1 Introduction
The Sr isotope ratio is a geochemical tracer widely used in Surface Earth Sciences to determine the nature and the origin of weathering fluxes carried by rivers (e.g., [1,14,25,34]). More recently, several studies have shown the potential of the (234U/238U) activity ratio to attain similar objectives (e.g., [5,7,9,13,29,30,36]). Nevertheless, such applications impose to characterise precisely the influence of the rainfall contributions on the U and Sr budget of river waters, which in turn requires a better understanding of the U and Sr systematics in rain waters.
Previous studies dealing with Sr in rainwater (e.g., [12,15,18,23–25]) pointed out the existence of quite important spatial and temporal variations of the 87Sr/86Sr ratio. Such variations imply the contribution of several Sr sources in rainwater. Identification and quantification of the contribution of these different sources is important for a precise estimate of rainfall contribution on Sr budget of river water. They could also be very informative to better constrain the chemical dynamics within rainfalls (see [12]). Compared to Sr, only a few studies dealt with uranium in rainwater (e.g., [6,20,21]). Some studies outline the low U content of rainwater and suggest an insignificant contribution on the U budget of river water (e.g., [7,30]). More systematic investigations are now required to confirm this point, and more generally, to constrain the origin of U in rainfall, and the parameters that control its (234U/238U) activity ratio.
The aim of this paper is to present results of the first investigation of spatial and temporal variations of U and Sr isotope ratios in rainwater performed at a regional scale. This study, which is conducted by analysing rainwater samples in Alsace (eastern France) and Luxembourg, brings new constrains on parameters controlling the U and Sr isotope variability in rainwater.
2 Sample locations and previous studies
Rain samples were collected in three different locations (Fig. 1) marked by quite contrasted climatic and pollution conditions. The first site is the Aubure site, a small-forested watershed (80-ha area) developed on granitic bedrocks and used since 1986 as an environmental observatory (http://ohge.u-strasbg.fr). It is located at a mid altitude in the Vosges Mountains, 60 km southwest of Strasbourg, in a zone quite well preserved from direct pollution sources. For the study, Aubure rainwater was collected in a meadow/glade close to the outlet of the watershed at 880 m height. The second sampling location is an urban site, in Strasbourg (140 m a.s.l.). The samples were collected in the Botanical Garden of the Strasbourg University, and also on the roof of the ‘Centre de géochimie de la surface’ (CGS) of the Strasbourg University. Both locations are close to each other and located about 1 km from the centre of the town and 2 km from industrial zones. The third sampling location is a peri-urban site located in an area of well developed industrial activity in Esch-sur-Alzette (20 km southwest of Luxembourg City) in Luxembourg, about 2 km away from industrial area with iron and steel industry as well as cement works.

Location of rainwater samples.
Localisation des échantillons d'eau de pluie.
Several studies have already presented geochemical data on rainwater collected in the same locations as for this work. Thus, Sanusi et al. [32] gave a first insight into the chemical variability of rainwater in eastern France, by studying rainwater from several locations, including Strasbourg and Aubure. Herckes [16] and Herckes et al. [17] made a detailed study of the chemical composition (major elements and trace elements) in rain- and fog-falls in the same three locations as for our study. In addition, for the Aubure site, several other studies gave analyses of rainwater for major element concentration and also for trace elements (Sr, REE) and Sr or O isotope ratios (e.g., [2–4,19,26,27]). Sampling location for these previous studies was nevertheless different from that chosen in the present study, as rainwater was collected on the top of the experimental Strengbach watershed, i.e. at a 300-m higher altitude. The new data presented for this work certainly constitute one of the first combined U and Sr isotope studies never performed at a regional scale. Several of the samples collected for our studies have been used for a study of variations of Ca isotopes in rainwater [33].
3 Sampling procedure and analytical methods
All the rain samples from Esch-sur-Alzette and a part of those from Strasbourg (i.e. samples collected in the Botanical Garden) were collected with an automated wet-only precipitation collector classically used for the WMO–GAW (World Meteorological Organisation–Global Atmosphere Watch) network, and more specifically used in this case for a survey of rain- and fog-water geochemical composition in Alsace and Luxembourg [16]. The other precipitation samples from Strasbourg (i.e. samples collected on the CGS roof) and all samples from Aubure were collected with permanently open collectors. At Aubure, Herckes [16] and Herckes et al. [17] verified that no bias was induced due to these different sampling procedures for the major element and metal results. The open collectors used for this study were composed of a plastic funnel (40-cm diameter) above a polypropylene container, both intensively cleaned by distilled HCl and distilled water. They were fixed on the ground with an elevation of the funnel from the ground of approximately 80 cm. The sampling duration ranged from one week to two months in order to collect for each sample several water litres (2 to 8 l).
