1 Introduction
Silicon (Si) is the second most abundant element (after O) in the Earth's crust (Epstein, 1999), making up 27% of the lithosphere by mass. Silicon is a key component of global biogeochemical cycles. As an essential nutrient for photosynthetic marine diatoms (Yool and Tyrrell, 2003), it indirectly plays a major role in governing the oceanic CO2 storage capacity (Smetacek, 1999). Eighty-five percent of the dissolved silicon (DSi) supplied to the ocean is delivered by rivers (Tréguer et al., 1995; Fig. 1). The riverine dissolved load originates for ∼45% from chemical weathering of continental silicate rocks (Stumm and Wollast, 1990). Importantly, chemical weathering of Ca and Mg silicate rocks controls atmospheric CO2 concentration, thus regulating climate on geological time scales (Berner, 1995).

Schematic diagram of the silicon (Si) biogeochemical cycle showing the main reservoirs and processes involved in the continental Si transfer to the ocean. BSi = biogenic Si, DSi = dissolved Si. Plant and oceanic BSi production values are from Conley (2002) and Tréguer et al. (1995), respectively.
Représentation schématique du cycle biogéochimique du silicium (Si) indiquant les principaux réservoirs et les processus impliqués dans le transfert du silicium des continents vers les océans. BSi = Si biogénique, DSi = Si dissous. Valeurs de production de BSi par la végétation et par les diatomées tirées de Conley (2002) et de Tréguer et al. (1995), respectivement.
Time, lithology, climate, vegetation and erosion are the key factors, which govern chemical weathering rate (mass change km−2 year−1) of silicate rocks, thereby controlling continental chemical fluxes to the ocean (Gaillardet et al., 1999a; White, 2011). Time or the age of the soil parent material dictates the duration of the weathering reaction, while lithology affects the solubility of silicate rocks (e.g., basalt weathers more rapidly than the other continental silicate rocks; Dessert et al., 2003). Climate agents such as rainfall and temperature enhance weathering reactions. Vegetation also increases weathering rates; solute fluxes exported from vegetated areas are about four times larger than those measured in barren lands (Moulton et al., 2000). By continuously supplying fresh particulate materials that can partially dissolve, erosion constitutes an important component of the regional and global chemical weathering fluxes (Gaillardet et al., 1999b; West et al., 2005).
Knowledge of the weathering processes controlling continental transfer of DSi to the ocean is fundamental to the estimate of the long-term atmospheric CO2-budget. Until the late 1980s, continental DSi fluxes were thought to be controlled exclusively by abiotic processes of silicate weathering and clay mineral neoformation (Drever, 1988). However, it is now acknowledged that the accumulation of Si in plants as biogenic opal (or phytolith; Alexandre et al., 1997; Lucas, 2001) represents a non-negligible source of DSi in rivers (Derry et al., 2005). The production of biogenic Si (BSi) by vegetation (∼60–200 Tmol.yr−1; Conley, 2002) is in the same range as that originating from marine diatoms (∼240 Tmol. yr−1; Tréguer et al., 1995), and is significant relative to the annual riverine Si supply to the ocean (∼5 Tmol.yr−1; Tréguer et al., 1995; Fig. 1). All together, a better understanding of biogeochemical Si transfers within the Earth's Critical Zone (CZ), which is defined as the soil-plant-water system occupying the volume extending from the upper limit of vegetation down to the lower limit of groundwater (Brantley et al., 2007), can shed new light on the factors which control continental DSi weathering fluxes.
Stable Si isotope (28Si, 29Si, and 30Si) studies offer an exciting avenue for investigating the complex abiotic and biotic controls on continental DSi fluxes. Significant fractionation of Si isotopes occurs during processes such as secondary mineral formation and plant uptake (Ding et al., 2005; Georg et al., 2007a; Opfergelt et al., 2006; Ziegler et al., 2005a). The past 10 years have seen a sharp increase in Si isotope measurements in various matrices (rock, soil, plant, water), thanks to the advent of high precision mass spectrometers. The range of Si isotopic compositions found in a variety of terrestrial materials and in the Earth's surface Si reservoirs has been compiled by several authors (Basile-Doelsch, 2006; Ding et al., 1996; Douthitt, 1982). More recently, Cornelis et al. (2011) discussed the application of Si isotopes to assess the origin of the DSi flux exported from soil-plant systems towards rivers. Reynolds (2011) emphasised the analytical requirements needed to perform routine measurements of Si isotope compositions in terrestrial samples.
The effect of weathering processes on the distribution of Si isotopes in soils and in rivers has been examined at the soil profile (Bern et al., 2010; Cornelis et al., 2010; Opfergelt et al., 2010; Steinhöfel et al., 2011; Ziegler et al., 2005a,b) and at the catchment scale, respectively (Alleman et al., 2005; Cardinal et al., 2010; Ding et al., 2004; Engström et al., 2010; Georg et al., 2006a, 2007a; Hughes et al., 2011a, 2012). These investigations provide important information regarding the effect of weathering on Si isotopes under local weathering conditions (soil age, lithology and climate). However, the question arises whether findings from local studies can be applied to explain the global pattern of Si isotope distribution evolution upon chemical weathering. Here we evaluate the potential of Si isotope measurements for better constraining spatially- and temporally-variable weathering processes controlling continental DSi fluxes to the ocean.
The review begins with a short description of the methods deployed in Si isotope measurements (Section 2). We then provide an overview of Si isotope variations observed at the Earth's surface (Section 3). Building on available Si isotope data obtained for soils weathering sequences and major rivers, we review Si isotope fractionations associated with weathering processes, taking into account:
- • the effect of time, lithology and climate on abiotic weathering processes in the CZ (Section 4.1);
- • the overprint of biotic processes on Si isotope signature inherited from abiotic weathering processes in the CZ (Section 4.2);
- • the impact of physical erosion on chemical weathering fluxes in major world rivers (Section 4.3).
We conclude the article by exploring further research directions.
2 Measurements of silicon isotopes
2.1 Basic principles
Silicon (z = 14, atomic mass = 28.0855) has three stable isotopes of atomic mass units (AMU) 28Si (27.976927), 29Si (28.976495) and 30Si (29.973770), with respective abundance of 92.23, 4.67 and 3.10% (Faure and Mensing, 2005). The relative mass differences are 7.8 (30Si, 28Si) and 3.5 (29Si, 28Si). Originally, Si isotopic analyses were reported against the isotopic composition of the CalTech Rose Quartz Standard (RQS; Douthitt, 1982). Nowadays, the Si isotopic composition is expressed in the δ-notation (in ‰ units) relative to NBS28, which is the accepted reference material for Si isotopes (National Institute of Standard and Technology RM #8546; Carignan et al., 2004). Although not certified for its Si isotopic composition, the NBS28 is certified for its δ18O composition and has long been used as the O isotope reference for silicate solid samples (Chapligin et al., 2011). RQS and NBS28 have been shown to be isotopically similar, thus allowing direct comparison between datasets (Georg et al., 2007b). Various secondary Si isotope reference materials (Diatomite, Big Batch, BHVO-1 and BHVO-2, LMG-08) are also now available (Abraham et al., 2008; Hendry et al., 2010a; Reynolds et al., 2007).
The Si isotopic composition of any sample can be expressed as:
| (1) |
| (2) |
Terrestrial Si isotope variations are described in terms of mass-dependent fractionations in a δ29Si vs. δ30Si plot. In this representation, the mass-dependent fractionations fall on a straight line. Any deviations from this trend may be attributed to a non-mass dependent isotope effect (Clayton et al., 1973), such as in extraterrestrial SiC grains in meteorites (Stone et al., 1991; Zinner et al., 1989). Two major isotopic fractionation types are distinguished: kinetic fractionation and equilibrium fractionation. Kinetic fractionation occurs during an irreversible chemical reaction or physical process, as opposed to equilibrium fractionation, which is associated with a reversible chemical reaction or physical process. For terrestrial samples, the slope (β) of the straight line in a δ29Si vs. δ30Si plot depends on the kinetic or equilibrium fractionation, and is calculated knowing the exact atomic masses (m) of Si isotopes according to (Young et al., 2002):
| (3) |
| (4) |
Based on the β value derived from the δ29Si vs. δ30Si plot, δ29Si values are converted into δ30Si values using a multiplying factor (1/β) of 1.96 in the case of kinetic mass-dependent fractionation, or 1.93 in the case of equilibrium mass-dependent fractionation. The latter is usually preferred since equilibrium fractionation has been observed to occur in natural rivers (Georg et al., 2006a, 2007a).
