1 Introduction
Water is the most abundant fluid on earth and the major constituent of organisms (70% of total weight in most cases). The understanding of hydrophilic and hydrophobic effects is of prime importance. These effects are related to the solvent properties of water and to the fact that some compounds are more or less soluble in water. The solvent abilities of water arise primarily from two properties: its tendency to form hydrogen bonds (very short characteristic lifetime, between 10−13 and 10−12 s) and its dipolar character [3]. The high dielectric constant , already alluded to, results from its dipolar character.
2 Hydrophilic interactions
2.1 Hydrogen-bond formers
Any molecules that carry groups capable of forming hydrogen bonds can do so with water; such groups will tend to make these molecules hydrophilic. Water readily dissolves hydroxyl compounds, amines, sulphydryl compounds, esters, ketones, and a wide variety of other organic compounds.
In the presence of a solid substratum, water may also form bonds. This is the case, for instance, of glasses where silanol groups SiOH are present at the interface water–silica. Let us mention porous materials [2], such as Vycor glass, silica gels, and zeolites.
2.2 Ionic compounds
Water is also an excellent solvent for ionic compounds. The explanation lies in the dipolar nature of the water molecules. Dipoles interact with ions such that cations and anions in aqueous solutions are surrounded with hydration shells. Careful neutron scattering experiments allowed us to determine the characteristics of the hydration shell around many ions, that is to say the distance between the ion and the water molecule, the tilt angle and the hydration number [16]. Around a cation, the water molecule is oriented with the oxygen close to the cation corresponding to a minimisation of the energy between the ion and the water dipole. Instead, around an anion, the hydrogen atoms are close to the ion. The hydration number, i.e. the number of water molecules inside the first hydration shell, and the lifetime of this shell depend on the nature of the solute and on its concentration. Layer-like clays minerals [32,36] have been the objects of many studies.
3 Hydrophobic interactions
The dipolar nature of the water molecule also contributes to dissolve non-ionic, but apolar molecules. Substances like hydrocarbon that are non-polar and non-ionic and cannot form hydrogen bonds show only limited solubility in water. Such hydrophobic molecules do not form hydration shells, as hydrophilic substances do. Instead, the regular water lattice forms ‘cages’ of ice-like clathrate structure about non-polar molecules, probably with some geometry close to that of polyhedra such as icosahedra [20].
4 Hydrophilic/hydrophobic interactions
Amphipathic molecules simultaneously exhibit both hydrophilic and hydrophobic properties. They have a head group that is strongly hydrophilic, coupled to a hydrophobic tail, usually a hydrocarbon. When one attempts to dissolve them in water, amphipathic substances form peculiar structures. Possible structures are a monolayer on the water surface, a micelle, and a bilayer vesicle, with water both inside and out. Examples of other structures that impose spatial restrictions on water molecules include polymer gels and micro-emulsions. In these cases, since the hydrophobic effect is the primary cause for the self-organisation of these structures, obviously the configuration of water molecules near the hydrophilic–hydrophobic interfaces is of considerable relevance [23].
An important field of interest of the subtle hydrophilic–hydrophobic interplay is that of associated water-soluble polymers. There is a novel type of polymers, namely telechelic polymers, where the hydrophilic chain is end-capped by hydrophobic short blocks. Their unique properties in aqueous systems make them very useful materials as rheology modifiers, suspension stabilisers, and drug carriers in pharmaceutical applications [40].
5 Structure and dynamics of confined water
In many technologically important situations, water is not in its bulk form, but instead attached to some substrates or filling small cavities. Common examples are: water in porous media, such as rocks or sandstones, and water in biological material as in the interior of cells or attached to surfaces of biological macromolecules and membranes. This is what we define here as the ‘confined’ or the ‘interfacial water’.
The structure and dynamics of water are modified by the presence of surfaces, by a change of hydrogen bonding, but also by modification of the molecular motion that depends on the distance of water molecules from the surface. Therefore, a detailed description of these properties must take into account the nature of the substrate and its affinity to form bonds with water molecules, and also the number of water layers or hydration shells. How are the water properties modified when water is in contact with hydrophilic or hydrophobic interfaces? Or both? How does the intracellular water behave? In this section, we give some examples of model systems developing either hydrophilic or hydrophobic interactions, or both, with water. In the following, the structural and dynamic properties of confined water relative to various systems are presented.