All the rainwater samples were filtered at 0.22 μm in the laboratory after collection. Aliquots for analyses of trace-element concentrations, as well as for analyses of U and Sr isotope ratios were stored in polypropylene bottles, previously cleaned with concentrated HCl, and acidified with double distilled HCl to a pH of 1. A non-acidified aliquot of filtered water was stored in polyethylene bottles to determine the major element concentration, the alkalinity, the conductivity and the dissolved organic carbon (DOC) content, following the techniques classically used at the CGS (see for instance [29]). Due to the very low trace-element concentration, U, Rb, Ba, Sr and metal concentrations were determined at CRPG using a SCIEX/ Perkin ELAN 6000 ICP–MS, as reported by Simonetti et al. [35]. Related uncertainties are generally better than 10%, except U, for which very low concentrations were measured so that uncertainty mostly range between 10 and 20%. Accuracy was controlled by repeated analyses of the SLRS-4 reference material.
The Sr isotopic compositions were determined on a sample volume equivalent to 150 ng to 1 μg of Sr, following the procedure used at the CGS (e.g., [29]). The regular measurements of the NBS 987 standard gave a mean Sr isotope ratio of (, ). The U isotope ratios (234U/238U) were also analysed at CGS on a VG Sector mass spectrometer equipped with a Daly detector operating in analogical mode, on 2 to 10 ng of U following the technique detailed in [29]. Due to the low U concentration in rainwater about 2 l of water or more are required for the analysis. Because of the low amount of U recovered for the analyses, analytical uncertainty ranges between 2 and 4% ( error). Regular measurements of the HU1 U reference material over the analyses period give a mean (234U/238U) a.r. of (, ) (see discussion in [8] for more details). The blanks of the whole procedure were less than 40 pg U.
4 Geochemical variations and their origin
Major element data are reported in Table 1 for the three studied sites, while trace elements, uranium and strontium isotope data are given in Table 2.
Major species contents in the rainwater of Strasbourg, Aubure and Esch-sur-Alzette. Data are expressed in μmol l−1
Concentration en éléments majeurs des pluies de Strasbourg, Aubure et Esch-sur-Alzette. Les données sont exprimées en μmol l−1
| Sample | Sampling period | pH | Na+ | K+ | Mg2+ | Ca2+ | Alc + H+ | Cl− | H4SiO4 | |||
| STRASBOURG | ||||||||||||
| EPS 0 | 26/9–5/10/98 | 4.79 | 51 | 8 | 4 | 3 | 10 | 1 | 11 | 26 | 37 | 1 |
| EPS 1 | 22/10–30/10/98 | 6.29 | 7 | 9 | 1 | 2 | 13 | 18 | 11 | 6 | 5 | <dl |
| EPS 2 | 30/10–10/11/98 | 5.38 | 27 | 19 | 2 | 4 | 16 | 2 | 22 | 19 | 29 | <dl |
| EPS 3 | 10/11–21/11/98 | 4.43 | 25 | 8 | 1 | 1 | 9 | 0 | 15 | 20 | 39 | <dl |
| EPS 4 | 26/3–12/4/99 | 6.29 | 92 | 16 | 5 | 6 | 31 | 25 | 22 | 36 | 67 | <dl |
| EPS 5 | 18/3–17/5/99 | 6.26 | 71 | 11 | 3 | 3 | 19 | 21 | 13 | 23 | 48 | <dl |
| EPS 6 | 17/5–9/7/99 | 5.99 | 53 | 5 | 5 | 4 | 25 | 14 | 8 | 22 | 40 | 2 |
| EPS 7 | 12/8–8/10/99 | 5.89 | 49 | 6 | 2 | 2 | 12 | 10 | 8 | 17 | 29 | 1 |
| AUBURE | ||||||||||||
