1 Introduction
The chemical element osmium (Os, atomic number 76) has seven stable isotopes, of which187Os has a wide range of relative abundances in nature because part of the 187Os found in a geological sample comes from the radioactive decay of 187Re (half-life of about 42 billion years; Smoliar et al., 1996) and varies as a function of both lithology and geological age. Osmium has a limited number of anthropogenic uses, which should theoretically limit its dispersal in the environment. Nevertheless, osmium contamination does occur and the principal sources of osmium inputs to the environment seem to be derived from the industrial production of metals (Chen et al., 2009; Rodushkin et al., 2007), automotive exhaust catalysts (Rauch et al., 2004; Poirier and Gariépy, 2005) and biomedical facilities emitted from hospital incinerators due to the use of OsO4 as a fixative for electron microscopy of organic material (Esser and Turekian, 1993). High osmium concentrations have been reported in coastal and estuarine sediments, and observed shifts in isotopic compositions have supported the conclusion that anthropogenic Os contamination into the environment is occurring (Esser and Turekian, 1993; Ravizza and Bothner, 1996). Dissolved aquatic Os is believed to be rapidly scavenged onto particle surfaces in reducing environmental redox settings, so that Os dispersion in such aquatic environment is controlled by particle transfers (Williams et al., 1997). Surface seawater was even reported as having been globally contaminated by anthropogenic Os (Chen et al., 2009). This chemical behaviour has quickly generated interest in the 187Os/188Os ratio as a tracer of anthropogenic inputs to sediments (Esser and Turekian, 1993; Ravizza and Bothner, 1996; Williams et al., 1997).
Our study area is the surroundings of the Grande-Baie aluminum smelter (G-B), operated by Rio Tinto Alcan (RTA) near the town of La Baie (Québec, Canada). Located on the shores of the Baie des Ha!-Ha! (BHH, Fig. 1), this smelter started operating in 1980. Aluminum smelting is a major industrial process within the realm of base metal production. The process of aluminum production involves the electrolytic reduction of alumina to Al (liquid) by reacting with a carbon anode in a cell containing a bath of molten alumina and cryolite (Na3AlF6), used as a fusion flux. In this specific case, the main process atmospheric emissions are, in order of abundances: CO2, gaseous fluorides, sulfur dioxide (SO2) and dust (Divan Junior et al., 2008). Thus, the hundred-meter-long pot rooms are subject to dust emission associated with the produced gases. Raw pot gases and dust are recovered directly from the cells and then scrubbed at gas treatment centres (GTC), with bag filters and injections of fresh alumina that reacts with the fluoride in the gas (part of that fluorinated alumina is then re-used for the electrolytic reduction). This process is > 99.5% efficient at removing dust and gaseous or particulate fluorides (Boullemant, 2011). Emitted dust comes mainly from alumina and molten bath particulate matter entrained to stacks with the carbon dioxide created during anode consumption. During maintenance operations on pots (especially during anode replacing), particulate matter that is neither recovered nor treated can be emitted via roof vents. These emissions are most probably deposited in the local environment of G-B (emissions of low velocity and low temperature “large” particles–i.e. > 2.5 μm). The fume treatment centre (FTC), located in another area of the plant, recovers fumes from the anode-baking furnace, where green anodes from the paste plant – where the anode paste (pitch and coke) is crushed, ground, and mixed – are shaped and baked (Boullemant, 2011). The fumes are then scrubbed with the same process as GTC. G-B smelter also has two effluents: 1201 for rainwater and 1202 for wastewater from the plant. They join together to form the 1203A effluent, which successively discharges into two artificial sedimentation lakes (Neree and Poleon lakes) and emerges as 1203B effluent to join BHH via the Mars river (Fig. 2).

Grande-Baie aluminum smelter location.