5.1 Water in hydrophilic systems
Water in porous materials such as Vycor glass, silica gel, and zeolites has been actively under investigation because of its relevance in catalytic and separation processes. In particular, the structure of water near layer-like clay minerals [32,36] condensed on hydroxylated oxide surface [22], confined in various types of porous silica [3] has been studied by neutron and/or X-ray diffraction. Water molecules adsorbed on ionic surfaces have been investigated by FT–IR, quasi-elastic neutron scattering and dielectric relaxation techniques [35]. Water in cement (hydrated tricalcium silicate) has been the subject of several studies by quasi-elastic neutron scattering [17].
The structure of water in Vycor has been investigated as a function of the hydration level and temperature. We present here some of our results for two levels of hydration: for full hydration (0.25 g water/g dry Vycor) and 25% [5]. The latter one corresponds roughly to monolayer coverage.
Results for two levels of hydration of Vycor demonstrate that the fully hydrated case is almost identical to the bulk water and the partially hydrated case is of little difference. It seems that the confinement of the water favours the nucleation of cubic ice that appears superimposed on the spectrum of liquid water and whose proportion can be deduced from the intensity of the (111) Bragg peak. The proportion of cubic ice increases with decreasing temperature. In fact, at , the spectrum of confined water looks similar to that of cubic ice (Fig. 1). This is in sharp contrast to bulk water that always nucleates into hexagonal ice. In Fig. 2, we show a spectrum that gives a clear evidence that liquid water is present below the Bragg peaks at , obtained by subtraction of the weighted spectrum of the same sample cooled down to .
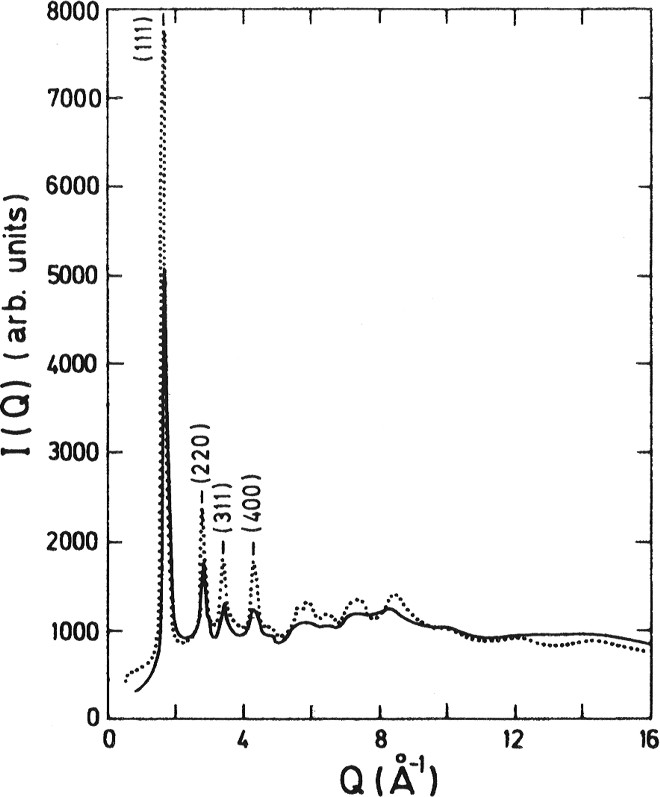
Spectrum of cubic ice (−198 °C) (dotted line) compared with that of confined D2O at −100 °C from fully hydrated Vycor (full line).
Comparaison entre le spectre de la glace cubique (−198 °C) (traits pointillés) et celui de l'eau (D2O), à −100 °C, confinée dans le Vycor, à hydratation totale (trait plein).
