1 Introduction
Due to increased anthropogenic activity, including industrial production (e.g., waste incineration, power plants, metal refining, etc.) and vehicular traffic (leaded petrol combustion), the input of heavy metals (HM) to the atmosphere has increased significantly over the past sixty years (Nriagu and Pacyna, 1988; Patterson and Settle, 1987). Epiphytic lichens are commonly used in biomonitoring studies because they derive their nutrients, and consequently the entire range of atmospheric fallout (dry and wet), from the atmosphere (e.g., Ayrault et al., 2007; Bergamaschi et al., 2002; Carreras et al., 1998; Cercasov et al., 2002; Conti et al., 2004; Gandois et al., 2014; Getty et al., 1999; Hissler et al., 2008; Monna et al., 1997; Palomaki et al., 1992). The HM concentration recorded in lichens has long been used to monitor the evolution of atmospheric inputs (Garty, 2001; Getty et al., 1999).
Among the different HM, Pb is widely used to monitor anthropogenic inputs to the atmosphere. In addition to Pb concentrations, Pb isotope ratios can be used to identify different sources and determine their relative contributions to the atmosphere (Aberg et al., 1999; Haack et al., 2002; Le Roux et al., 2008; Reimann et al., 2008). Lead has four stable isotopes (204, 206, 207, 208), three of which are derived from radioactive decay of U and Th 238,235U (206Pb, 207Pb) and 232Th (208Pb). Pb isotopes are not significantly fractionated by anthropogenic activity, such as smelting/refining (Baron et al., 2009; Cui and Wu, 2011; Gale and Stos-Gale, 1996) and their isotope ratios reflect the age of the source material. Consequently, differences in Pb isotope composition can be used to discriminate different sources. The Pb isotope technique has been employed in a number of environmental studies often using airborne particulates (e.g., Aberg et al., 1999; Bollhofer and Rosman, 2001; Deboudt et al., 1999; Flament et al., 2002; Graney and Landis, 2013; Haack et al., 2002; Lahd Geagea et al., 2008a, b; Mihaljevic et al., 2011; Monna et al., 1997; Negrel et al., 2015; Simonetti et al., 2000; Véron et al., 1999; Weiss et al., 1999). Since the 1995 publication of the pioneering study by Carignan and Gariepy (1995), numerous studies have highlighted the advantages of using Pb isotope ratios in lichens as a powerful tool for identifying atmospheric source end-members (Chiaradia and Cupelin, 2000; Dolgopolova et al., 2006; Doucet and Carignan, 2001; Monna et al., 1997; Simonetti et al., 2003; Spiro et al., 2004). However, few studies have applied the technique in the vicinity of urban areas (Cloquet et al., 2006a,b; Cloquet et al., 2009; Gueguen et al., 2012; Hissler et al., 2008; Lahd Geagea et al., 2007). The main goal of the present study was to evaluate whether temporal and/or spatial trends in atmospheric fallout occurred over a decade in areas affected by local to long-range atmospheric contamination. To reach this objective, we:
- • monitored trace element concentrations in lichens in an urban area and its surroundings over a 10-year period;
- • used enrichment factors to evaluate the anthropogenic contribution to large spatial and temporal gradients;
- • used Pb isotopes to identify the different sources of metals in the atmosphere.
2 Sampling and methods
This study presents elemental concentrations of selected metals and Pb isotope compositions in epiphytic lichens collected during five sampling campaigns conducted between 2001 and 2009 in northeastern France. The study area (Fig. 1) covers around 900 km2 and contains about 400,000 inhabitants. The territory was divided into four geographical area – rural, suburban, urban and industrial – as described in Estrade et al. (2010a,b). In brief, the urban area (8 sites) is represented by the city of Metz (130,000 inhabitants). The suburban area (17 sites) extends from the city to the rural area (13 sites), and includes the flatlands to the south, east, and west. To the north of Metz, a large industrial valley has been developed, which contains mostly steel industries and power plants (13 sites). The dominant wind directions are from the southwest and northeast and winds strengthen from south to north. During the 2009 field campaign, only the industrial area was sampled.