| EDP AE 1/2 | 9/10–19/10/98 | 5.54 | 11 | 16 | 5 | 5 | 12 | 3 | 17 | 13 | 17 | <dl |
| EPA 1 | 2/11–11/11/98 | 4.67 | 6 | 15 | 1 | 2 | 2 | 0 | 21 | 8 | 14 | <dl |
| EPA2 | 11/11–1/12/98 | 6.28 | <dl | 40 | 31 | 11 | 26 | 20 | 60 | 22 | 19 | <dl |
| EPA 3 | 11/5–8/6/99 | 5.79 | 33 | 5 | 7 | 2 | 5 | 19 | 6 | 10 | 14 | <dl |
| EPA 4 | 29/6–18/7/99 | 5.02 | 39 | 8 | 5 | 1 | 3 | 0 | 7 | 18 | 23 | <dl |
| ESCH | ||||||||||||
| EPL 1 | 19/3–2/5/99 | 6.8 | 25 | 22 | 4 | 12 | 91 | 121 | 25 | 27 | 39 | 15 |
| EPL 2 | 21/6–9/7/99 | 6.95 | 40 | 10 | 4 | 8 | 47 | 84 | 12 | 18 | 26 | 6 |
| EPL 3 | 26/7–6/10/99 | 6.7 | 26 | 12 | 8 | 6 | 31 | 47 | 13 | 14 | 24 | 70 |
Trace-element concentrations and U and Sr isotopic composition in the rainwater of Strasbourg, Aubure and Esch-sur-Alzette. Concentrations are expressed in μg l−1. The uncertainty on Sr isotopic measurements is 0.0015% (2 σ). (234U/238U) activity ratios were calculated from the measurement of the 234U/235U isotopic ratio and by using a 235U/238U isotope ratio of 137.88
Concentrations des éléments en trace et composition isotopique de l'U et du Sr des eaux de pluie de Strasbourg, d'Aubure et d'Esch-sur-Alzette. Les concentrations sont exprimées en μg l−1. Les incertitudes sur la mesure des rapports isotopiques du Sr sont de 0.0015% (2 σ). Les rapports d'activité (234U/238U) ont été calculés à partir de la mesure du rapport 234U/235U et en admettant un rapport isotopique 238U/235U de 137,88
| Sample | U | Rb | Ba | Sr | Cd | Cr | Co | Cu | Mn | V | Zn | Al | (234U/238U) | 87Sr/86Sr | |
| STRASBOURG | |||||||||||||||
| EPS 0 | 0.18 | 3 | 1.22 | 0.33 | 0.20 | 3.68 | 3.41 | 25 | 1.185 | 0.020 | 0.70880 | ||||
| EPS 1 | 0.0005 | 0.06 | 1.82 | 1.3 | 0.01 | 0.04 | 0.06 | 0.13 | 0.88 | 0.34 | 5.0 | 0.26 | 1.143 | 0.015 | 0.70860 |
| EPS 2 | 0.0020 | 0.09 | 5.02 | 2.14 | 0.04 | 0.13 | 0.40 | 11.0 | 4.60 | 0.74 | 21.5 | 3.7 | 1.148 | 0.017 | 0.70891 |
| EPS 3 | 0.0050 | 0.07 | 2.9 | 1.1 | 0.22 | 0.13 | 0.22 | 1.78 | 2.85 | 1.20 | 14.0 | 12.9 | 1.104 | 0.015 | 0.70889 |
| EPS 4 | 0.0013 | 0.32 | 12.35 | 4.1 | 0.08 | 0.13 | 0.35 | 1.61 | 9.1 | 1.05 | 30 | 3.65 | 1.385 | 0.011 | 0.70881 |
| EPS 5 | 0.0014 | 0.26 | 4.31 | 2.54 | 0.08 | 0.11 | 0.04 | 1.00 | 1.91 | 0.63 | 10.0 | 3.57 | 1.121 | 0.025 | 0.70881 |
| EPS 6 | 0.0011 | 0.21 | 6.31 | 2.34 | 0.19 | 0.13 | 0.12 | 1.05 | 7.73 | 0.48 | 18.5 | 3.91 | 1.304 | 0.025 | 0.70898 |
| EPS 7 | 0.70904 | ||||||||||||||
| AUBURE | |||||||||||||||
| EDP 0 | 1.6 | 2.4 | 1.3 | 0.60 | 0.10 | 6.00 | 2.30 | 4.3 | 1.170 | 0.020 | |||||
| 1.158 | 0.020 | ||||||||||||||
| EPA 1 | 0.0040 | 0.12 | 3.6 | 0.53 | 0.20 | 0.04 | 0.02 | 1.27 | 4.90 | 0.20 | 6.6 | 3.3 | 1.177 | 0.008 | 0.71080 |
| EPA2 | 0.0035 | 4.77 | 3.99 | 6.26 | 0.07 | 0.14 | 0.05 | 1.65 | 21 | 0.29 | 23 | 1.43 | 0.71200 | ||
| EPA 3 | 0.0009 | 1.98 | 2.32 | 0.71 | 0.02 | 0.05 | 0.02 | 0.52 | 6.40 | 0.21 | 4.8 | 1.77 | 1.170 | 0.022 | 0.71459 |
| EPA 4 | 0.0005 | 0.93 | 2.12 | 0.38 | 0.05 | 0.07 | 0.02 | 0.99 | 4.63 | 0.19 | 19.5 | 1.65 | 1.178 | 0.032 | 0.71295 |
| 1.181 | 0.021 | 0.71350 | |||||||||||||
| ESCH | |||||||||||||||
| EPL 1 | 0.0009 | 0.33 | 8.83 | 7.35 | 0.16 | 0.22 | 0.02 | 1.24 | 11.5 | 1.54 | 69 | 5.4 | 1.053 | 0.012 | 0.70881 |