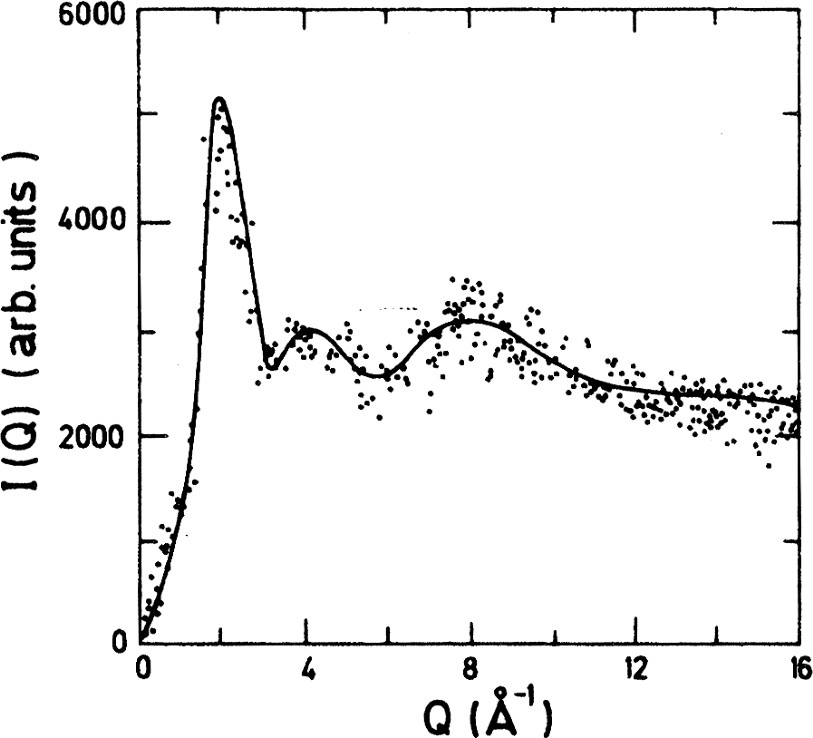
Spectrum of confined D2O at −18 °C from fully hydrated Vycor after subtraction of Bragg peaks. There is 23% liquid water [5].
Spectre de l'eau (D2O) à −18 °C, confinée dans le Vycor, à hydratation totale, après soustraction des pics de Bragg. Il y a 23% d'eau liquide [5].
Results relative to a 25% hydrated Vycor glass corresponding to a monolayer of water molecules indicate that, at room temperature, interfacial water has a structure similar to that of bulk supercooled water at a temperature of about 0 °C, which corresponds to a shift of about 30 K [3]. Therefore, the structure of interfacial water is characterized by an increase of the long-range correlations, which corresponds to the building of the H-bond network, as it appears in low-density amorphous ice [4]. There is no evidence of ice formation when the sample is cooled from room temperature down to (liquid nitrogen temperature). Nevertheless, despite the fact that interfacial water and LDA show the same position of the first sharp diffraction peak (FSDP), their structures are not strictly identical. Such structural differences could be related to a topologically distorted hydrogen-bond network, as already invoked in the case of other interfacial water systems.
Fig. 3 gives the Arrhenius plots of for hydration water at the surface of a protein [8] as compared with those of water in Vycor at different levels of hydration [8] and bulk water [37].
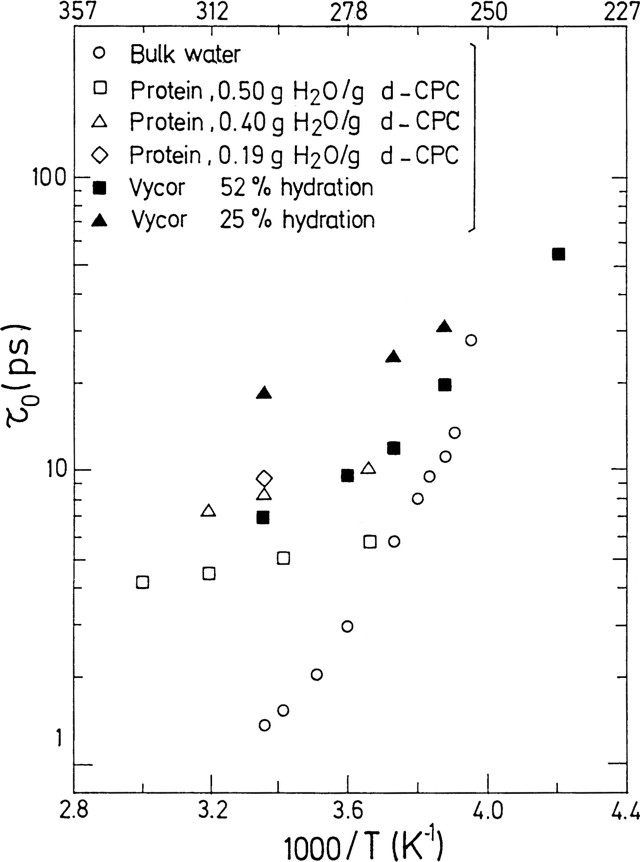
Arrhenius plot of the residence time for different levels of hydration: water at the surface of H2O-hydrated d-CPC protein (empty symbols); water contained in hydrated Vycor (solid symbols); as compared with bulk water (empty circles) [8,37].
