1. Introduction
Multicomponent reactions (MCRs) have seen a surge in interest due to their significant abilities of building complex scaffolds from easy-to-access starting materials, all the while respecting the principles of green chemistry [1]. A subclass of this field is the isocyanide-based multicomponent reactions (IMCRs), the Passerini [2] and Ugi [3] reactions being prime examples. They employ the unique properties of isocyanide moieties: they classically first react as nucleophiles, to generate nitrilium species, which can then be trapped by other nucleophiles, to efficiently construct complex patterns in one step [4].
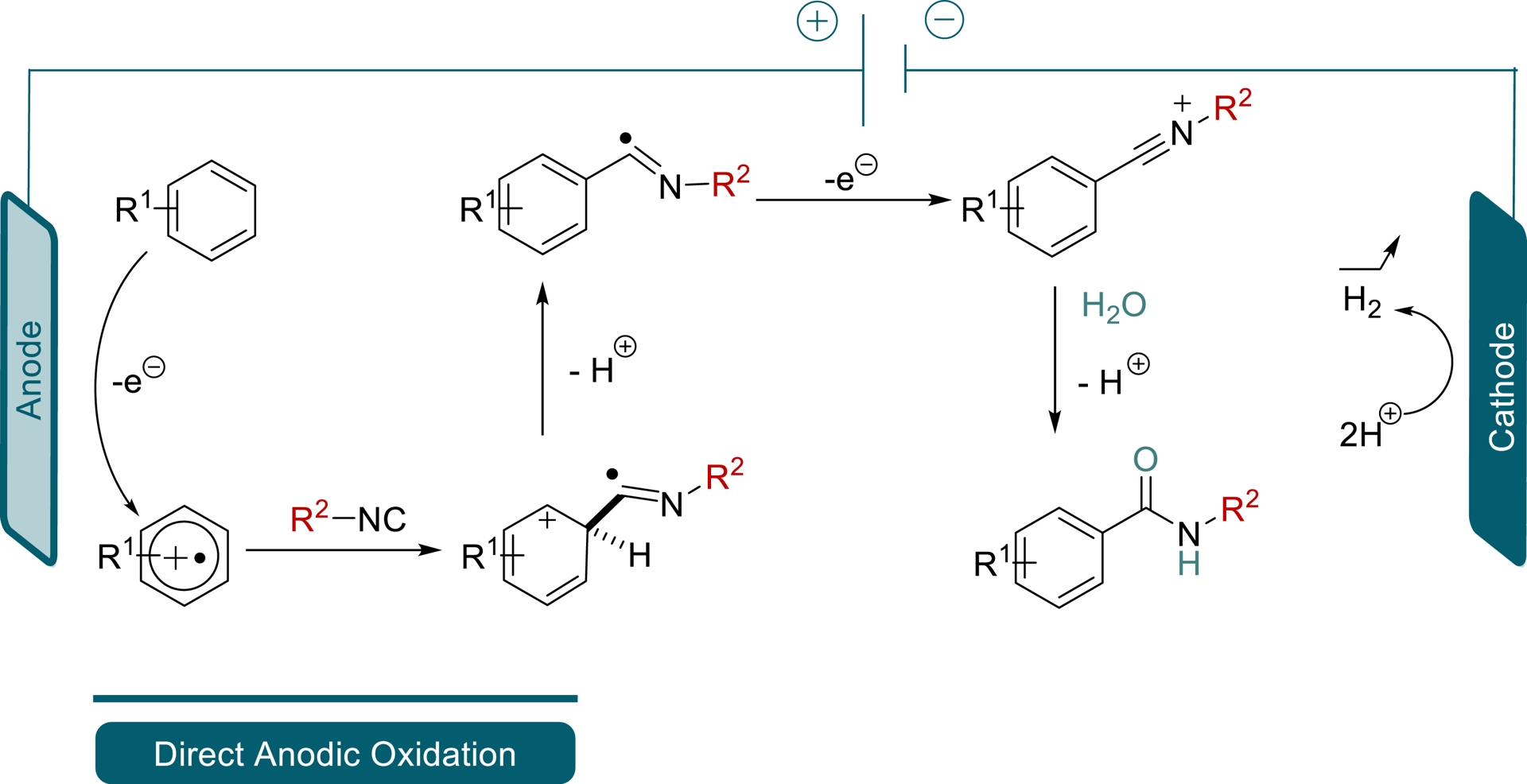
Electrosynthesis can also be a major contributor to respecting green chemistry principles, by replacing costly and stoichiometric oxidants (often toxic and with poor atom-economy), or rare-earth-based catalysts [5, 6]. In this endeavour, our group [7, 8, 9, 10] and many others [11] merged the two concepts, electrosynthesis and IMCR, to avoid the use of unstable substrates, additives, or catalysts, toward the synthesis of Passerini and Ugi adducts among other structures. However, synthetizing a benzamide group from a non-substituted aromatic compound without using a metal-based catalysis and functionalization of the aromatic ring remained elusive. Inspired by previous electro-induced C(sp2)–H bond functionalizations [12, 13, 14, 15] (Scheme 1), we proposed a method merging electrosynthesis and IMCR toward the production of arylcarboxamides.