| EPL 2 | 0.0022 | 0.25 | 13.82 | 4.46 | 0.16 | 0.14 | 0.01 | 1.27 | 5.90 | 1.02 | 29 | 5.56 | 1.107 | 0.018 | 0.70905 |
| EPL 3 | 1.34 | 1.065 | 0.013 | 0.70903 |
4.1 Chemical composition
Major element concentrations as well as trace element concentrations of rainwater analyzed for our study compare quite well with previously published data for the same studied sites [16,17]. The data therefore confirm that the chemical composition of these rainwater samples departs from seawater chemical composition (Fig. 2), implying the contribution to these rainwater samples of continental components of natural and/or human origin. For instance, the high amounts of , and in Alsatian rainwater, as well as the high or ratios (Fig. 2) result from the incorporation into and/or reaction with rainwater of nitrogenous and sulfur compounds of industrial and automobile origin [17,32]. Similarly, the high trace metal enrichments measured in these samples especially for Cu, Zn and Cd (Table 3) can be related to the heavily industrialized character of western Europe, close to the observations for rainwater of industrialized zones of northeastern America [35]. These metals (Cu, Zn, Cd) were also reported in excess (relative to Al) in lichens from the Vosges Mountains [10]. Combined to Pb isotope data, an anthropogenic origin was suggested for these metals measured in lichens. The second information that our data confirm is that the different study sites have quite contrasted chemical characteristics. Thus, pH of rainwater is higher in Esch-sur-Alzette than in the two other sites, and the dominant cations and anions are different. In the Esch-sur-Alzette rainwater, the dominant cations and anions are Ca2+ and , respectively, whereas and, at a lower degree, Ca2+ are the dominant cations in Aubure and Strasbourg rainwater, and and the dominant anions. The origin of the high level of Ca2+ and in Esch-sur-Alzette rainwater was discussed by Herckes [16]. It could result from the input of carbonates particles into rainwater from neighbouring industries, i.e. iron and steel works, cement works. Such carbonate inputs would also explain the relatively high concentrations of this rainwater in Sr and Ba [16]. Finally, the third information brought by our data is a confirmation that the U concentration of rainwater is still very low, much lower than the U concentration found in river water of studied geographical area [13,29], with concentration around a ppt or a few ppt in rainwater of the three sites (Table 2).

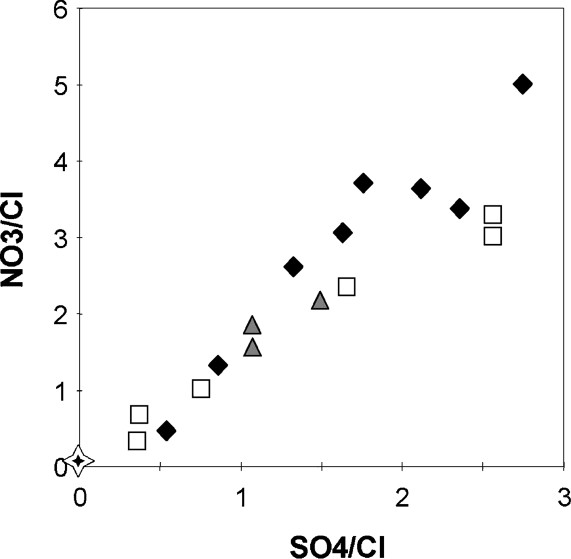
Variations of chloride and sodium concentrations in the rainwater of Strasbourg (black diamonds) Aubure (white squares), Esch-sur-Alzette (grey triangles) and comparison with the domain of seawater-like rain composition as defined by Möller [22], i.e. molar Na/Cl ratios between 0.5 and 0.876.
Variations des concentrations en Cl et Na des eaux de pluie de Strasbourg (losanges noirs), Aubure (carrés blancs), Esch-sur-Alzette (triangles gris) et comparaison avec la composition des eaux de pluie d'origine marine donnée par Möller [22], i.e. Na/Cl entre 0,5 et 0,876.