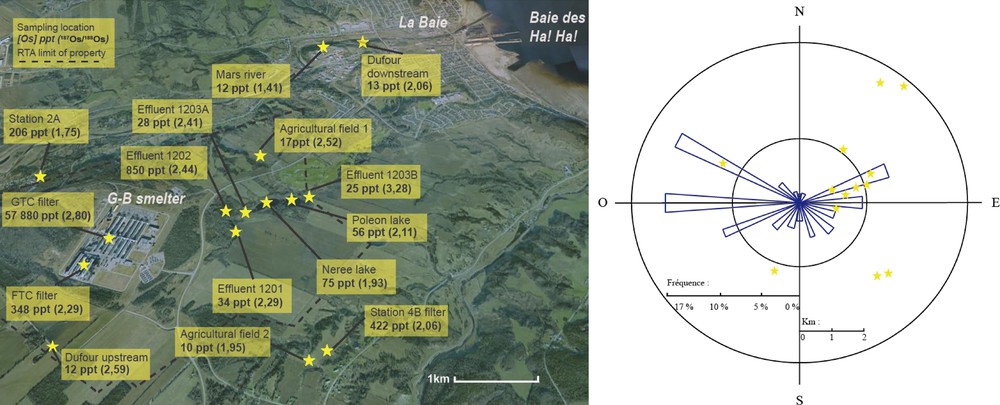
(Colour online.) Close-up view of sampling location (modified Google map image) and radial diagram of winds distribution for the Grande-Baie's smelter.
Modified from GENIVAR, 2008.
Osmium is present as a significant natural impurity in the anode, because of its fossil fuel origin (i.e. distillation residue from petroleum refining). The emission process of this heavy metal (atomic mass ∼ 190.2 g/mol) will not change the isotopic composition of the metal to any significant degree. Thus, depending on the age and composition of local country rock and soils, the isotopic signature of the emitted dust can be different and detectable from the surrounding environment. The carbon anode is an essential component of the electrolysis process used in aluminum smelters. The fact that it is fully consumed during the oxygen exchange reaction (2Al2O3 + 3Canode → 4Al(l) + 3CO2(g); see below) suggests that trace elements present in it can be emitted at the stacks or the roof vent, being drawn in and transported by the generated gas. The anode being a carbon-rich by-product of petroleum refining industry (petroleum coke and pitch), one can expect it to contain a significant amount of radiogenic osmium (e.g., Selby et al., 2005). Coke and pitch have high Re/Os elementary ratios, and thus, over their burial time (many million years) develop large quantities of 187Os from in situ disintegration of 187Re. Consequently, the 187Os/188Os ratio of coke/pitch will be much higher than those of ultramafic deposits, which are the typical anthropogenic platinum group metal sources and have 187Os/188Os ratios closer to that of the Earth's mantle (0.1–0.2). Therefore, it should be possible to follow emissions of osmium in the atmosphere from smelters using its isotopic fingerprint. An anode used in aluminum smelting at G-B was measured at 187Os/188Os = 2.393 ± 0.005 (2σ) (Boullemant, 2011), yielding a radiogenic value as expected, which is significantly different from usual anthropogenic sources (< 0.2) (Chen et al., 2009; Peucker-Ehrenbrink and Ravizza, 2000), and from average eroding continental crust (about 1.4; Peucker-Ehrenbrink and Jahn, 2001).
2 Experimental
We measured the uppermost 80 cm corresponding to the last ca. 300 years of a piston core raised in Baie des Ha! Ha! during the 1997 cruise of the CSS Martha L. Black. This core was raised immediately following a local flash flood in 1996 that deposited ca. 10 cm of sediments (St-Onge and Hillaire-Marcel, 2001). A complementary box core from the same location was obtained in June 2010 onboard R/V Coriolis II, in order to obtain sedimentation record up to the present day, and hence get a more complete picture of the sedimentary history for the area. Soil and sediment samples from nearby agricultural fields, smelter's effluents, sedimentation lakes, and a stream and a river close to the smelter were collected in August 2010 (Fig. 2). Fiberglass filters used for 2.5 μm particulate matter (PM2.5), direct sampling of the exhaust stacks of Grande-Baie's gas treatment centre (in the Al-reduction sector) and fumes treatment centre (in the anode-baking sector) have been also obtained. Gases containing particulate matter were sampled in the chimneys, just before the exhaust to the atmosphere. The amount of air sampled by the fibreglass filters was about 800 m3. Because we wanted the solid-dust fraction only, ice was placed before the filters in the sampling system in order to minimize condensation on the fiberglass filter (otherwise the filter would have gotten wet and clogged). Filters from two air-quality monitoring stations located outside the industrial property were also collected. These glass fiber filters have captured PMtotal present in the ambient air for a week. The respective locations of these stations appear on Fig. 2 under the names Station 2A and Station 4B. A new piece of anode used in the smelting process was analyzed in order to obtain an Os input signal. Fresh alumina, roof vent dust, and final produced aluminum were also analysed to determine G-B's osmium annual balance.