Previous and current strategies for direct oxidative C(sp2)–H functionalization.
Indeed, building on the mechanistic studies of Gooßen et al. [13], we surmised that a radical cation, produced at the anode from the direct oxidation of an arene, could be trapped by an isocyanide and a water molecule to build arylcarboxamides. The released protons would in turn be converted into dihydrogen at the cathode, closing the electrical circuit (Scheme 2). This original carboxamide synthesis would avoid the use of catalyst and stoichiometric oxidants, using green energy (94% of low-carbon electricity in France’s electricity mix in 2024, 96% in 2025) to convert available substrates into high-value products [16].
2. Results and discussion
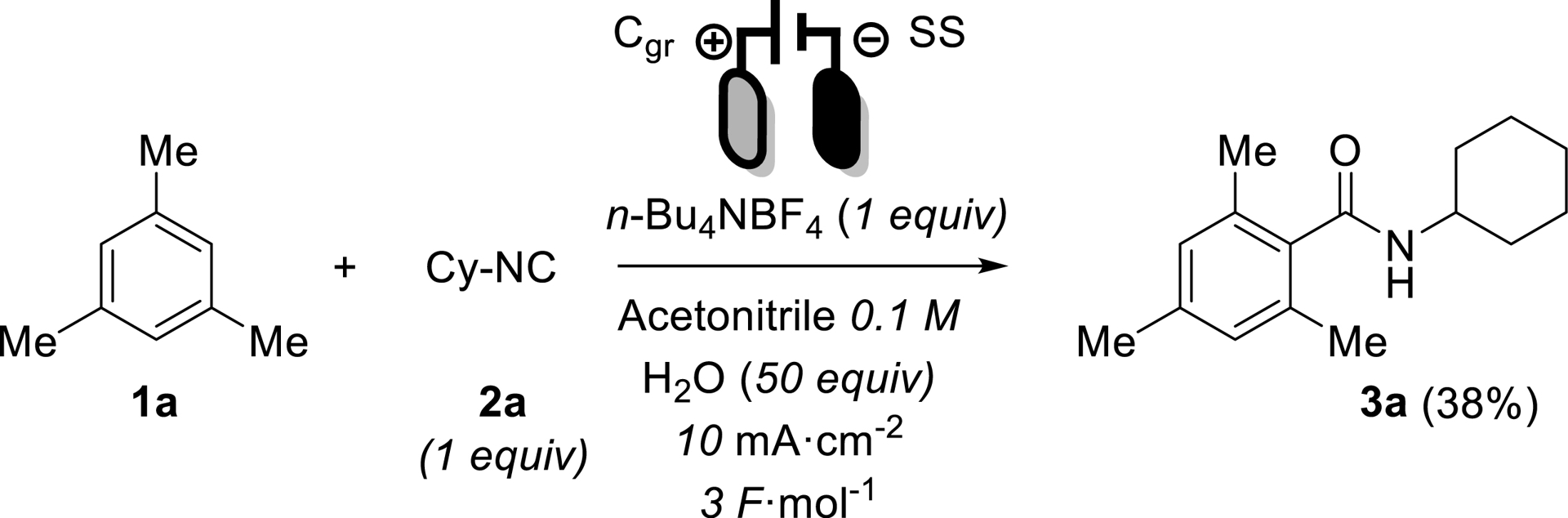
With this strategy in mind and using our in-house expertise on electro-induced IMCRs, we selected mesitylene 1a as a prototypical oxidizable arene along with cyclohexyl isocyanide (Cy-CN) 2a as partner in acetonitrile as solvent. A quick pre-optimization led to choose N-tetrabutylammonium tetrafluoroborate (n-Bu4NBF4) as supporting electrolyte, graphite as cathode and stainless steel as anode. These conditions led to the isolation of desired product 3a in 38% yield, which constituted the starting point of the optimization process.