Average of metal-enrichment factor (EFc) in rainwaters by sampling site, following the definition given in Duce et al. [11] , using the Rudnick and Gao's compilation for the mean crustal abundance [31]
Moyenne des facteurs d'enrichissement (EFc) en métaux des pluies par site d'échantillonnage, calculés selon la méthode donnée par Duce et al. [11] , et en utilisant la compilation de Rudnick et Gao [31] pour la composition chimique moyenne de la croûte continentale
| Sampling sites | Cd | Cr | Co | Cu | Mn | V | Zn |
| Strasbourg (N=6) | 24 034 | 44 | 388 | 2232 | 2 | 306 | 8501 |
| Aubure (N=5) | 44 224 | 40 | 83 | 1770 | 5 | 173 | 9939 |
| Esch/Alzette (N=2) | 26 445 | 29 | 13 | 667 | 2 | 197 | 10 945 |
4.2 Variations of Sr and U isotope ratios
The Sr and U isotopic data confirm that rainwater from the three studied sites have geochemical characteristics different from seawater and different from one site to another, as illustrated in the plot of (234U/238U) activity ratio against 87Sr/86Sr isotope ratio, where the three sites define three very different variation domains (Fig. 3). The Aubure rainwater exhibits variable Sr isotope ratios (between 0.71080 and 0.71459) systematically higher than the seawater ratio, and quite constant U activity ratios slightly superior to seawater. By contrast, rainwater from the two other sites (Strasbourg and Esch-sur-Alzette) have much less variable Sr isotope ratios with values inferior to seawater and quite variable U activity ratios with USr variation trends different for the two sites. These results certainly outline the important role of local components to explain the geochemical characteristics of rainwater at a regional scale. The origin of the variation for each site is discussed below.
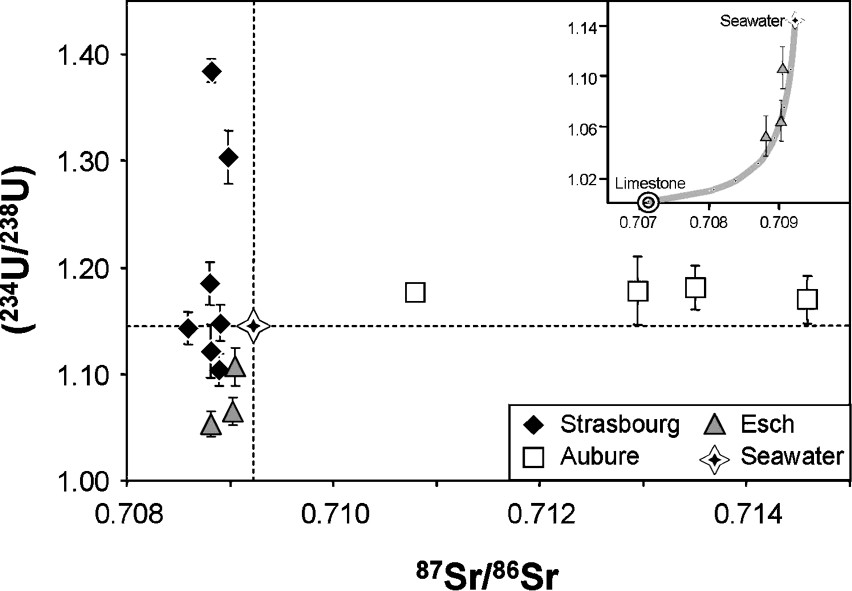
(234U/238U) activity ratios versus 87Sr/86Sr isotope ratios in the rainwater of Strasbourg (black diamonds) Aubure (white squares), Esch-sur-Alzette (grey triangles); star: seawater. Insert: The USr isotope variations of the Esch-sur-Alzette rainwater can be explained as a mixing between a seawater-like end-member and a carbonate end-member with USr characteristics of a Jurassic carbonate in secular (234U/238U) equilibrium (i.e., [U] = 2 ppm, [Sr] = 400 ppm, 87Sr/86Sr = 0.707 and (234U/238U) = 1).
Rapports d'activité (234U/238U) en fonction des rapports isotopiques 87Sr/86Sr dans les eaux de pluie de Strasbourg (losanges noirs), Aubure (carrés blancs), Esch-sur-Alzette (triangles gris) ; étoile : eau de mer. Insert : la variation USr des pluies d'Esch-sur-Alzette peut s'expliquer par un mélange entre un pôle de type eau de mer et un pôle de type carbonate proche d'un carbonate jurassique à l'équilibre séculaire (i.e., [U] = 2 ppm, [Sr] = 400 ppm, 87Sr/86Sr = 0,707 et (234U/238U) = 1).
4.3 Esch-sur-Alzette
The previous study by Herckes [16] proposed that the Esch-sur-Alzette rainwater is marked by the dissolution of carbonate particles, a large part of them could be emitted by local industry such as cement works. The chemical data obtained in our work for the Esch-sur-Alzette rainwater is consistent with carbonate aerosol dissolution. The U and Sr isotope data can also be interpreted in this way. Indeed, the Sr isotope ratios of the three analysed rainwater are inferior to seawater value, which is consistent with an input into rainwater of Sr from dissolution of carbonate dusts coming from old marine sediments, as used by local industry (iron and steel works, cement works). Furthermore, such carbonate dust dissolution should not fractionate the 234U and 238U isotopes and will therefore induce a concomitant U flux with no or only very limited U disequilibrium – (234U/238U) activity ratios close to 1. This explains therefore the U activity ratios of the Esch-sur-Alzette rainwater lower than seawater value and also the USr covariation that seems to appear in the USr diagram (Fig. 3). Interpretation of this covariation in terms of mixing between an end-member with seawater U and Sr isotope ratio characteristics and a carbonate end-member close to carbonates found in the Paris Basin allows us to infer that around 10% of Sr and between 30 to 60% of U of these waters would be controlled by dissolution of the carbonate dusts (insert in Fig. 3).