Samples were ground to a fine powder in an alumina mortar and pestle apparatus. About 1 g of powdered sample (less for the anode) was mixed with a 190Os–185Re spike and inverse aqua regia (3 parts HNO3, 2 parts HCl) in a quartz vessel. This mixture was digested for 3 h 30 at 300 °C under a pressure of 120 bars of nitrogen in a High Pressure Asher (Anton Paar). This precludes the escape of gases (including volatile OsO4) from the vessels, even under oxidizing conditions and high temperatures (Meisel et al., 2003a,b; Paul et al., 2009). After digestion, osmium was extracted using liquid Br2 (Birck et al., 1997) and purified by micro-distillation (Roy-Barman and Allègre, 1994). Prior to mass spectrometry, Os samples were loaded with a small quantity of activator (0.5 μL of freshly prepared barium hydroxide in NaOH solution) on Pt filaments previously outgassed in the air. Isotopic analyses were performed by negative thermal ionization mass spectrometry (N-TIMS) on a Thermo Triton equipped with an O2 bleeding valve (Creaser et al., 1991; Völkening et al., 1991). This instrument is equipped with a dedicated sample turret and ion source for Os measurements. Samples were measured by peak jumping on a secondary-electron multiplier. We measured all Os isotopes (except 184Os) as oxides and monitored 185Re16O3 for potential interference of 187Re16O3 on mass 235. The oxygen-bleeding rate into the mass spectrometer source was optimized for each sample, and typically resulted in a source pressure of 2 × 10−7 mbar. Normalization of isotopes ratios was done offline using 192Os/188Os = 3.08271 (Nier, 1937) after spike and oxygen contribution corrections. During the course of this study, full-procedure blanks (n = 16) were negligible and yielded between 0.02 and 0.71 pg (average = 0.15 pg) of total osmium with 187Os/188Os average ratios of 1.02 ± 0.5 (2σ). Repeated osmium DROsS standard measurements (n = 12) yielded an average ratio of 187Os/188Os = 0.1607 ± 0.0003 (2σ) for ca. 10 pg size loads.
3 Results and discussion
Isotopic compositions and concentrations of osmium from sediment core samples between years 1700 and 1900 were obtained from the piston core. These correspond to a natural (pre-industrial) background for the regional environment of G-B: 187Os/188Os ∼ 2.4 and [Os] = 30 ppt (Fig. 3). This sedimentary 187Os/188Os ratio is higher than the average upper continental crust (Peucker-Ehrenbrink and Jahn, 2001) and, unexpectedly, very close to the one measured in anodes (Table 1). This radiogenic composition is most likely a consequence of the local geology: with a geological age of ∼ 1 Ga, the Grenville geological province is a belt of metamorphic rocks containing large masses of intrusive granitic rocks outcropping at the southern margin of the Canadian Shield (Hocq and Dubé, 1994). However, occurrences of sedimentary sequences from the Paleozoic St-Laurent lowlands platform, which are known to contain black shale units, have been recognized outcropping in the region (MERN, 2015). This may contribute, perhaps through glacial till deposits around BHH, to the high natural 187Os/188Os ratio shown by pre-industrial