Représentation d'Arrhénius du temps de résidence pour différents taux d'hydratation : eau à la surface d'une protéine deutériée (d-CPC) (symboles vides) ; eau contenue dans du Vycor hydraté (symboles pleins) ; comparaison avec l'eau volumique (cercles vides) [8,37].
The residence times of confined water from 25% hydrated Vycor and from hydrated proteins are always longer than the residence time of bulk water, at the same temperature. They increase rapidly as either the temperature or the level of hydration decreases. For example, for the 25% hydrated Vycor sample, at .
The hydrogen-bond lifetimes for confined water are close to that of bulk water [37]. They have an Arrhenius temperature dependence (Fig. 4), while the residence time does not exhibit such a behaviour (Fig. 3).
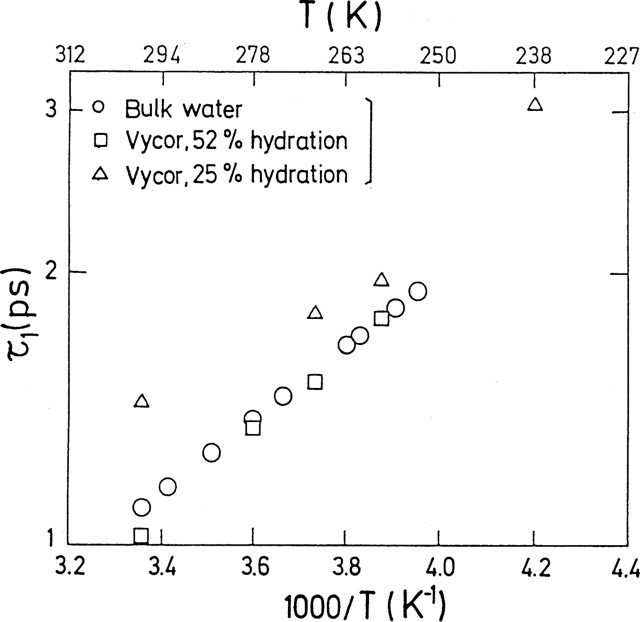
Arrhenius plot of the hindered rotations characteristic time, . This time can be associated with the hydrogen-bond lifetime [8,37].
Représentation d'Arrhénius du temps caractéristique des rotations gênées, . Ce temps peut être associé au temps de vie de la liaison hydrogène [8,37].
Fig. 3 gives the evolution of the residence time for confined water as compared with bulk water, and Fig. 4 that of hydrogen-bond lifetime.
5.2 Water in hydrophobic systems
Among hydrophobic model systems, one experimental investigation of particular interest concerns the structure of water contained in a carbon powder [7]. The structure of water has been determined both by X-ray and neutron diffraction, as functions of hydration levels, from room temperature down to 77 K. In agreement with previous work [5,6,14,38], this study gave support to the existence of a region near the interface where the properties of water are markedly different from those of the bulk liquid. A crude determination from the specific area indicates that, for a hydration equal to 50%, the thickness does not exceed 5 Å. This value must be compared with the computer simulations data [27], which indicate that structural modifications do not extend beyond 10 Å from the solid surface.
When partially hydrated, samples are cooled down to 77 K, no crystallisation peak is detected by differential thermal analysis. X-ray and neutron scattering show that an amorphous form of water is obtained. Its structure is different from those of low- and high-density amorphous ices already known [4]. This phenomenon looks similar in both hydrophilic and hydrophobic model systems.
5.3 Water in hydrophilic/hydrophobic systems [32]
The water/dimethyl-sulphoxide (DMSO) mixture is a model system to study the structure and dynamics of water under simultaneous hydrophilic/hydrophobic hydration.
The local quasi-tetrahedral structure of pure water has been found to be largely preserved in the presence of DMSO (X-ray and neutron diffraction). However, there is a transfer of hydrogen bonds from water to DMSO, in agreement with a greater hydrogen-bond affinity for water/DMSO than for water/water. Water HH pair-correlation functions of pure water and at the eutectic composition have been determined: the local coordination number is maintained [33].