4.4 Aubure
The Sr isotope ratios of the Aubure rainwater are characterized by radiogenic values higher than the seawater value. These rainwater data, collected in 1998 and 1999, are in the range of the Sr data published by Aubert et al. [3] for a similar collection period (1997–1998), but at a higher altitude. On the other hand, they are higher than the Sr data reported by Probst et al. [27] for rainwater collected between 1989 and 1992 (Fig. 4a). All these samples have nevertheless Sr values superior to the seawater value, which indicates the contribution to the Aubure rainwater of a radiogenic Sr source. The simplest explanation would be the contribution of a Sr input of dusts from local granitic bedrocks and soils that are radiogenic (e.g., [3]). Nevertheless, the lack of a simple relationship between the Sr isotope ratios and the rainwater Al concentrations or the Al/Sr ratios (which, in first approximation, can be used as an index of dust concentrations) questions this first interpretation. Moreover, the high Sr isotope ratios of our dataset is associated with quite high Ca/Sr and K/Sr ratios, which are different from the ratios found in the Aubure soils and granite, but much closer to the value classically found in plants and throughfall, as well illustrated in Fig. 4a and b. Such observations are certainly strong arguments to consider radiogenic Sr in Aubure rainwater as a result of recycling of local Sr through plants by a mechanism that remains to be defined, but that could certainly be plant exudation. The fact that for our samples the highest Sr isotope ratios are found in the rainwater samples collected in summer and fall reinforce this point. The observation that the rainwater data obtained by Probst et al. [27] for samples collected between 1989 and 1992, that is at a time when the trees were much less developed at the top of the site than today, have less radiogenic Sr values (Fig. 4a), agrees also with such an interpretation. At least, variations of Ca isotope data from Aubure rainwater are also consistent with this scenario [33]. Nevertheless, when all Aubure rainwater samples are considered, it appears that the Sr from the Aubure rainwater cannot be only explained by a simple binary mixing between a seawater-like end-member and a ‘plant’ end-member, but that a third end-member with low Sr isotope value and high Ca/Sr ratios has to be involved (Fig. 4a and b). This third end-member has geochemical characteristics quite close to sedimentary carbonates, as observed in the Esch-sur-Alzette samples, and can therefore be assimilated to a Sr flux from sedimentary carbonate dusts, which must come from outside the granitic Aubure watershed. The Sr isotopic data of Aubure rainwater confirm therefore the multiple origins of chemical components carried by rainwater with a local component introduced into the rain through the vegetation, and two other end-members of more regional origin, which are external to the Aubure watershed.
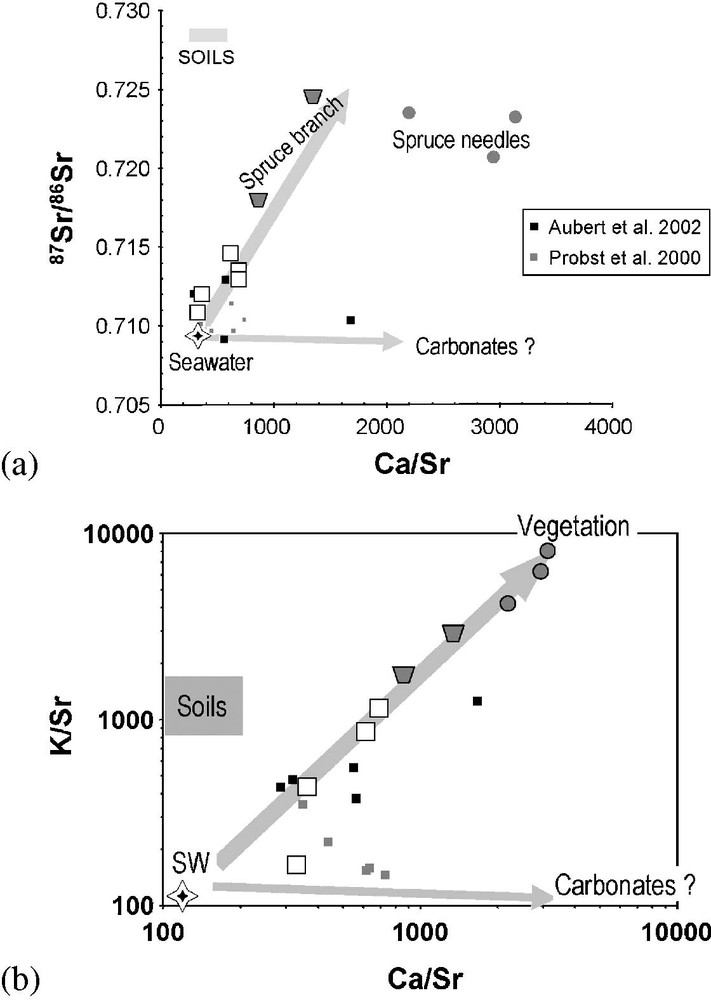
(a) Variations of Sr isotope ratios in Aubure rainwater against Ca/Sr ratios. (b) Variations of molar K/Sr ratios against molar Ca/Sr ratios for Aubure rainwater samples (Spruce branch and needles data: Pierret et al., unpublished data).
