and pilot-scale. This work also allowed identifying opportunities of process improvement.
In particular, the conception of the bioreactor (dimensions, stirring, air injection, mode of handling the solid support within the reactor) was shown to be a key parameter for the industrial implementation of the ferrous iron bio-oxidation. This is all the more important that some critical requirements are somehow antagonist. For example, it is required to avoid bacterial washout from the reactor in order to maintain high bio-oxidation performances so fluid turbulences should be limited to avoid biofilm detachment from the solid support. However, a good homogenization is a prerequisite to ensure high mass transfer, in particular for gas, and then high bio-oxidation performances. It is also essential to consider the management of precipitates when designing the bioreactor in order to maintain the performances over long periods of time and to limit the effects of the maintenance phase. Last but not least, the design of the bioreactor has a strong impact on the CAPEX and on the OPEX. For example, a Stirred Tank Reactor allows high mass transfer but leads to high CAPEX and OPEX. Using a pond with air diffusers (which could be considered as the industrial equivalent of the bubble column) has the advantages of low CAPEX and OPEX but may lead to hydrodynamic short circuits and then to low mass transfers.
Regarding the operating conditions, results showed that the microorganisms used in the bioprocess have a strong robustness, which is a really positive feature since it means that they can be adapted to changes in operating conditions without decreasing their performances, and that a brutal change in operating conditions (due to an unexpected issue for example) does not lead to a loss in biomass. In particular, regarding the effluent composition, it was observed that it is possible to adapt the biomass to high concentrations of elements by first diluting the effluent and progressively decreasing the dilution level. This parameter is easier to evaluate in continuous mode rather than in batch mode, as the change in effluent composition is less abrupt. Moreover, results showed that the temperature and the enrichment of air in CO2, which highly determines the biomass formation, are very important during batch phases such as the start-up or the restart after a maintenance operation. They are not so important during the bio-oxidation phase. These phases should then be carefully considered during the scale-up and later for the industrial exploitation of the process, as they are decisive for the on-site implementation, not only from a technical point of view but also from an economic point of view.
Results obtained in this study highlighted the need to have and to maintain shake flasks for checking the toxicity of any new effluent or material that will have to be placed within the bioreactors. These shake flasks could also be used to assess the composition of the effluent towards the biomass resistance in case of an unexpected change in the mine process.
Another lesson learned from this study is that it is essential to have a good knowledge of the biofilm properties. Monitoring the overall process parameters (oxidation yield, total Fe and Fe2+ concentrations, etc.) does not allow understanding the mechanisms related to the biofilms (such as formation, growth, clogging, detachment), which are nevertheless a pivotal point of this bioprocess. Changes in biomass at the microscopic scale may results in high variations of the behavior and performances of the whole process at macroscopic scale. Better understanding the biofilm properties often requires access to cutting-edge characterization equipment not available on the industrial sites, so strong collaboration with research laboratories should be encouraged.
Finally, the definition of Key Performance Indicators (KPIs) quite early in the implementation strategy was proven to be very effective to guide the planning of the experimental testwork for the upscaling of the bioprocess. They also constitutes the basis of an iterative approach for the update of the techno-economic feasibility of the process, considering the information obtained at the different development steps, allowing the identification of technical and economic hot spots as soon as they appear, which need then more research work for their optimization.
6. Conclusions
The development of the bio-oxidation of ferrous iron to improve uranium recovery was initiated with theoretical works and with laboratory-scale tests performed on synthetic effluent to evaluate its potential. From these first results, the main steps for its upscaling and its implementation on a mining site were defined. The main results from each of these steps are presented, discussed and put into perspective in order to support an iterative approach for the design of the bioreactor and for the update of the techno-economic feasibility of the process with the final objective to define the most appropriate operational technology and flowsheet.
Even if this study was stopped at the 700 L piloting stage due to an internal decision of Orano Mining, it still allowed improving process know-how and operational experience that could then be used for the industrial implementation of any bioprocess. In particular, several lessons can be learned and allow identifying opportunities to improve the investigated bioprocess and the approach used for its upscaling.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
This study was performed under the project IronBiox (“Bioaugmentation en fer ferrique”) funded by Orano Mining.
Acknowledgments
The authors thank all the persons who have contributed to this project and in particular Mohamed Kariman, Allasane Issaka Oumarou and Moussa Sabiou from Somaïr; Dominique Morin, Yannick Ménard, Christopher Bryan, Jérôme Jacob, Catherine Joulian, Pierre Gallé, Dominique Breeze and Patrick d’Hugues from BRGM; Jean-Luc Rey, Véronique Dutheil, Alexandre Michaut, Magali Celier and Pascal Nardoux from Orano Mining.





 CC-BY 4.0
CC-BY 4.0