The degree of isotope fractionation (in ‰ units) between two materials A and B for a given process can be reported as α or ɛ. The fractionation factor α is defined as:
| (5) |
| (6) |
For small isotope fractionations such as those usually found in terrestrial environments, the Si isotopic discrimination between materials A and B (Δ30Si A-B) offers a good approximation for 30ɛ if equilibrium is reached:
| (7) |
Two approaches are used to model isotopic fractionation: the Rayleigh model (or closed system), and the steady-state model (or open system). The Rayleigh model assumes a finite Si reservoir without replenishment by external sources (De La Rocha et al., 1997). The evolution of the DSi isotopic signature (δ30SiDSi) and of the associated Si precipitate (δ30Sisolid) can then be described by:
| (8) |
| (9) |
δ30Siinitial is the Si isotope composition in the initial Si reservoir, ƒ is the fraction of DSi remaining in the Si reservoir (f = [Si]/[Si]initial), and 30ɛ is the fractionation factor between the dissolved and the solid phase. In contrast, a steady-state model assumes a continuous supply of Si from the same external source (Varela et al., 2004). The isotope signatures of the DSi (δ30SiDSi) and of the Si precipitate (δ30Sisolid) are represented by:
| (10) |
| (11) |
2.2 Analytical development
Before the advent of multicollector inductively coupled plasma mass spectrometry (MC-ICP-MS), Si stable isotopes were measured by gas source isotope ratio mass spectrometry (IRMS) and secondary ion microprobe mass spectrometry (SIMS) (Ding, 2004; Reynolds, 2011). The first Si isotopic measurements were obtained by IRMS after conversion of the Si-containing solid samples into SiF4 (Reynolds and Verhoogen, 1953). This was achieved via:
- • BaSiF6 decomposition (Reynolds and Verhoogen, 1953);
- • direct fluorination using F2 and HF (Taylor and Epstein, 1962);
- • or direct fluorination using BrF5 (Clayton and Mayeda, 1963).
More recently, silica was converted into SiF4 after decomposition of Cs2SiF6 obtained by addition of HF and CsCl (Brzezinski et al., 2006). IRMS has been applied to the analysis of Si isotopes in meteorites and terrestrial rocks (Epstein and Taylor, 1970; Molini-Velsko et al., 1986). The precision (standard deviation, 2σ) on IRMS measurements has greatly improved since the 1950s (Fig. 2), and approaches ∼0.1‰ (Brzezinski et al., 2006). Compared to IRMS, SIMS technique is more rapid, has a higher sensitivity and involves smaller sample size. However, the technique suffers from a poorer precision (0.2‰; Basile-Doelsch et al., 2005; Huneke et al., 1983; Zinner et al., 1987; Fig. 2), although this can be partially offset by the possibility to perform a high number of replicates.
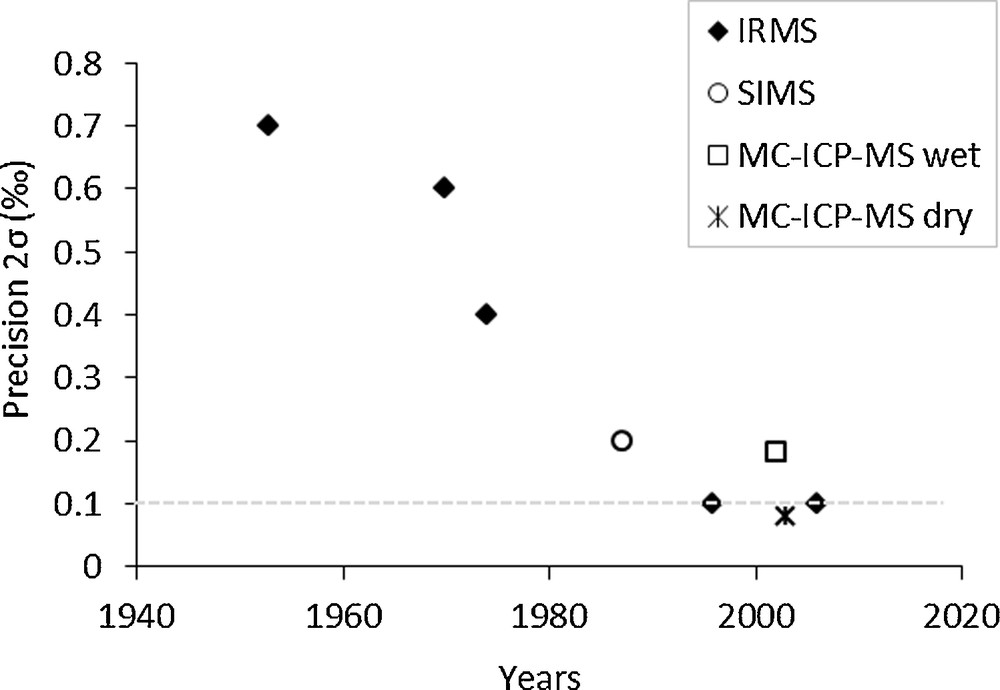
Evolution of the analytical precision (standard deviation, 2σ) for silicon (Si) isotope determinations since the 1950s with gas source isotope ratio mass spectrometry (IRMS), secondary ion microprobe mass spectrometry (SIMS), and multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS). IRMS: Reynolds and Verhoogen (1953); Epstein and Taylor (1970); Clayton et al. (1974); De La Rocha et al. (1996); Brzezinski et al. (2006); SIMS: Zinner et al. (1987); MC-ICP-MS wet plasma: De La Rocha (2002); MC-ICP-MS dry plasma: Cardinal et al. (2003). Masquer
Evolution of the analytical precision (standard deviation, 2σ) for silicon (Si) isotope determinations since the 1950s with gas source isotope ratio mass spectrometry (IRMS), secondary ion microprobe mass spectrometry (SIMS), and multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS). IRMS: Lire la suite
Évolution de la précision analytique (déviation standard, 2σ) sur la mesure des isotopes du silicium (Si) depuis les années 1950 avec la spectrométrie de masse à source gazeuse (IRMS), la sonde ionique (SIMS), et le spectromètre de masse à multicollection (MC-ICP-MS). IRMS : Reynolds et Verhoogen (1953) ; Epstein et Taylor (1970) ; Clayton et al. (1974) ; De La Rocha et al. (1996) ; Brzezinski et al. (2006) ; SIMS : Zinner et al. (1987) ; MC-ICP-MS plasma humide (wet) : De La Rocha (2002) ; MC-ICP-MS plasma sec (dry) : Cardinal et al. (2003). Masquer
Évolution de la précision analytique (déviation standard, 2σ) sur la mesure des isotopes du silicium (Si) depuis les années 1950 avec la spectrométrie de masse à source gazeuse (IRMS), la sonde ionique (SIMS), et le spectromètre de masse à multicollection (MC-ICP-MS). ... Lire la suite
The use of MC-ICP-MS for measuring Si isotopes is increasingly adopted (Table 1). The development of MC-ICP-MS has significantly improved the precision of Si isotope measurements, thereby allowing the study of terrestrial samples characterized by small isotopic variations. While in the wet plasma mode a precision of 0.18‰ is normally achieved (De La Rocha, 2002), a precision less than 0.1% can be obtained using a dry plasma (Cardinal et al., 2003; Fig. 2). Over the last decade, polyatomic isobaric interferences on 30Si have been resolved by operating the MC-ICP-MS in high mass resolution (m/Δm > 2000; Reynolds et al., 2006; Zambardi and Poitrasson, 2010), or pseudo-high resolution mode (m/Δm > 1000; Abraham et al., 2008; Engström et al., 2006; van den Boorn et al., 2006).