local sediments. Such a local isotope signature makes it more difficult to discern the signal of the anode within the recent sediments. However, with the exception of the 1996 coarse grain enriched flood layer (80% silt + sand), concentrations of osmium in these sediments are slowly increasing and coupled with a simultaneous decrease in isotopic ratio since 1850 (Fig. 3), suggesting small gradual increase in unradiogenic Os input during the industrial era, most probably from global diffuse Os (Chen et al., 2009). The slight increase in Os content beneath the low level of the flood layer of 1996 could be the result of early diagenetic processes, related to the redox affinities of osmium: the sediments brought in the Baie des Ha!-Ha! by the 1996 flood created a layer of freshly oxygenated detrital material from which Os diffused down to the underlying more organic-rich layer (Poirier, 2006). The osmium content of the surface sediment (1997–2010) seems to be confirming this, with Os contents lower than pre-1996 layers. Natural variability seems to be the dominating factor for the osmium changes observed in the sediments from Baie des Ha! Ha!, thus suggesting a rather weak influence, if any, of G-B on the regional Os budget. Using pre-1900 sediments as the present-day natural background (with the Os isotope ratio oscillating between 1.9 and 2.9), one can calculate the residual anthropogenic fraction for the most recent points (from the core raised in 2010). These data define a binary mixing line that can be used to calculate a theoretical fraction due to the smelter (assuming it has a constant 187Os/188Os = 2.393). Such calculations suggest that a maximum of 2–3 × 10−12 g/g Os in the Baie des Ha!-Ha! sedimentary levels would originate from the smelter, with an uncertainty budget encompassing zero, because of the high variability of the natural background, indicating very little impact on a regional scale. When focusing on samples from soils and stream/lake sediment from the very nearby environment of the smelter, we observe a gradual decrease in concentrations of Os (from 850 to 13 ppt) away from the factory (Fig. 4). A value of 25 ppt is measured at the sampling point closest to the boundary of the property of RTA (effluent 1203B), which is below the average natural level of the eroding continental crust (30 ppt), and very close to the natural local background. The wastewater cleaning process set up by G-B, which includes two sedimentation lakes, thus seems efficient for particulate osmium removal. Although the concentration of osmium on filters from the GTC's stacks is relatively high, the amount of osmium released annually in the form of solid particles (∼ 2.5 g of Os per year for GB, less than a kilogram for all Al-smelting activities, see below) when accounting for the particulate matter emission flow rate at stacks, is low in comparison to the potential Os emitted from all base metal smelters (up to a few thousand kilograms; Chen et al., 2009).
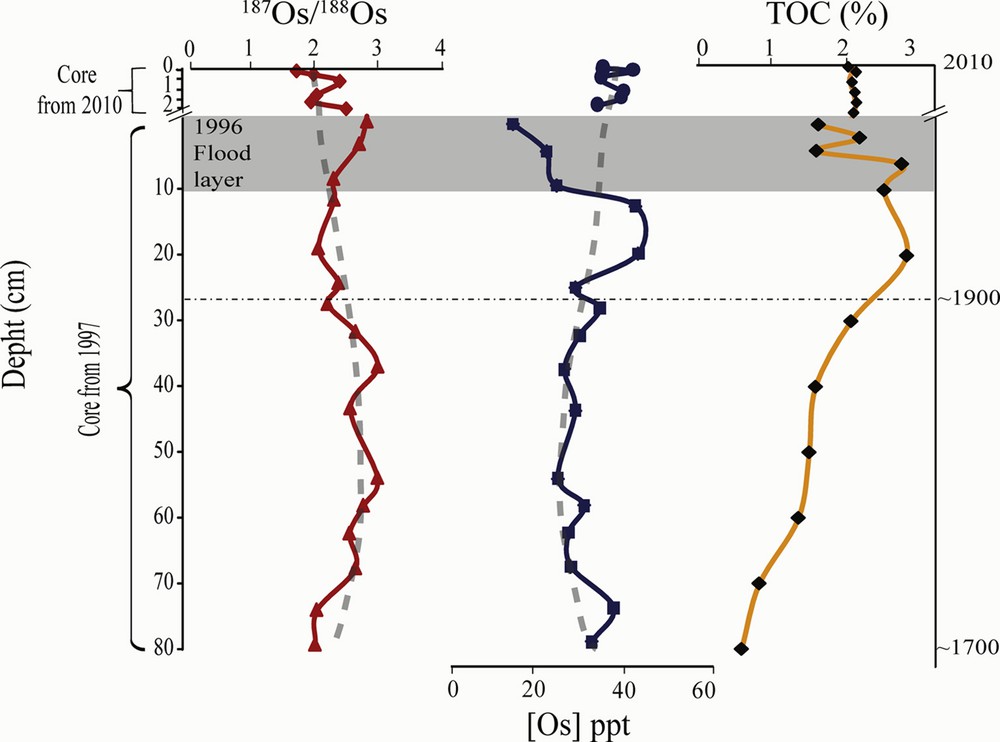
(Colour online.) Osmium content, organic carbon and isotopic composition of sediments from Baie des Ha! Ha!, Québec.