Subsequent tests fixed the temperature at 20 °C (a 41% yield at 60 °C was not significant enough to continue using high temperature) and showed that the stirring had no impact on the outcome. These observations fixed half of the reaction parameters (see Table S1 in Supplementary material), however, the rest of the parameters (in italic in Scheme 3) could hardly be optimized the usual way, one at a time, as they could be interdependent. Thus, we resorted to a machine learning model, as it was capable of optimizing several parameters at the same time [17, 18, 19, 20]. The model chosen was EDBO, created by the Doyle group in 2021 [21] for its low resource cost and availability (https://edboplus.org/). While this work was in progress, the Ackerman group also used machine learning to optimize an electro-induced annulation via palladium catalysis [22], while the Sigman group reported using EDBO for the design of palladium catalyst for the fluorination of arylboronic acids [23], proving the usefulness of this strategy. In order to use this model, several questions needed to be answered: (1) Which outcome(s) is (are) of concern to us, and whether its maximization or minimization were desired? (2) How to measure the chosen outcome, and how to interact with the model? (3) What is the research space for the model to investigate? (4) What is (are) the stop condition(s)?
In our case, maximizing the yield was the main goal, which would be measured by 1H NMR against an internal standard, the supporting electrolyte (it was proven to be stable with external standards). The research space was designed as neutral as possible, to avoid introducing human bias into the model (see Table 1). As an example, various amounts of the isocyanide led to different products, 3a and 4a (see Scheme 4, structure of 4a was elucidated by X-ray analyses). Thus, the number of isocyanide equiv had to be limited between 0.33′′ and 3 (0.33 experimentally means 1 equiv of isocyanide and 3 equiv of mesitylene).
Research space
| Parameters | Values | ||||||
|---|---|---|---|---|---|---|---|
| Cy-NC equiv | 0.33 | 0.5 | 0.75 | 1 | 1.5 | 2 | 3 |
| Water equiv | 1 | 5 | 10 | 25 | 50 | ||
| n-Bu4NBF4 equiv | 0.5 | 1 | 2 | ||||
| Current density (mA⋅cm−2) | 5 | 10 | 15 | 20 | 25 | ||
| Limiting reagent concentration (M) | 0.05 | 0.1 | 0.15 | 0.2 | |||
| Charge (F⋅mol−1) | 2 | 3 | 4 | 5 | 6 | ||
Products formed according to isocyanide equivalents.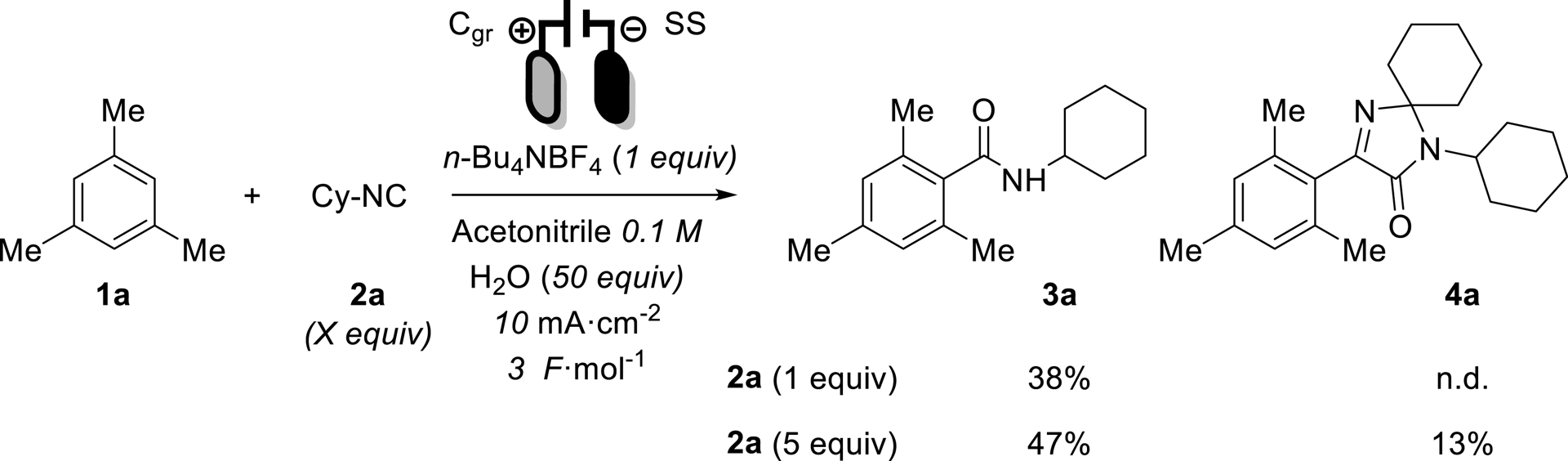
Similarly, using more than 50 equiv of water was meaningless, so its limits were set at 1 and 50 equiv. Standard values often found in the literature for the supporting electrolyte were between 0.5 and 2 equiv; for the concentration of the reagents, they were above 0.05 M and below 0.5 M, and the current density was bounded between 5 and 25 mA⋅cm−2. Finally, based on our proposed mechanism (see Scheme 2), this reaction would need at least 2 electrons per molecule of mesitylene. Using the same limits as for the isocyanide, the upper limit for the charge was set at 6 F⋅mol−1. These studies led to the following research space (Table 1).