Détails des méthodes de détermination des isotopes du silicium (Si) par MC-ICP-MS. LA = Ablation laser couplée au MC-ICP-MS.
| Group | Instrument | External doping | Desolvation plasma | Resolution | Si requirement (μg) | Precision (1σ) ‰ |
| De La Rocha, 2002 | Nu Plasma | Wet | Low | ∼ l60 | δ29Si ± 0.09 | |
| Cardinal et al., 2003 | Nu Plasma | Mg | Dry | Low | 2 | δ29Si ± 0.04 |
| Abraham et al., 2008 | Nu Plasma | Mg | Dry | Pseudo-high | 4 | δ30Si ± 0.07 |
| Reynolds et al., 2006 | Nu 1700 | Dry | High | 2 | δ30Si ± 0.10 | |
| Engström et al., 2006 | Neptune | Mg | Wet | Pseudo-high | 20 | δ30Si ± 0.25 |
| van den Boorn et al., 2006 | Neptune | Dry | Pseudo-high | 4 | δ30Si ± 0.18 | |
| Chmeleff et al., 2008 | Neptune | LA | Medium | 0.05 | δ30Si ± 0.12 | |
| Zambardi and Poitrasson, 2010 | Neptune | Mg | Wet | High | 3 | δ30Si ± 0.08 |
In parallel to recent instrument developments, a series of adjustments have been made to reduce matrix effects as these have a direct impact on measurement precision and reproducibility. For example, sample purification technique has been improved (Georg et al., 2006b), notably for samples with a S-rich matrix (van den Boorn et al., 2009). Solution pH is also controlled before the purification step (Fitoussi et al., 2009), while the mass bias introduced by anionic matrices is reduced by adding anions to both the sample and bracketing standards (Hughes et al., 2011b). External Mg doping is also used to control mass bias (Cardinal et al., 2003; Engström et al., 2006; Zambardi and Poitrasson, 2010).
3 Silicon isotope variability at the Earth's surface
Silicon belongs to the third period and the fourth family of the periodic table. While Si can occur as SiC in meteoritic grains, it is essentially found in combination with O as a tetrahedral silicate ion (SiO4) in natural conditions on Earth. Combined with a single valence (4 +), this explains the comparatively small range of terrestrial Si isotopic variations, in contrast to other light stable isotopic systems such as C, H, O, N and S (Ding et al., 1996). The lightest and heaviest Si isotope compositions on Earth have been reported for silcretes (δ30Si = −5.7‰, Basile-Doelsch et al., 2005) and rice grains (δ30Si = +6.1‰, Ding et al., 2005), respectively, which correspond to a range of 11.8‰ variation at the Earth's surface. The natural variation in δ30Si values found in various natural reservoirs shown in Fig. 3 can be attributed to three distinct processes of isotopic fractionation, which are detailed below:
- • rock-forming processes;
- • water-rock interaction;
- • biological processes.
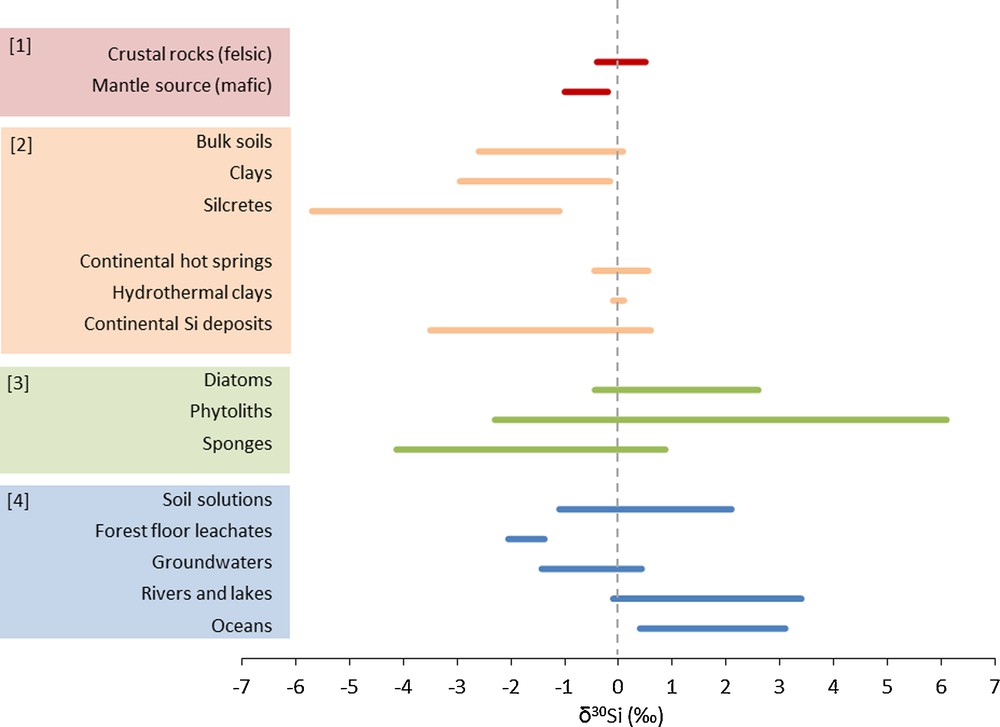
Range of silicon (Si) isotope variations at the surface of the Earth in relation to rock-forming processes [1], water-rock interactions [2], biological processes [3], water reservoirs [4]. References are given in the text: [1] Section 3.1, [2] Section 3.2, [3] Section 3.3, [4] Section 3.4.
Gamme de variations des isotopes du silicium (Si) à la surface de la Terre, en lien avec les processus de formation des roches [1], les interactions eaux-roches [2], les processus biologiques [3], les réservoirs en eaux [4]. Les références sont données dans le texte : [1] Section 3.1, [2] Section 3.2, [3] Section 3.3, [4] Section 3.4.
3.1 Rock-forming processes
Silicon isotopic fractionation associated with rock-forming processes results from two types of exchange reactions: solid–liquid (silicate mineral and silicate melt) or solid–solid (crystal–crystal). These rock-forming processes are associated with small Si isotopic fractionations of 0.2–0.5‰ (quartz-feldspar, feldspar-hornblende, feldspar-pyroxene; Ding et al., 1996). The Si isotope composition (δ30Si) of the bulk silicate Earth (BSE) as estimated from primitive undifferentiated meteorites (chondrites) is −0.50‰ (Armytage et al., 2011). Differentiation of the primitive Earth produced three main reservoirs. The Earth's mantle is rather homogeneous (Savage et al., 2010), as supported by Si isotope signatures of mafic igneous rocks (terrestrial and lunar basalts), peridotite, and lherzolite (δ30Si = −0.34‰; Armytage et al., 2011; Georg et al., 2007b). The distinct mantle Si isotope composition (δ30Si = −0.34‰) relative to that of BSE (δ30Si = −0.50‰) is attributed to preferential incorporation of light Si isotopes into the Earth's core before the Moon formed (Armytage et al., 2011; Georg et al., 2007b). A limited isotopic fractionation accompanies magmatic differentiation, leading to heavier δ30Si signature with increasing silica content and degree of silicate polymerisation (Ding et al., 1996; Douthitt, 1982; Savage et al., 2011). This explains the heavier signature of the felsic components of the Earth's crust (granite, gneiss, granulite; δ30Si = ∼0‰; Ding et al., 1996; Savage et al., 2012). So far, magma degassing has not been shown to produce fractionation of Si isotopes. Sedimentary rocks, including quartzite and sandstone, exhibit isotopic signatures in the same range as those reported in the Earth's crust (Ding et al., 1996).
3.2 Water-rock interaction
Water-rock interaction during weathering or hydrothermal alteration involves liquid-solid exchanges between monosilicic acid (H4SiO4) and neoforming minerals such as clays, oxy-hydroxides and inorganic silica. Although a small Si isotopic fractionation (0.5‰) is typically associated with such exchanges, the final fractionation may be large (up to 3‰) due to successive dissolution/precipitation or adsorption/desorption cycles. Most of the currently available studies of Si isotope fractionation associated with water-rock interactions have been obtained at the soil profile and catchment scales.