The dynamic behaviour of water in dimethyl-sulphoxide solutions has been studied [9]. There is evidence of some slowing down of the translational diffusive motion of water. At the opposite, the effect on hydrogen bonding is not significant. A similar behaviour has been observed in water/trehalose [21] and water/pyridine solutions [1].
5.4 Water confined in biological systems
In the field of biology, the effects of hydration on equilibrium protein structure and dynamics are fundamental to the relationship between structure and biological function [11,24,28,31,34].
The assessment of perturbation of liquid water structure and dynamics by hydrophilic and hydrophobic molecular surfaces is fundamental to the quantitative understanding of the stability and enzymatic activity of globular proteins and functions of membranes.
The surface exposed by macromolecules to their aqueous environment consists mainly of hydrophilic domains that attract water dipoles [26,29]. However, superficial apolar domains also induce the perturbation of structure of water in contact with them, generally into a clathrate-like form. According to Wiggins and MacClement [39], significant amounts of water are retained in a highly ‘structured form’ inside hydrophobic pockets. The same authors defined this water, as ‘structured water’, this water being commonly designated as interfacial or (vicinal) water [15]. Because this form of water generally is difficult to extract, it is also called ‘bound water’. The properties of this water have been described extensively [6,15,25,38,39]. This distinction in terms of two classes of water, bound water and free water, has been often used.
Hydrophilic–hydrophobic interactions control the equilibrium of biological systems. It is worth noting that, in the presence of biological macromolecules, such as peptides, enzymes, proteins, DNA, all these behaviours can be found depending on the more or less hydrophilic or hydrophobic nature of each site or residue. Bonds, in particular, play a major role in the structure of these macromolecules.
The dynamics of water molecules on the surface of a protein [8] is slowed down. A similar behaviour is observed for water confined in a porous hydrophilic model system [8] (see Figs. 3 and 4).
Why is it so important to understand the effects of water on the shapes of biological molecules and/or that of biological molecules on water? On a practical level, understanding the relationship between structure, dynamics, and function of biological molecules in water may one day help researchers design new drugs that act by blocking or enhancing various biochemical pathways.
In cells, because of the considerable macromolecular crowding and the high values of the macromolecular surface/volume ratio, the properties of water are influenced by hydrophilic/hydrophobic interactions [25]. Water molecules, because they are electric dipoles, are attracted and oriented in the electric fields produced by polar or charged domains of macromolecules. In the region of apolar domains, which have little interaction with water, water molecules organise themselves in special structures, in order to compensate for the absence of equilibrium that exists at the frontier. In all cases, this water adopts a very different structure from that of bulk water. Similarities with water in model systems (Vycor, carbon powder, at low hydration) are observed.
6 Conclusion
From the present results, it appears that the structure and the dynamics of water are deeply perturbed when hydrophilic or hydrophobic interactions, or both, are developed in a medium. From the more recent findings, combining various techniques and molecular dynamics simulations, one gets the following picture of confined water. Water, in the vicinity of a hydrophilic surface, is in a state equivalent to bulk water at a lower temperature. As previously demonstrated, the structure and dynamics of confined water depend on the degree of hydration of the sample. In particular, at room temperature, interfacial water shows a slow dynamics similar to that of bulk water at a temperature 30 K lower, such that it behaves like bulk supercooled water.
In order to understand the microscopic origin of the confinement and slowing down of motions of water molecules and the exact role played in this context, the theory of kinetic glass transition in dense supercooled liquids [12,19] has been recently used. This theory leads to some description of the dynamics of confined water in terms of correlated jump diffusion [10] instead of jump diffusion [37]. This description looks consistent with molecular dynamics simulations of supercooled water [18] and has been confirmed by high-resolution quasi-elastic neutron scattering experiments of water from hydrated Vycor [41] and from hydrated C-phycocyanin protein [13].
This more sophisticated way shows a large distribution of residence times for water molecules in the cage formed by the neighbouring molecules, which is a more realistic view than the sharp separation of water molecules into two classes, according to their mobility [8]. Short-time dynamics results about hydrated myoglobin have been recently interpreted by using this same theory of kinetic glass transition in dense supercooled liquids [30].