The resulting number of possible combinations was 10 500, and, as an example, a human would conduct 29 (0.3% of the research space) experiments with this table: one experiment for each value of each parameter, with all other parameters fixed, following the classical OFAT (one factor at a time) method [24]. For the purpose of this study, we decided to fix the total number of experiments to 15, which represents half the effort required with the OFAT method (below 0.15% of the research space). The results of this EDBO-led optimization are displayed in Figure 1.
Evolution of yield with EDBO.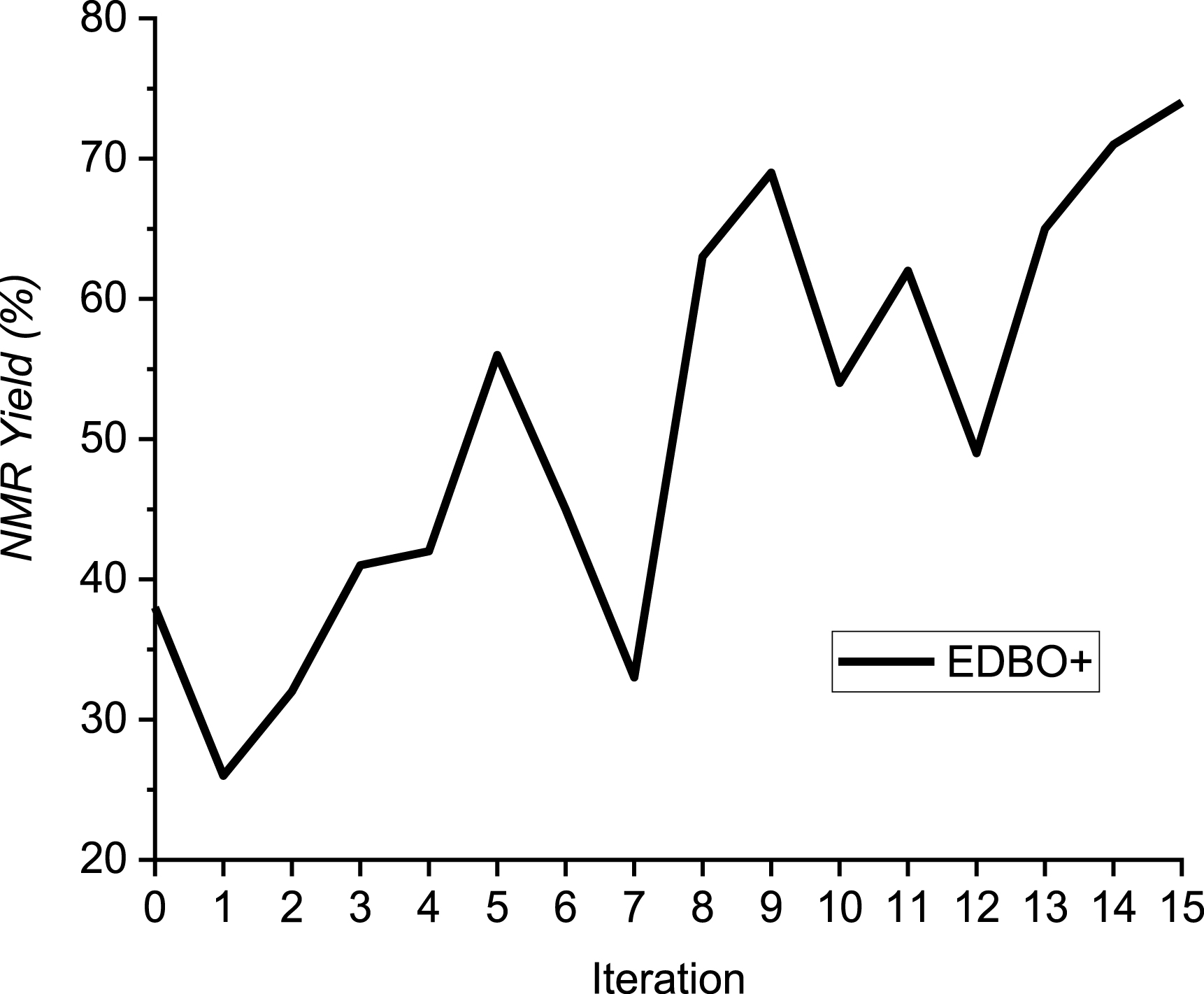
It became clear that EDBO was able to quickly increase the yield from the starting 38%, reaching 50% after 5 experiments, 70% at the 9th, and stopping at its 15th experiment, in which the starting yield of 38% was doubled to reach a high yield of 74%. The differences between the starting point (Scheme 3) and the best conditions found by EDBO (Scheme 5) were counter-intuitive compared to commonly used values (isocyanide in default, high current density and concentration), highlighting the importance of an unbiased design of the research space.
Final conditions found by EDBO.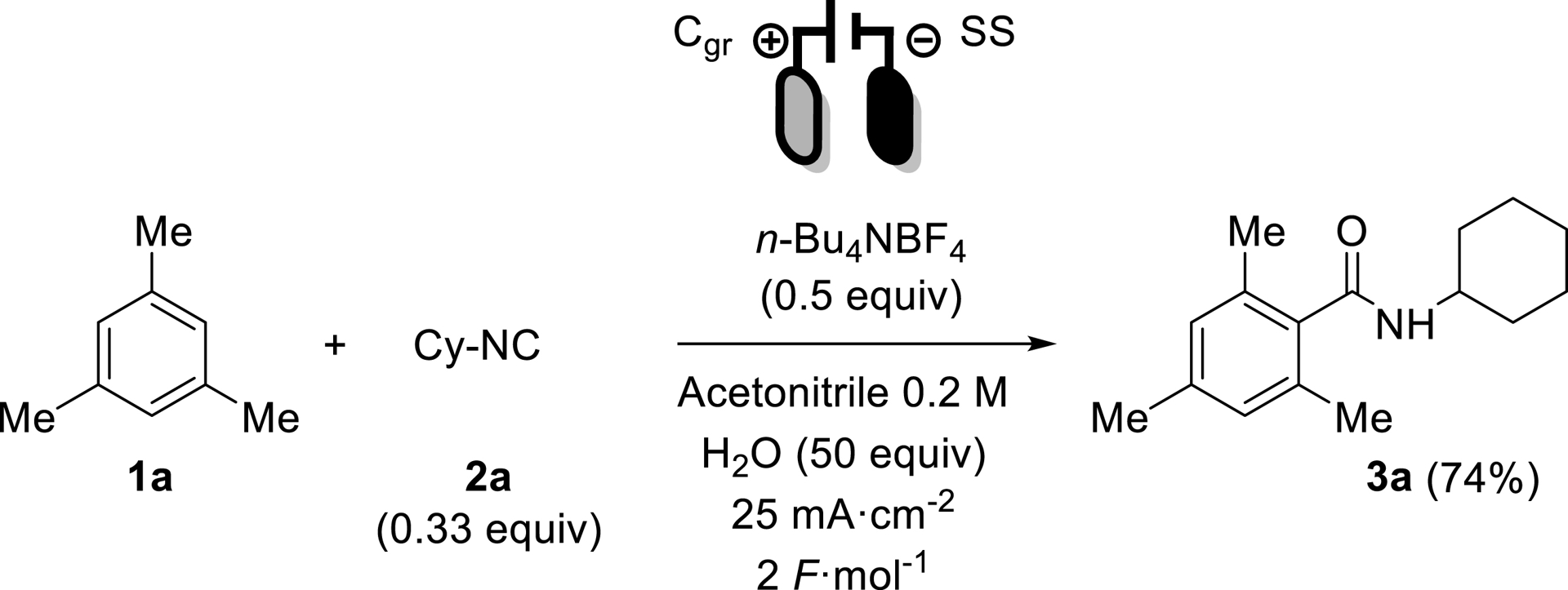
With these optimized conditions, the scope of the reaction’s applicability was then tested (Scheme 6).
Scope of the studied reaction.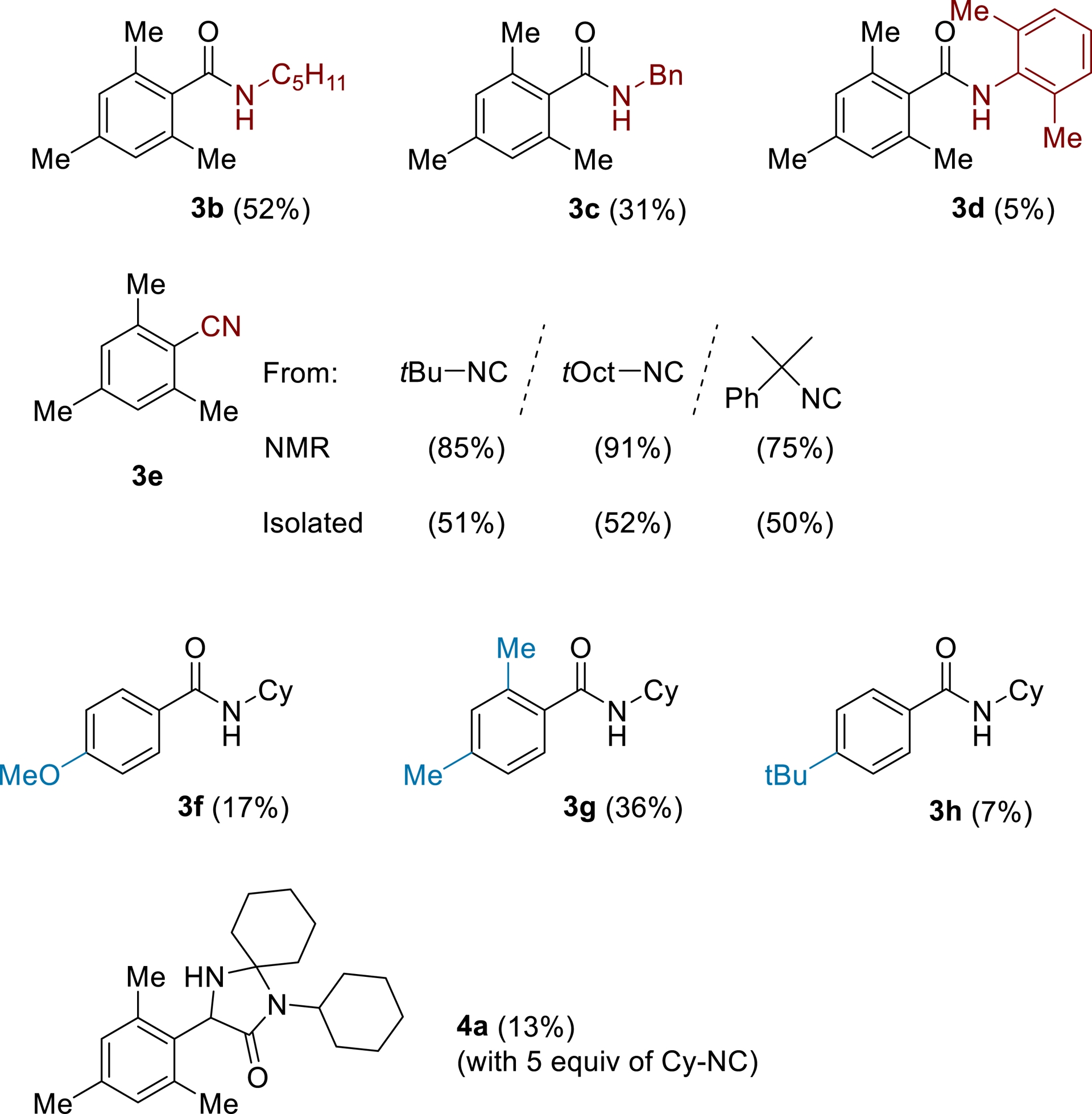