In general, the Si isotopic composition of clay minerals formed during weathering is lighter (δ30Si = −2.95 to −0.16‰) than that of the parent silicate material (Fig. 3; Bern et al., 2010; Cornelis et al., 2010; Douthitt, 1982; Georg et al., 2007a, 2009a, 2009b; Opfergelt et al., 2010; Steinhöfel et al., 2011; Ziegler et al., 2005a,b). This was shown to be the result of preferential incorporation of the light Si isotopes during mineral formation (Georg et al., 2007a; Ziegler et al., 2005a,b). Currently, the fractionation factor associated with the formation of secondary phases is only available for kaolinite and allophane; it remains undetermined for other clay minerals abundant in soils such as smectite, illite, chlorite and vermiculite. The 30ɛ values have been inferred from field measurements for kaolinite (30ɛ = −2‰; Ziegler et al., 2005b) and experimentally for allophane (30ɛ = −1.8‰; Ziegler et al., 2005a) (Fig. 4). An equilibrium isotopic fractionation of 30ɛ = −1.6‰ was estimated from theoretical calculations between kaolinite and quartz (Méheut et al., 2007). This value matches that derived from field based-measurements of silicate weathering in Iceland (30ɛ = −1.5‰; Georg et al., 2007a).
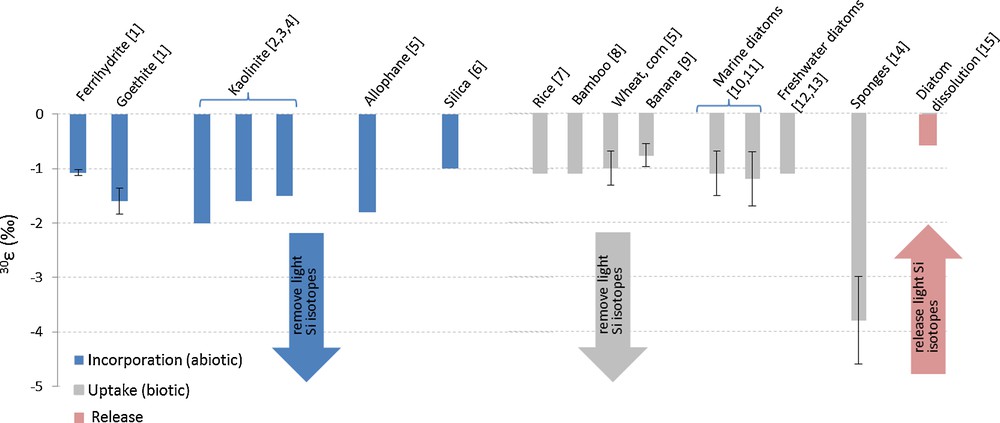
Experimental silicon (Si) isotope fractionation factors (30ɛ) for abiotic and biotic processes. [1] Delstanche et al. (2009); [2] Ziegler et al. (2005b); [3] Méheut et al. (2007); [4] Georg et al. (2007a); [5] Ziegler et al. (2005a); [6] Li et al. (1995); [7] Ding et al. (2008a); [8] Ding et al. (2008b); [9] Opfergelt et al. (2006); [10] De La Rocha et al. (1997); [11] Fripiat et al. (2011); [12] Alleman et al. (2005); [13] Opfergelt et al. (2011); [14] De La Rocha (2003); [15] Demarest et al. (2009). Masquer
Experimental silicon (Si) isotope fractionation factors (30ɛ) for abiotic and biotic processes. [1] Delstanche et al. (2009); [2] Ziegler et al. (2005b); [3] Méheut et al. (2007); [4] Georg et al. (2007a); [5] Ziegler et ... Lire la suite
Facteurs de fractionnement isotopique en silicium (Si) expérimentaux (30ɛ) des processus abiotiques et biotiques. [1] Delstanche et al. (2009) ; [2] Ziegler et al. (2005b) ; [3] Méheut et al. (2007) ; [4] Georg et al. (2007a) ; [5] Ziegler et al. (2005a) ; [6] Li et al. (1995) ; [7] Ding et al. (2008a) ; [8] Ding et al. (2008b) ; [9] Opfergelt et al. (2006) ; [10] De La Rocha et al. (1997) ; [11] Fripiat et al. (2011) ; [12] Alleman et al. (2005) ; [13] Opfergelt et al. (2011) ; [14] De La Rocha (2003) ; [15] Demarest et al. (2009). Masquer
Facteurs de fractionnement isotopique en silicium (Si) expérimentaux (30ɛ) des processus abiotiques et biotiques. [1] Delstanche et al. (2009) ; [2] Ziegler et al. (2005b) ; [3] Méheut et al. (2007) ; [4] Georg et al. (2007a) ; [5] Lire la suite
Based on these findings, it can be concluded that the relative proportions of primary and secondary silicate minerals exert a strong control on the bulk Si isotopic signature of a soil. Consequently, low soil δ30Si values are indicative of strong weathering (Opfergelt et al., 2010; Ziegler et al., 2005a,b). This trend is enhanced further due to partitioning of Si isotopes during Si adsorption onto secondary Fe oxy-hydroxide minerals (Delstanche et al., 2009; Opfergelt et al., 2009). Indeed, the adsorption of Si onto Fe oxy-hydroxides is accompanied by preferential retention of the light Si isotopes, with larger fractionation observed with increased degree of crystallinity, e.g., ferrihydrite: 30ɛ = −1.08 ± 0.06‰ and goethite: 30ɛ = −1.60 ± 0.24‰ (Delstanche et al., 2009) (Fig. 4). While this behaviour was confirmed experimentally for natural Fe oxy-hydroxides (Opfergelt et al., 2009), Al-oxides still await further investigation. We note that these compounds are twice as much more effective in sorbing Si than Fe-oxides (Jones and Handreck, 1963).
Precipitation of inorganic silica depletes the original solution in light Si isotopes (30ɛ = −1.0‰; Li et al., 1995; Fig. 4). In contrast, silicate dissolution releases the light Si isotopes preferentially, as shown for basaltic glass (Ziegler et al., 2005a). As mentioned earlier, the lightest Si isotopic reservoir on Earth is represented by silcretes with a mean δ30Si of −3.8‰ (Basile-Doelsch et al., 2005). Silcretes are formed through cycles of secondary quartz precipitation/dissolution, and this explains the large fractionation observed and hence, the strong depletion in heavy Si.
A direct consequence of the preferential incorporation of the light Si isotopes in secondary weathering products is that the soil solutions are enriched in heavy Si isotopes relative to the parent silicate material (Ziegler et al., 2005a; Fig. 3). However, caution is needed when generalizing this trend as recent work indicates that the dissolution of secondary clay minerals release light Si isotopes in solution (Cornelis et al., 2010). Of note, a few studies have emphasized that the δ30Si signature of a soil may also be influenced by aeolian dust input, which acts as a source of weathering-resistant minerals such as quartz and mica (Bern et al., 2010; Opfergelt et al., 2010), notably in strongly weathered environments (Chadwick et al., 1999; Section 5.3). In addition, Si recycling by plants is another important control on the distribution of Si isotopes in topsoils (Bern et al., 2010; Opfergelt et al., 2010; Ziegler et al., 2005a; Sections 3.3 and 4.2).
Only a few studies have measured the Si isotopic composition of secondary minerals deposited from water–rock interaction in hydrothermal systems (Fig. 3). Ding et al. (1996) measured hydrothermal clay minerals (kaolinite) with δ30Si values between −0.1 and +0.1‰ (Ding et al., 1996). The heavier δ30Si values for hydrothermal clay minerals relative to the δ30Si of clay minerals derived from meteoric weathering processes likely reflects a Si contribution from continental hydrothermal fluids (hot spring water: δ30Si = −0.45 to +0.55‰; Ding et al., 1996; Douthitt, 1982; Opfergelt et al., 2011). Silicifications in such hydrothermal environments were shown to display a large range of Si isotope variations (δ30Si = −3.5 to 0.6‰; Ding et al., 1996; Douthitt, 1982; van den Boorn, 2008).
3.3 Biological processes
Fractionation of Si isotopes associated with biological processes is related to H4SiO4 uptake and formation of BSi. Silicon biomineralisation in plants, diatoms and sponges has been shown to favour incorporation of the light Si isotopes (De La Rocha et al., 1997; De La Rocha, 2003; Ding et al., 2005; Opfergelt et al., 2006).