Chronology of 1997 core modified from St-Onge and Hillaire-Marcel, 2001.
Elemental concentrations of osmium, total organic carbon and isotopic composition of osmium.
| Sample | Depth (cm) | 187Os/188Os ± (2σ) |
[Os] (ppt) | TOC (%) | Latitude | Longitude |
| 1997 core | 0.5 | 1.84 | 48°22.0 N | 70°46.2 W | ||
| 1997 core | 1.5 | 2.75 ± 0.01 | 14 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 2.5 | 2.11 | 48°22.0 N | 70°46.2 W | ||
| 1997 core | 4.5 | 1.71 | 48°22.0 N | 70°46.2 W | ||
| 1997 core | 6.5 | 2.45 | 48°22.0 N | 70°46.2 W | ||
| 1997 core | 7.5 | 2.64 ± 0.00 | 22 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 10.5 | 2.08 | 48°22.0 N | 70°46.2 W | ||
| 1997 core | 12.5 | 2.25 ± 0.01 | 24 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 15.5 | 2.25 ± 0.01 | 42 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 20.5 | 2.25 | 48°22.0 N | 70°46.2 W | ||
| 1997 core | 22.5 | 2.01 ± 0.00 | 43 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 27.5 | 2.32 ± 0.01 | 29 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 30.5 | 2.15 ± 0.01 | 34 | 1.72 | 48°22.0 N | 70°46.2 W |
| 1997 core | 34.5 | 2.58 ± 0.01 | 30 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 39.5 | 2.92 ± 0.00 | 26 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 40.5 | 1.38 | 48°22.0 N | 70°46.2 W | ||
| 1997 core | 45.5 | 2.50 ± 0.02 | 29 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 50.5 | 1.3 | 48°22.0 N | 70°46.2 W | ||
| 1997 core | 55.5 | 2.92 ± 0.01 | 25 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 59.5 | 2.70 ± 0.01 | 31 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 60.5 | 1.23 | 48°22.0 N | 70°46.2 W | ||
| 1997 core | 63.5 | 2.49 ± 0.02 | 27 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 68.5 | 2.58 ± 0.01 | 28 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 70.5 | 0.89 | 48°22.0 N | 70°46.2 W | ||
| 1997 core | 74.5 | 1.99 ± 0.01 | 37 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 79.5 | 1.96 ± 0.00 | 32 | 48°22.0 N | 70°46.2 W | |
| 1997 core | 80.5 | 0.73 | 48°22.0 N | 70°46.2 W | ||