Primary and secondary isocyanides were found to work under these conditions (74% for cyclohexyl isocyanide, 52% for 1-pentyl isocyanide, and 31% for benzyl isocyanide), while the aromatic isocyanide gave a low amount of the desired product (5%). Interestingly, tertiary isocyanides were found to fragment to give the cyano compound in good yield (up to 91% NMR yield, 52% isolated yield)1 . Its formation is supposed to take place when the iminyl radical is produced: this structure may fragment to release a stabilized tertiary carbon-centered radical and the benzonitrile compound, favoring this elimination.
Alternative aromatic compounds were also tested, and m-xylene was functionalized with an acceptable 36% yield. Anisole was found to polymerize (black tar formation), resulting in low yield (17%), while tert-butylbenzene also gave the para-product in low yield (7%, also prone to polymerization). Several other aromatic compounds were tested, and none produced the desired amide in detectable amounts.
3. Conclusion
In this article, we report a previously unknown electro-induced isocyanide-based multicomponent reaction toward the synthesis of arylcarboxamides, with an AI-guided optimization. In respect with green chemistry objectives, this work avoids the use of chemical oxidants, coupling agents, or catalysts by relying on electrosynthesis. The optimization was conducted with a minimal number of experiments, thanks to guidance by a Bayesian optimization-based model (EDBO). Overall, this work opens a path toward more challenging electro-induced methods and the use of AI in the laboratory. Subsequent work on other electro-induced IMCRs is currently under investigation in our team.