Importantly, phytoliths display heterogeneous Si isotopic composition. Reported δ30Si values show large variations ranging from -2.3 to +6.1‰ (Bern et al., 2010; Cornelis et al., 2010; Ding et al., 1996, 2005, 2008a,b; Douthitt, 1982; Engström et al., 2008; Hodson et al., 2008; Köster et al., 2009; Opfergelt et al., 2006, 2008, 2010; Sun et al., 2008; Ziegler et al., 2005a; Fig. 3). The large δ30Si variability in phytoliths is attributed to several factors. Plant uptake favours light Si isotopes, as determined experimentally in banana (30ɛ = −0.77 ± 0.21‰; Opfergelt et al., 2006), rice (30ɛ = −1.1‰; Ding et al., 2008a), bamboo (30ɛ = −1.1‰; Ding et al., 2008b) and wheat and corn (30ɛ = −1.00 ± 0.31‰; Ziegler et al., 2005a) (Fig. 4). In addition, it has been shown that intra-plant Si isotope fractionation in rice, bamboo and banana is associated with evapotranspiration (Ding et al., 2005, 2008a, b; Opfergelt et al., 2006). As a result, the Si isotope composition of phytoliths depends on their location in the plant, with heavier signatures found in upper shoots (Opfergelt et al., 2008). Further, since both the soil parent material lithology and the soil weathering degree dictate the isotopic composition of DSi taken up by the plant, they also control that found in phytoliths (Opfergelt et al., 2008). Isotopic fractionation during phytolith dissolution has not been determined, but is likely to favour the release of light Si isotopes, as observed during dissolution of diatom-derived BSi (Demarest et al., 2009) or basaltic glass (Ziegler et al., 2005a). However, further studies on Si isotope fractionation associated with BSi dissolution should determine if isotopic fractionation is due to preferential release of light Si isotopes from BSi with a homogeneous isotope composition, or if it results from dissolution of BSi with heterogeneous isotope compositions.
Silicon is also an important component of marine biogenic matter. Diatoms are the main BSi producer in oceans and rivers (Conley, 1997; Ragueneau et al., 2000), strongly impacting the Si isotope budget in these environments. Laboratory studies of marine diatoms have established that fractionation of Si isotopes during Si uptake (30ɛ = −1.1 ± 0.4‰; De La Rocha et al., 1997) is not species- or temperature-dependent (Fig. 4). A similar fractionation factor was derived from in situ measurements of both marine (30ɛ = −1.2 ± 0.5‰; Fripiat et al., 2011) and freshwater diatoms (30ɛ = −1.1‰; Alleman et al., 2005; Opfergelt et al., 2011). Dissolution of diatom-derived BSi results in isotopic fractionation of 30ɛ = −0.55‰ (Demarest et al., 2009), which is about half the magnitude observed during Si uptake. These findings led Opfergelt et al. (2011) to conclude that seasonal dissolution of lacustrine diatoms partly controls continental Si fluxes exported to rivers and oceans.
Compared to diatoms, Si isotope fractionation during opal formation in marine sponges is accompanied by a much larger isotopic fractionation in the range 30ɛ = −3.8 ± 0.8‰ to 30ɛ = −6.02‰ (De La Rocha, 2003; Wille et al., 2010). The Si isotope fractionation associated with uptake in marine sponges is constant at −1.34‰, whereas fractionation during spicule formation increases as a function of external seawater DSi concentration (Hendry et al., 2010b; Wille et al., 2010). Marine sponges exhibit significant δ30Si variations ranging from −4.13 to +0.87‰ (De La Rocha, 2003; Douthitt, 1982; Hendry et al., 2010b; Wille et al., 2010; Fig. 3).
Overall, production of BSi by biomineralisation has been shown to produce a large range of isotope variations of 9.8‰ (Fig. 3), which represents 83% of the whole terrestrial isotopic range (11.8‰). Given the larger annual Si fluxes derived from plants (Conley, 2002) and diatoms (Tréguer et al., 1995) as compared to the annual riverine Si supply to the ocean (Tréguer et al., 1995), this suggests a significant control of the biogenic Si pool on continental Si isotopic budget (Fig. 1).
3.4 River waters and lakes
Dissolved Si in fresh water bodies (rivers and lakes) and seawater is enriched in 30Si relative to the Earth's crust. The δ30Si signature of fresh waters ranges between −0.1 and +3.4‰ (Alleman et al., 2005; Cardinal et al., 2010; De La Rocha et al., 2000; Ding et al., 2004,2011; Engström et al., 2010; Georg et al., 2006a, 2007a; Hughes et al., 2011a, 2012; Ziegler et al., 2005a; Fig. 3), probably reflecting preferential incorporation of light Si isotopes during weathering and biological processes. In comparison, seawater displays δ30Si values between +0.4 and +3.1‰ (Cardinal et al., 2005; De La Rocha et al., 2000; Fripiat et al., 2007; Varela et al., 2004). Rivers and lakes can show temporal and spatial variations in Si isotope composition due to mixing of base flow and superficial runoff (Georg et al., 2006a) and/or biological uptake and release (Ding et al., 2004; Engström et al., 2010; Hughes et al., 2011a, 2012; Opfergelt et al., 2011). Lower riverine isotopic signatures (close to δ30Si = 0‰) such as measured in the black rivers from the Congo basin were attributed to dissolution of clay minerals in organic-rich waters (Cardinal et al., 2010).
On a global scale, riverine Si isotope compositions are mainly influenced by the lithology of the bedrock, biological uptake/dissolution (diatoms and plants) and weathering (dissolution and secondary mineral neoformation) (Ding et al., 2011; Hughes, 2011). Biogenic Si formation by diatoms can induce a seasonal effect on riverine δ30Si, which is superimposed on a constant abiotic δ30Si value throughout the year (Hughes et al., 2011a). Detailed investigations at the catchment scale are needed in order to augment our understanding of the contribution of CZ weathering processes to riverine Si fluxes.
Relative to surface waters, groundwater has lighter δ30Si values comprised between −1.43 and +0.43‰, possibly the result of secondary clay minerals and silcrete dissolution (Georg et al., 2009a,b; Fig. 3). In some regions, chemical outputs at the catchment scale originate largely from groundwaters (e.g., Mule Hole watershed, India; Maréchal et al., 2011). Since the δ30Si of groundwater is typically lighter than that of surface water (Fig. 3), Si isotope studies may offer a mean to quantify the contribution from groundwater to the chemical weathering budget and therefore, may help to shed light on hydrological budget at the catchment scale. To achieve this goal, a better assessment of seasonal variations of Si isotope compositions in surface waters is required.
4 Application of silicon isotopes to investigate continental silicon transfer to the ocean
4.1 Soil weathering degree and abiotic weathering processes
Most of the Si isotopic studies available in soils have been carried out at the soil profile scale (Bern et al., 2010; Cornelis et al., 2010; Opfergelt et al., 2010; Steinhöfel et al., 2011; Ziegler et al., 2005a,b). In general, these data indicate that the δ30Si of soil decreases with increasing weathering degree, which is a measure of the extent to which chemical elements have been stripped from the soil parent material at a given time. In a soil chrono-climo-toposequence with homogeneous parent material (e.g., Hawaii or Guadeloupe), the soil weathering degree was shown to be dictated primarily by soil age and climate (Chadwick et al., 1999; Colmet-Daage and Lagache, 1965). Available Si isotope studies on soil weathering sequences report the effect of weathering on Si isotopic compositions under local weathering conditions (soil age, lithology, climate). However, weathering conditions vary worldwide, thereby also impacting on chemical weathering rates.