| 2010 core | 0 | 1.67 ± 0.01 | 35 | 1.93 | 48°21.0 N | 70°48.1 W |
| 2010 core | 0.5 | 1.93 ± 0.01 | 42 | 2.04 | 48°21.0 N | 70°48.1 W |
| 2010 core | 1 | 2.34 ± 0.01 | 34 | 2.02 | 48°21.0 N | 70°48.1 W |
| 2010 core | 1.5 | 2.04 | 48°21.0 N | 70°48.1 W | ||
| 2010 core | 2 | 1.98 ± 0.01 | 39 | 2.06 | 48°21.0 N | 70°48.1 W |
| 2010 core | 2.5 | 1.89 ± 0.01 | 39 | 2.03 | 48°21.0 N | 70°48.1 W |
| 2010 core | 3 | 2.44 ± 0.00 | 34 | 1.95 | 48°21.0 N | 70°48.1 W |
| Dufour upstream | 2.59 ± 0.00 | 12 | 48°17.878 N | 70°55.856 W | ||
| Dufour downstream | 2.06 ± 0.01 | 13 | 48°20.023 N | 70°53.682 W | ||
| Effluent 1201 | 2.29 ± 0.01 | 34 | 48°18.317 N | 70°54.828 W | ||
| Effluent 1202 | 2.44 ± 0.01 | 850 | 48°18.584 N | 70°54.894 W | ||
| Effluent 1203A | 2.41 ± 0.00 | 28 | 48°18.556 N | 70° 54.692 W | ||
| Effluent 1203B | 3.28 ± 0.01 | 25 | 48°18.652 N | 70°54.217 W | ||
| Agricultural field 1 | 2.52 ± 0.00 | 17 | 48°18.768 N | 70°54.685 W | ||
| Agricultural field 2 | 1.95 ± 0.02 | 10 | 48°17.772 N | 70°54.105 W | ||
| Neree lake | 1.93 ± 0.01 | 75 | 48°18.600 N | 70°54.500 W | ||
| Poleon lake | 2.11 ± 0.01 | 56 | 48°18.600 N | 70°54.300 W | ||
| Mars river | 1.41 ± 0.01 | 12 | 48°20.033 N | 70°53.854 W | ||
| Station 2A filter | 1.75 ± 0.01 | 207 | 48°18.620 N | 70°57.100 W | ||
| Station 4B filter | 2.06 ± 0.02 | 422 | 48°17.750 N | 70°54.100 W | ||
| GTC filter (n = 4) | 2.81 ± 0.01 | 70 939 | 48°18.320 N | 70°55.500 W | ||
| 2.85 ± 0.00 | 53 462 | 48°18.320 N | 70°55.500 W | |||
| 2.68 ± 0.01 | 63 115 | 48°18.320 N | 70°55.500 W | |||
| 2.86 ± 0.01 | 44 007 | 48°18.320 N | 70°55.500 W | |||
| FTC filter (n = 2) | 2.31 ± 0.02 | 264 | 48°18.250 N | 70°55.700 W | ||
| 2.77 ± 0.00 | 433 | 48°18.250 N | 70°55.700 W | |||
| Anode (n = 3) | 2.04 ± 0.01 | 201 | ||||
| 2.12 ± 0.01 | 211 | |||||
| 2.53 ± 0.01 | 211 | |||||
| G-B roof vent dust | 2.61 ± 0.01 | 6 369 | ||||
| Aluminum paper | 2.46 ± 0.01 | 31 |
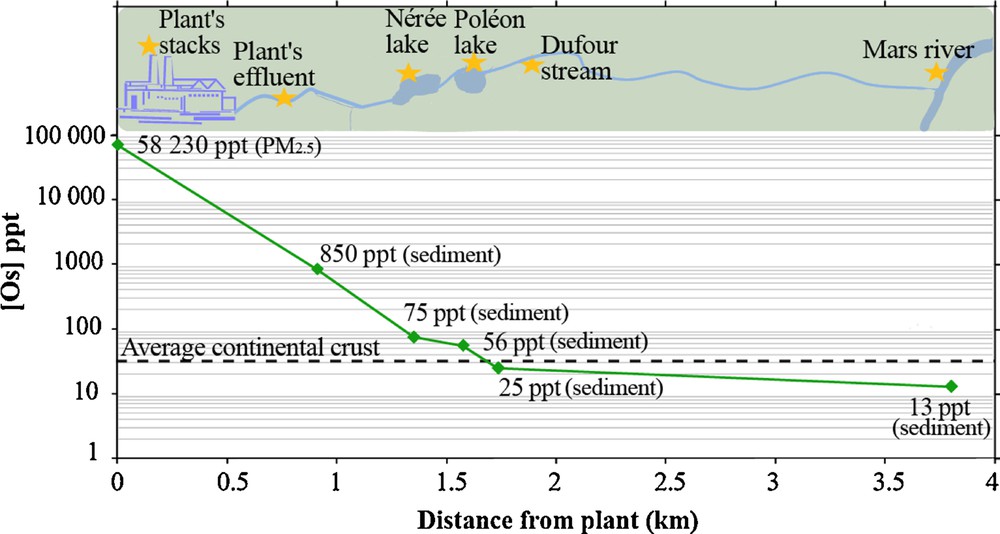
(Colour online.) Decrease of osmium content in the surroundings of G-B smelter.