(a) Variations du rapport isotopique du Sr dans les pluies d'Aubure en fonction du rapport molaire Ca/Sr. (b) Variations du rapport molaire K/Sr en fonction du rapport molaire Ca/Sr dans les pluies d'Aubure (Branches et aiguilles d'épicéas : Pierret et al., unpublished data).
Compared to the Sr isotope data, the U activity ratios of Aubure rainwater is constant, within the analytical precision of 2 to 4%, implying either a unique U flux into the rainwater or several fluxes with very similar signatures. On the basis of our study, it is difficult to choose between these two possibilities; nevertheless, the fact that U concentrations of the Aubure rainwater, even if very low, decrease when the Sr isotopic ratios or the Ca/Sr ratios increase would suggest that the plant contribution is very low in the U budget of Aubure rainwater. The U activity ratios of the Aubure rainwater would therefore correspond to a regional geochemical background, whose mean value could indicate that it is slightly different from a purely seawater origin.
4.5 Strasbourg
Compared to Aubure rainwater, the Strasbourg precipitations are marked by much more variable (234U/238U) activity ratios and much less in 87Sr/86Sr isotope ratios, with U values between and , and 87Sr/86Sr isotope ratios between 0.70860 and 0.70904 (Fig. 3). Similarly to the two other localities, the geochemical characteristics of the Strasbourg rainwater cannot be explained by the contribution of only one geochemical end-member. The scatter of the Strasbourg data points in the 87Sr/86Sr vs. Na/Sr or Ca/Sr diagrams implies the contribution of at least three different Sr sources to the Strasbourg rainwater. If we assume the contribution of a Sr seawater end-member in the Sr budget of this rainwater, as proposed by the works by Herckes [16] and Sanusi et al. [32], then two other end-members with lower Sr isotope ratios have to be invoked (Fig. 5a). From their Sr isotope ratios, these two end-members could correspond to an input of aerosol of carbonate origin. The U activity ratios suggest nevertheless that this aerosol contribution cannot be identified with dissolution of carbonate aerosols, since in this case we should expect, as observed in the Esch rainwater samples, a decrease in the U activity ratios with an increase in the carbonate dissolution. Such a covariation is not observed with our data. On the contrary, the samples with high Ba/U ratios are marked by high U activity ratios (Fig. 5b). Determination of the origin of this material is not obvious on the basis of the lone data. It would be tempting to consider these end-members (or one of them) as representing chemical fluxes linked to human activity (automobile emission, particles from local incinerators, etc.), especially because samples like EPS6 or EPS1 exhibit quite high enrichment factors in some metals (Cd, Mn) (Table 3). Nevertheless, we are conscious that the low number of data does not allow us, at this time, to give a clear answer to this point, and that a detail study of rainwater at the scale of the Alsace plain is now required to resolve this question. Such a study should also help to understand the processes accounting for U activity ratios in rainwater higher than in seawater.