Weathering degree not only controls the Si isotope composition of soils, but also that of DSi exported from the CZ (Section 3.2). As found by Ziegler et al. (2005a), soil solutions become isotopically heavier with increasing weathering degree. However, evaluating the effect of soil weathering degree on the DSi isotopic composition in different weathering conditions is impeded by a paucity of δ30Si measurements on DSi in soil weathering sequences. To evaluate whether the findings of local studies on bulk soils can be extrapolated globally, we compiled available Si isotope data (n = 120) from soil weathering sequences in various weathering conditions worldwide. These soils span a wide range in age, from ∼1 ka to > 2.5 Ma (Fig. 5a) and have developed from volcanic (andesite in Guadeloupe and basalt in Iceland, Cameroon and Hawaii) or plutonic (granite in Puerto Rico) rocks under contrasted climates, i.e., temperate in Iceland, tropical for the other sites. The δ30Si data are plotted against three weathering indices (Fig. 6), namely TRB (Total Reserve in Bases, in cmolc.kg1; Herbillon, 1986), WIP (Weathering Index of Parker, with volumetric concentration of the oxides gr.cm−3, Parker, 1970) and CIA (Chemical Index of Alteration, Nesbitt and Young, 1982), defined as follows:
| (12) |
| (13) |
| (14) |

Map showing the locations of the sites studied with silicon (Si) isotopes: (a) in soil weathering sequences (the soil age is given) from Guadeloupe ([1] Opfergelt et al., 2012), Cameroon ([2] Opfergelt et al., 2008), Iceland ([3] Opfergelt, unpublished), Hawaii ([4] Bern et al., 2010) and Puerto Rico ([5] Ziegler et al., 2005b); (b) in major world rivers including Congo River ([1] Cardinal et al., 2010, [2] Hughes et al., 2011a), Amazon ([3] De La Rocha et al., 2000, [4] Hughes, 2011), Brahmaputra ([5] Georg et al., 2009a), Ganges ([6] Georg et al., 2009a) and Mekong ([7] Hughes, 2011). Masquer
Map showing the locations of the sites studied with silicon (Si) isotopes: (a) in soil weathering sequences (the soil age is given) from Guadeloupe ([1] Opfergelt et al., 2012), Cameroon ([2] Opfergelt et al., 2008), Iceland ([3] Opfergelt, ... Lire la suite
Carte localisant les sites étudiés avec les isotopes du silicium (Si) : (a) dans les séquences d’altération de sols (l’âge des sols est précisé) en Guadeloupe ([1] Opfergelt et al., 2012), au Cameroun ([2] Opfergelt et al., 2008), en Islande ([3] Opfergelt, unpublished), à Hawaii ([4] Bern et al., 2010), et à Puerto Rico ([5] Ziegler et al., 2005b) ; (b) dans quelques rivières majeures au monde comme le Congo ([1] Cardinal et al., 2010, [2] Hughes et al., 2011a), l’Amazone ([3] De La Rocha et al., 2000, [4] Hughes, 2011), le Brahmaputre ([5] Georg et al., 2009a), le Ganges ([6] Georg et al., 2009a), et le Mékong ([7] Hughes, 2011). Masquer
Carte localisant les sites étudiés avec les isotopes du silicium (Si) : (a) dans les séquences d’altération de sols (l’âge des sols est précisé) en Guadeloupe ([1] Opfergelt et al., 2012), au Cameroun ([2] Opfergelt et al., 2008), en ... Lire la suite
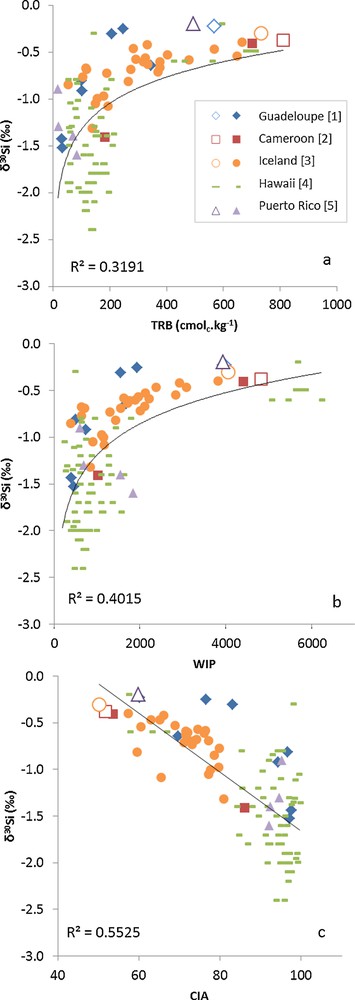
Plot of soil silicon (Si) isotope compositions as a function of weathering degree. Three weathering indices are used: (a) Total Reserve in Bases (TRB = [Na] + [Mg] + [Ca] + [K]); (b) Weathering Index of Parker (WIP = [(2Na2O/0.35) + (MgO/0.9) + (2 K2O/0.25) + (CaO/0.7)] × 100); (c) Chemical Index of Alteration (CIA = [(Al2O3)/(Al2O3 + CaO + Na2O + K2O)] × 100). Parent materials (open symbols) and soils (full symbols). Data from [1] Opfergelt et al. (2012); [2] Opfergelt et al. (2008); [3] Opfergelt (unpublished); [4] Bern et al. (2010); [5] Ziegler et al. (2005b) and White et al. (1998).
Diagramme montrant la composition isotopique en silicium (Si) du sol en fonction du degré d’altération. Trois indices d’altération sont utilisés : (a) la réserve totale en bases (TRB = [Na] + [Mg] + [Ca] + [K]) ; (b) l’indice d’altération de Parker (WIP = [(2Na2O/0,35) + (MgO/0,9) + (2 K2O/0,25) + (CaO/0,7)] × 100) ; (c) l’indice d’altération chimique (CIA = [(Al2O3)/(Al2O3 + CaO + Na2O + K2O)] × 100). Matériaux parentaux (symboles vides) et sols (symboles pleins). Données tirées de [1] Opfergelt et al. (2012) ; [2] Opfergelt et al. (2008) ; [3] Opfergelt (unpublished) ; [4] Bern et al. (2010) ; [5] Ziegler et al. (2005b) et White et al. (1998). Masquer
Diagramme montrant la composition isotopique en silicium (Si) du sol en fonction du degré d’altération. Trois indices d’altération sont utilisés : (a) la réserve totale en bases (TRB = [Na] + [Mg] + [Ca] + [K]) ; (b) l’indice d’altération de Parker (WIP = [(2Na2O/0,35) + (MgO/0,9) + (2 K2O/0,25) + (CaO/0,7)] × 100) ; (c) l’indice d’altération chimique (CIA = [(Al2O3)/(Al2O3 + CaO + Na2O + K2O)] × 100). Matériaux parentaux ... Lire la suite
Both the TRB and WIP decrease as weathering increases, whereas CIA follows an opposite trend due to leaching of base cations (Mg2+, Ca2+, Na+, K+) upon weathering.
As depicted in Figs. 6a–c, bulk soil δ30Si seems to be inversely correlated to weathering degree. This result, obtained for samples corresponding to different lithologies, climates and ages, lends further credence to the idea that preferential incorporation of light Si isotopes in secondary mineral phases gradually depletes the soil in isotopically heavy Si isotopes (Bern et al., 2010; Cornelis et al., 2010; Georg et al., 2007a, 2009a,b; Opfergelt et al., 2010; Steinhöfel et al., 2011; Ziegler et al., 2005a,b). It also underlines that the factors, which modify weathering rates, such as time, lithology and climate do not alter this general tendency. Interestingly, the δ30Si values of highly weathered soils (i.e., Hawaii with TRB < 200, WIP < 1500, CIA > 90) display significant scattering (range δ30Si −1.0 to −2.4‰). Further depletion of the soil in light Si isotopes may be the result of successive cycles of secondary mineral dissolution/precipitation at very advanced weathering degree, i.e., when the weatherable mineral reserve becomes exhausted.
It should also be noted that the data used in Fig. 6 are dominated by soils from volcanic islands (Guadeloupe, Iceland, Hawaii) and therefore, caution must be exerted when extrapolating the trend observed to other soils worldwide. Nevertheless, it has been suggested that ∼45% of the suspended river material carried to the ocean originates from volcanic islands (Milliman and Syvitski, 1992). By determining the influence of abiotic weathering processes on δ30Si of soil particles in volcanic island settings, the source(s) of particulate Si in river suspended matter could be better resolved (Section 5.1).