Average crust value from Peucker-Ehrenbrink and Jahn, 2001).
Finally, when trying to understand the G-B smelter's internal balance of osmium, we end up with a significant deficit on the output side (ca. 65%) (Table 2). We are still investigating this issue, but excluding an analytical artefact, we suggest that some of the exhausted osmium could be released as gaseous specie(s) from GTC. Osmium tetroxide (OsO4, with Os in +VIII valence state) has a boiling point of < 130 °C and is known to be volatile at room temperature. It is known to be a very oxidizing/reactive chemical species and would thus be reduced to its non-volatile form quickly once released in open air and deposited with particulate matter and ambient dust. On the other hand, the thermodynamics of the fluoride-rich process of aluminum production might favour the generation of low-boiling-point (< 50 °C) osmium fluoride species (Haynes, 2011). To help clarify this internal balance, more work is needed to sequester and isolate gaseous emissions from the stacks at different condensation temperatures, in order to measure their respective osmium contents. If G-B is representative of average aluminum smelting plants worldwide, then using our osmium results in the particulate matter annual emissions and worldwide production of primary aluminum (51.6 M tons; Wang et al., 2012), we can calculate that ca. 600 g of particulate osmium would be released by the global aluminum industry. Although this amount is low when compared to global Os emissions to the environment, it is interesting to include it into the annual global budget of anthropogenic osmium released into the environment, as it is a rare source of highly radiogenic anthropogenic osmium. Because of this feature, aluminum smelting activities could mask the apparent impact estimates of other industries based on the sole 187Os/188Os ratio in densely industrialized areas with multiple sources of osmium contamination, as it could shift the more typical anthropogenic Os isotopic values from mantle-like signature towards un-impacted continental crust values.
Mass balance of G-B smelter's input and output osmium.
| Output | ton/year | Os concentration (g/g) | Os (g/year) | % of the osmium inputa |
| Anode butts (not recycled) | 877 | 2.08 × 10−10 | 0.182 | 0.7 |
| Aluminum produced | 215 000 | 3.12 × 10−11 | 6.717 | 26.2 |
| Roof vents dust (PM total) | 182.84 | 6.37 × 10−09 | 1.164 | 4.5 |
| PM2.5 from GTC | 20.2 | 7.09 × 10−08 | 1.433 | 5.6 |
| PM2.5 from FTC | 3.65 | 4.33 × 10−10 | 0.002 | < 0.1 |
a There is a potential 25.66 g of osmium liberated from 123,492 tons of consumed anode per year in G-B smelter. See main text.
4 Conclusion
This study presented the potential of osmium isotopes and concentration as tracers of particulate atmospheric heavy metal emissions from an aluminum smelter. Many anthropogenic heavy metal inputs to the natural environment have been quantified by previous studies, but the use of radiogenic isotopes as tracers of industrial sources is not yet widespread, but very promising. This study allowed us to follow the PM2.5 emissions from the stack of the smelter, into its nearby vicinity, until natural background level was found again. When trying to understand the osmium internal balance within the smelter, we noted a deficit on the output side. This apparent paradox can be resolved if the osmium is released as gaseous specie(s) from the smelter. More work is required regarding this hypothesis and to find out the exact volatile chemical species involved (both fluoride and oxide compounds being thermodynamically possible within this particular setting). More generally, the strong radiogenic signature of the raw material (anode) involved in the Al-smelting process makes osmium a remarkable forensic-like tool to fingerprint the impact of such industrial input to the environment, and eventually to discriminate its impact from those coming from other (base metal ore) smelting activities.
Acknowledgment
This article is dedicated to the friendship and memory of Dr. Jean Carignan. This work has been supported by a FQRNT-NSERC scholarship to J. Gogot. Rio Tinto Alcan is acknowledged for their financial and technical support (with special thanks to Dr. Rachel Dosnon-Olette and Jean-Michel Jolas for pre-reviewing the original manuscript). We acknowledge a thorough review by Dr. L. Reisberg.