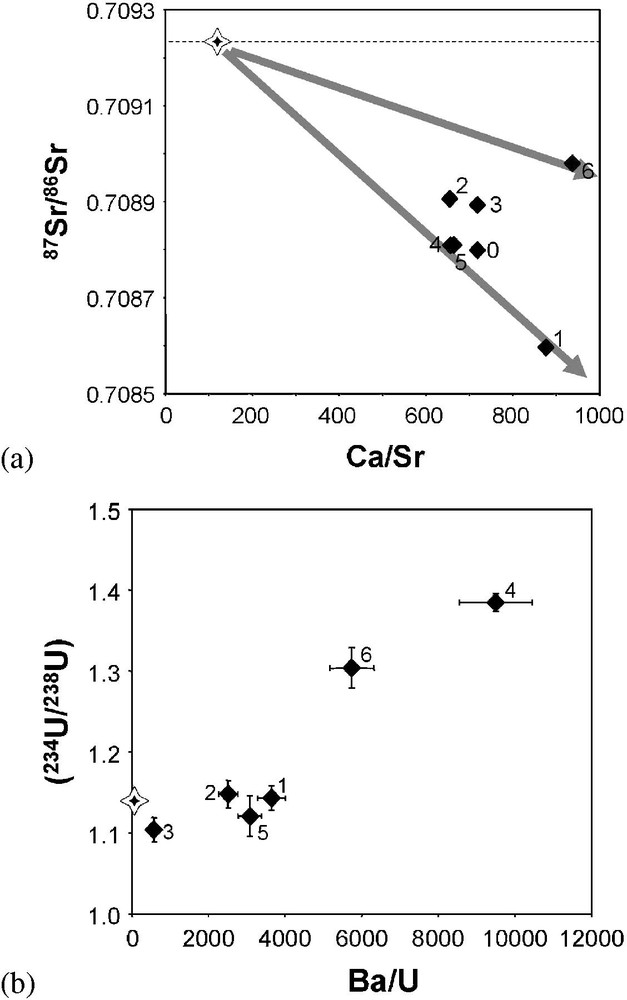
Sr and U isotope variations in Strasbourg rainwaters. (a) Variations of Sr isotope ratios against molar Ca/Sr ratios. (b) Variations of U activity ratios against Ba/U ratios.
Variations des rapports isotopiques du Sr et de l'U dans les eaux de pluie de Strasbourg. (a) Variations du rapport isotopique du Sr en fonction du rapport molaire Ca/Sr. (b) Variations du rapport d'activité de l'U en fonction du rapport Ba/U.
5 Implications and conclusions
The study presented in this work outlines a quite large spatial and temporal variation of chemical and isotopic compositions of rainwater at a regional scale. It also suggests an important influence of local chemical inputs on the creation of the spatial variability of rainwater chemistry. In quite densely populated and/or industrialized areas the local component(s) is (are) certainly related to the human and industrial activity. This seems to be well documented in the Esch-sur-Alzette rainwater. By contrast, in scarcely populated and forested areas such as at Aubure, the vegetation through the canopy would be an important source of elements in rainwater, inducing into the rainwater a local terrigenous flux, which significantly influences the budget of alkali and calc-alkali. As a corollary, in forest watersheds, such as the Aubure watershed, a precise estimate of the rainwater contribution in the chemical budget of streamwaters should require as a first prerequisite a precise estimate of the recycling flux related to the vegetation. This should be crucial for elements such as K, Rb, Ca, and should be taken into account for all studies willing to model the biogeochemical functioning of forest ecosystems. Furthermore, as our data outline that there is an important time variation of the Aubure chemical and Sr isotopic composition, determination of the recycling component (as well as the two other components) in the Aubure rainwater will necessitate a detailed study of the variation of chemical and isotopic composition of the rainwater over at least one hydrological cycle. In addition, the large spatial variability of chemical composition of rainwater, pointed out by our study, could also question the reliability of the method classically used to estimate the rainwater contribution on the river chemical budget. This should be crucial when the considered watersheds are constituted by very contrasted bedrocks, and/or are marked by an inhomogeneous anthropogenic activity within the watershed.
Another important result of our study is the systematically quite low U concentration of rainwater, whatever the sampling location. This observation confirms the a priori low, even insignificant, contribution of wet deposition to the U budget of river waters, as already outlined in previous studies (e.g., [28–30]). The U isotopic data obtained for the Aubure rainwater certainly gives a definitive demonstration of this point. Indeed, Riotte and Chabaux [29] observed a decrease in U activity ratio in the dissolved load of the Strengbach stream at the outlet of the watershed from 1.02 to 0.9, when the discharge of the stream increases. The U activity ratios obtained for the Aubure rainwater, with values systematically higher than 1.14, clearly demonstrate that the increase of rainwater contribution cannot result in a decrease in the (234U/238U) ratio of the streamwater. The variation of the U activity ratio, when the stream discharge increases, represents therefore the variable contribution of at least two different U sources from soils and rocks. This result thus confirms the interesting property of U to be a geochemical tracer specific of weathering fluxes carried by rivers.
Acknowledgements
We would like to thank D. Million for his help in measuring major element concentrations and Sekhar Muddu for his comments on the manuscript. P.H. was supported by the ‘Ministère de la Recherche du Luxembourg’. We are grateful for the help of the ‘Administration de l'environnement du Luxembourg’ for sample collection in Esch-sur-Alzette. The reviews by Mathieu Roy-Barman, Sophie Ayrault and Bassam Ghaleb are highly appreciated. This is a CGS–EOST contribution.