4.2 Overprint of biotic processes on the δ30Si signature inherited from abiotic weathering processes
In addition to abiotic weathering processes in the CZ, plants and microorganisms also contribute to rock weathering, thereby supplying mineral nutrients to ecosystems (Bonneville et al., 2009; Cochran and Berner, 1992; Parsons et al., 1998). While the impact of microorganisms on weathering fluxes exported from the CZ remains poorly quantified (van Schöll et al., 2008), vegetation is thought to increase weathering rates significantly. According to Moulton et al. (2000), solute fluxes exported from vegetated areas are about four times larger than those measured in barren lands. The influence of plants on weathering can be explained by:
- • conversion of DSi into BSi via phytolith precipitation; the subsequent decrease in DSi concentration in the soil solution being balanced by an increase in soil weathering;
- • nutrient uptake by plant roots and concomitant decrease in cation concentrations in the rhizosphere, hence favouring mineral dissolution;
- • production of root exudates (organic acids and protons) acting as weathering agents (Kelly et al., 1998).
Phytoliths represent a significant contribution to the riverine DSi flux (Derry et al., 2005). Struyf et al. (2010) showed that the conversion of forested area into cultivated areas is accompanied by a short-term pulse in DSi flux, followed by a long-term decline. The initial increase in DSi flux was interpreted by these authors to be the result of dissolution of BSi stored in soils (formed under forest); with time this pool shrinks due to removal of crops and lack of fresh BSi input.
Systematic determination of the isotopic composition of DSi in the CZ can shed light on the timing and relative importance of biotic vs. abiotic weathering processes contributing to DSi flux exported from the soil-plant system (Cornelis et al., 2011). Unfortunately, the interpretation of bulk δ30Si values in the soil solution is not straightforward as both biotic (phytolith precipitation) and abiotic (e.g., clay mineral formation) processes lead to preferential incorporation of the light Si isotopes (Ding et al., 2005, 2008a,b; Georg et al., 2007a; Opfergelt et al., 2006; Ziegler et al., 2005a,b; Fig. 4). However, detailed characterisation of soil weathering degree and δ30Si in soil solutions can provide valuable information for disentangling the impact of biotic processes (which prevail in the soil surface) on DSi fluxes exported from the CZ from the effect of abiotic processes (which dominate at depth) (Ziegler et al., 2005a). Besides, further investigations aimed at capturing the seasonal variability of processes controlling Si fluxes and δ30Si in soil solutions and rivers are needed to determine the respective effect of biotic and abiotic processes on continental weathering. This approach was successfully applied by Hughes et al. (2011a), who explained the seasonal variations of dissolved δ30Si in the Congo River in terms of fluctuations in the diatom activity against a stable abiotic δ30Si background.
Further, a better understanding of the effect of phytolith formation and dissolution on weathering flux and Si isotope signature transferred to rivers may be gained by studying terrestrial environments where weathering is driven either biotically or abiotically. High DSi concentrations are found in catchments dominated by wetlands (Humborg et al., 2004; Struyf and Conley, 2009; Zakharova et al., 2007), although these ecosystems typically lack a significant reserve of weatherable minerals. This implies that Si fluxes exported from wetland regions are chiefly governed by biotic processes (McCarthy et al., 1989; Shotyk, 1988). The Si isotopic signature of chemical weathering fluxes emanating from wetlands is not known, and new δ30Si measurements are needed to confirm a biotic control. In addition, such type of data would also be useful for discussing the contribution of vegetation to continental Si weathering in areas where abiotic processes also produce DSi.
4.3 Impact of physical erosion on chemical weathering fluxes
In some instances, physical erosion is the dominant component of the continent-derived Si weathering flux (Gaillardet et al., 1999b; West et al., 2005). For the low-elevation lying Congo River, it has been estimated that physical erosion contributes ∼55% of the total denudation rate (TDR = physical + chemical weathering), but values up to 90% have been reported in high relief areas such as the Himalaya (White, 2011). At low erosion rates mineral supply limits weathering rate (transport-limited), whereas at higher erosion rates there is abundant material but kinetics and hence, climatic factors, dictate weathering rate (weathering-limited) (West et al., 2005).
For transport-limited weathering, the release of cations from silicate weathering is directly related to the supply of material by erosion, and primary minerals contribute to weathering fluxes in proportion to their abundance in the fresh regolith. In this scenario, the rate of clay mineral formation exceeds the rate of primary mineral dissolution. Since light Si isotopes are preferentially incorporated into clay minerals (Georg et al., 2007a; Ziegler et al., 2005a,b), DSi produced during transport-limited weathering with high chemical weathering rate will be enriched in the heavy isotopes (Georg et al., 2007a), providing that there is a limited influence from biological uptake or BSi dissolution. In contrast, in high erosion environments, rapid removal of material may not allow significant neoformation and accumulation of secondary phases. Thus, the comparatively low δ30Si values found for DSi in some Icelandic catchments have been interpreted in terms of high erosion rate, i.e., weathering-limited (Georg et al., 2007a). Based on these considerations, we posit that in areas poorly impacted by biological Si uptake or BSi dissolution, light dissolved Si isotopes compositions indicate either high erosion rate in weathering-limited environments, or low chemical weathering rate in transport-limited environments.
Silicon isotope studies on major rivers are limited to the Congo River (Cardinal et al., 2010; Hughes et al., 2011a), Amazon (De La Rocha et al., 2000; Hughes, 2011), Brahmaputra (Georg et al., 2009a), Ganges (Georg et al., 2009a) and Mekong (Hughes, 2011) (Fig. 5b). In Fig. 7, the δ30Si average values of these rivers are plotted against the corresponding silicate weathering fluxes (Gaillardet et al., 1999a). In order to establish if there is a relationship with erosion, we excluded from the dataset the measurements which explicitly reflected biological fractionation (Si uptake by diatoms). While diatom frustule dissolution cannot be ruled out, the significance of this process is probably limited when considering the pH-dependent solubility of amorphous silica. Amorphous silica is poorly soluble below pH 8 but solubility increases dramatically around pH 9.5 (Alexander et al., 1954), when the pH of the water is equal to or higher than the pK1 of monosilicic acid (pK125 °C = 9.51). The pH values reported for the major rivers above are systematically below 8 (Hughes, 2011). It can be noted from Fig. 7 that the Amazon, which is characterised by a high silicate weathering flux, displays a significantly lighter average δ30Si value compared to the other rivers, which also have lower silicate weathering fluxes. The high weathering flux of the Amazon may be sourced to a high total denudation rate (TDR ∼35 t.km−2.yr−1 for the Guyana shield) compared to a lower TDR for the Congo River (TDR ∼10 t.km−2.yr−1 for the African shield; West et al., 2005). Thus, the comparatively lighter DSi isotopic composition of the Amazon could reflect high erosion rate in a weathering-limited environment. Since TDR values are lacking for the Brahmaputra, Ganges and Mekong systems, it is not possible to confirm this preliminary result for other rivers.