CRediT authorship contribution statement
Virgile Rouffeteau: Data curation, Writing—original draft.
Clara Perrier: Data curation, Writing—review & editing.
Maximilian Fleck: Software, Writing—review & editing.
Geoffrey Gontard: Formal analysis, Writing—review & editing.
Maxime R. Vitale: Funding acquisition, Conceptualization, Supervision, Writing—review & editing.
Laurence Grimaud: Funding acquisition, Conceptualization, Supervision, Writing—review & editing.
Acknowledgments
Drs. A. Simon and S. Bachollet are thanked for early investigations.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
ENS-PSL, CNRS, and the Agence Nationale de la Recherche (ANR-20-CE07-0020) are thanked for financial support. VR thanks Ecole Polytechnique for a PhD grant (AMX). CP thanks CNRS for a placement grant. MF acknowledges support under the Major Research Program of PSL Research University “ChemAI” launched by PSL Research University and implemented by ANR with the references ANR-10-IDEX-0001.
Supplementary materials
Deposition Number CCDC 2492104 contain the supplementary crystallographic data for compound 4a. These data can be accessed free of charge via the Cambridge Crystallographic Data Centre (CDCC).
1 This benzonitrile is prone to sublimation, which explains the difference between the two yields.






 CC-BY 4.0
CC-BY 4.0