Plot of silicon (Si) isotope compositions (average ± 2σ) of major world rivers vs. corresponding silicate weathering flux (total dissolved cations from silicate weathering; Gaillardet et al., 1999a). Isotope data from [1] Cardinal et al. (2010) (n = 13), [2] Hughes et al. (2011a) (n = 10), [3] De La Rocha et al. (2000) (n = 2), [4] Hughes (2011) (n = 4), [5] Georg et al. (2009a) (n = 2), [6] Georg et al. (2009a) (n = 1), [7] Hughes (2011) (n = 2). Masquer
Plot of silicon (Si) isotope compositions (average ± 2σ) of major world rivers vs. corresponding silicate weathering flux (total dissolved cations from silicate weathering; Gaillardet et al., 1999a). Isotope data from [1] Cardinal et al. (2010) (n = 13), [2] Lire la suite
Diagramme montrant la composition isotopique en silicium (Si) (moyenne ± 2σ) de rivières majeures à l’échelle mondiale en fonction du flux d’altération des silicates (somme totale des cations dissous dérivés de l’altération des silicates ; Gaillardet et al., 1999a). Données isotopiques tirées de [1] Cardinal et al. (2010) (n = 13), [2] Hughes et al. (2011a) (n = 10), [3] De La Rocha et al. (2000) (n = 2), [4] Hughes (2011) (n = 4), [5] Georg et al. (2009a) (n = 2), [6] Georg et al. (2009a) (n = 1), [7] Hughes (2011) (n = 2). Masquer
Diagramme montrant la composition isotopique en silicium (Si) (moyenne ± 2σ) de rivières majeures à l’échelle mondiale en fonction du flux d’altération des silicates (somme totale des cations dissous dérivés de l’altération des silicates ; Gaillardet et al., 1999a). Données isotopiques tirées ... Lire la suite
5 Future research directions
5.1 Weathering, erosion and river suspended sediments
The continental Si transfer to the ocean includes dissolved and particulate Si. In this review, the focus has been placed on DSi originating from chemical weathering of continental silicate rocks, the process thought to regulate atmospheric CO2 level over geologic time scales. However the DSi flux to the ocean (67,857 × 103 T.y−1) is substantially lower than that of particulate Si (3,816,00 × 103 T.y−1; Oelkers et al., 2011; Viers et al., 2009). The flux of riverine sediments is not well constrained and ranges from 12.6 to 24 Gt.yr−1 (Viers et al., 2009; Walling, 2006). River suspended material is thought to integrate the whole weathering history within a basin, and not only the present day weathering fingerprint (Gaillardet et al., 1999b). This suggests that river suspended material may include particles originating from successive cycles of secondary mineral dissolution/precipitation at very advanced weathering degree. River suspended materials also include biogenic Si from phytoliths and diatoms (Cary et al., 2005; Conley, 1997; Hughes et al., 2011a; Opfergelt et al., 2009). Silicon isotope determinations of river suspended matter are available only for two major rivers, the Yangtze and the Yellow River, with δ30Si values ranging from −0.7 to +0.3‰ (Ding et al., 2004, 2011). After extraction of the BSi fraction from river suspended material, the Si isotope composition of the lithogenic fraction may be used to investigate the weathering degree of river suspended matter, as δ30Si measurements in soils are shown to reflect their weathering degree (Section 4.1). This approach would improve our ability to trace sources of particulate Si in river suspended matter, since the nature and amounts of particles produced from erosion is dictated by the weathering regime, i.e., weathering- or transport-limited (Section 4.3). When most particles are eroded from transport-limited environments, the δ30Si of river suspended matter is expected to reflect the weathering degree of the source. Conversely, when most particles are eroded from weathering-limited environments (high erosion rate), the δ30Si of river suspended matter is expected to reflect that of the bedrock.
5.2 Hydrothermal environments
In volcanic regions, chemical silicate weathering fluxes can be strongly influenced by hydrothermal water-rock interaction (Dessert et al., 2009; Jones et al., 2011; Louvat et al., 2011). Recently, Dessert et al. (2009) estimated that 25% of the total cationic weathering rate in the Kamchatka Peninsula, Russia, resulted from the hydrothermal contribution. Similarly, Jones et al. (2011) highlighted that the highest concentrations of dissolved solutes corresponded to rivers draining the active part of La Soufrière volcano on Montserrat. Importantly, the major control on chemical weathering rates in these areas is the input of volcanic acids, mainly H2SO4, rather than non-volcanic H2CO3 (Dessert et al., 2009; Gaillardet et al., 2011).
Additional work is needed in order to quantify the hydrothermal contribution to the weathering budget, and Si isotopic studies may constitute a useful tool. Based on the few analyses reported in the scientific literature, secondary clay minerals formed during hydrothermal alteration display notably heavier δ30Si values than their meteoritic counterparts (Fig. 3). Conversely, the isotopic composition of DSi in hydrothermal fluids seems to be lighter than that typically found for surface water (Fig. 3). Thus, using isotopic mass balances, Si isotopes may serve to extract the contribution from hydrothermal waters to the riverine DSi flux.
5.3 Dust contribution to continental chemical fluxes
Mineral dust sourced to soils in semi-arid and arid areas is a major constituent of airborne particles (Derry and Chadwick, 2007) and is suggested to play an important role in climate forcing by altering the radiation balance in the atmosphere (Mahowald et al., 2006; Miller and Tegen, 1998). Soil dust deposition may also impact nutrient cycles, including Si, in ocean and terrestrial ecosystems, thereby influencing the uptake of CO2 in the biosphere and atmospheric CO2 concentrations (Chadwick et al., 1999; Watson et al., 2000). About 10 Tmol (280 Tg) particulate Si reaches the ocean surface by eolian dust transport, of which 5–10% may dissolve in seawater (Tréguer et al., 1995). Thus, Si is effectively redistributed in the environment through transport of soil-derived dust in the atmosphere. Mineral dust deposition also can supply weatherable minerals, thereby contributing to chemical weathering fluxes (Pett-Ridge et al., 2007, 2009).
The vast majority of atmospheric dust is emitted from North African (50–70%) and Asian (10–25% deserts (Tanaka, 2011). However, global Quaternary records of dust suggest that different climatic periods are accompanied by a large variation in dust fluxes and possibly sources (Kohfeld and Harrison, 2001). In addition, soils disturbed by recent human activity have been shown to enhance eolian dust deposition in parts of the world (Neff et al., 2008). In order to document these changes and ultimately, to relate them to climate and environmental forcing, the sources of soil dust reaching the ocean or distal terrestrial ecosystems must be identified. Typically, the mineralogical and chemical fingerprints of potential source areas are matched with those of the dust sample under study; more rarely, radiogenic isotopes (Rb, Sr, Nd) have been applied (Kohfeld and Harrison, 2001).
Silicon isotopes may provide a powerful tool for tracing present and past sources of soil dust in the atmosphere as the Si isotopic signature is more specific than the mineral and chemical characteristics of source areas. As shown earlier, Si isotope measurements offer the possibility to estimate the degree and type (transport- vs. weathering-limited; as defined in Section 4.3) of weathering of the source area (Sections 4.1 and 4.3). This may be particularly useful when considering the radical changes in weathering regimes, which may have accompanied climate shift of the past. For example, Arnold et al. (1995) argued that the uplift, and subsequent cooling, of the Tibetan Plateau led to a change from a transport-limited to a weathering limited environment. This pattern should be recorded in the Si isotopic signature of the dust clay minerals deposited far away from the source.
5.4 Paleoweathering processes
It has been inferred in Section 4.1 that the soil Si isotopic signature reflects weathering degree, which in turn provides an indication of the extent to which mobile elements have been leached from the parent material. By analogy, the δ30Si of paleosols may provide valuable information on the intensity and types of weathering processes in the past. Paleosols are abundant throughout the geological record (Retallack, 2001, 2003), and it has been shown that the distribution of Si isotopes is not significantly affected by metamorphic processes (André et al., 2006). Thus, we suggest that δ30Si measurements may constitute a new tool for studying paleoweathering processes and therefore, for improving our understanding of variable continental chemical weathering fluxes to the ocean (Vance et al., 2009).
In addition, Si isotopic investigations may also provide new insights into Banded Iron Formations (BIFs). BIFs are prominent sedimentary deposits of the Precambrian, characterized by alterning Si-rich (cherts) and Fe oxide-rich layers. These deposits constitute exceptional records of the chemical conditions that prevailed in ancient oceans. However, BIF formation is not yet fully elucidated. Notably, it has been suggested that the Si in cherts is derived from continental weathering, while Fe in the Fe oxide rich layers is hydrothermal in origin (Hamade et al., 2003; Frei and Polat, 2007). Encouragingly, the results of recent studies suggest that δ30Si measurements can illuminate the factors driving Si contribution during BIFs formation (André et al., 2006; Steinhöfel et al., 2010).
Acknowledgements
We thank C. Bern for providing complementary information on Hawaiian soils, H. Hughes for sharing his compilation of Si isotope measurements on rivers and P. Ayris for drawing Fig. 1. S.O. has greatly benefited from discussions with her former and present colleagues from Royal Museum for Central Africa, University of Oxford, Université Libre de Bruxelles and Université catholique de Louvain. S.O. and P.D. acknowledge support from the Belgian Fonds de la Recherche Scientifique (Postdoctoral Researcher grant 1.7.048.09.F and MIS-Ulysse grant F.6001.11, respectively).



Vous devez vous connecter pour continuer.
S'authentifier