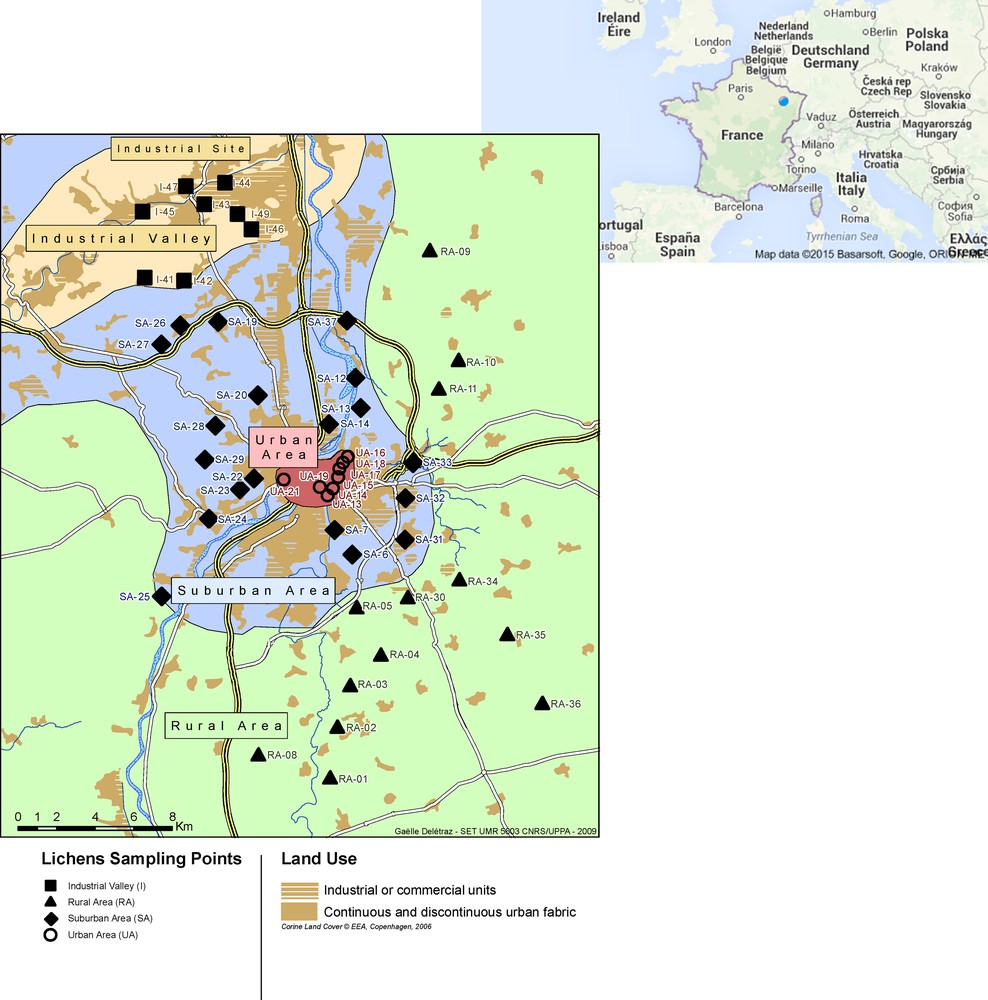
(Color online.) Locations of sampling sites and the different zones defined according to land occupation (modified from Estrade et al., 2010a, b).
A selection of epiphytic lichens (Evernia prunastri, Ramalina farinacea, and Hypogymnia physodes), representative of the different areas was collected from small tree branches, as previously described (Carignan et al., 2002; Cloquet et al., 2006a, 2009; Doucet and Carignan, 2001). Lichens were collected using pre-cleaned plastic tweezers and then placed inside sealed plastic bags for transport to the laboratory (Carignan and Gariepy, 1995; Cloquet et al., 2006b). Lichens were isolated from their substratum when necessary, and then dried at 105 °C for 24 h or freeze-dried. Lichens were digested using a mixture of HNO3, H2O2 and HF and diluted in LiBO2 prior to elemental determination (Cloquet et al., 2006a; Doucet and Carignan, 2001). Major and trace elements were determined at the “Service d’analyse des roches et des minéraux” (SARM), following the procedure described elsewhere (Carignan et al., 2001). A Thermo IRIS ICP-OES was used for major and minor element analyses, a PerkinElmer Elan 5000 and Thermo X7 ICP-MS were used for trace element determinations and CVAAS was used for Hg determination (Estrade et al., 2010b). Internal reference material and the international BCR-CRM 482 reference material were included and processed in each preparation batch for quality control purposes.
Lead from the lichens, digested as described above and then dissolved in HBr 0.8 M, was isolated from the rest of the matrix using the method described in Manhès et al. (1980). Lead isotopic analyses were performed on purified samples using the Micromass Isoprobe MC-ICP-MS at CRPG (Nancy, France), as detailed elsewhere (Baron et al., 2006; Cloquet et al., 2006a), and Tl external normalization (White et al., 2000). NIST SRM 981 Double Spike TIMS values were used as a reference (Thirlwall, 2002) and measurements of four BCR-CRM 482 digestions yielded values of 17.612; 15.574; 37.498; 1.1308; 2.1290 for 206Pb/204Pb; 207Pb/204Pb; 208Pb/204Pb; 206Pb/207Pb and 208Pb/206Pb, respectively. These values are consistent with previously published ones (Cloquet et al., 2006a; De Muynck et al., 2008). The two standard deviation of the mean calculated on Pb BCR-CRM 482 isotope ratios 206Pb/204Pb; 207Pb/204Pb; 208Pb/204Pb; 206Pb/207Pb and 208Pb/206Pb were 78 ppm; 65 ppm; 62 ppm; 61 ppm and 61 ppm, respectively.
The enrichment factor (EF) was determined using the following equation:
| (1) |
3 Results
When the whole dataset is examined (12 to 50 samples per year), it can be seen that the median elemental concentrations and enrichment factors (EF) (Table 1) remained comparable from 2001 to 2008 and then changed dramatically in 2009. The Ti and Al concentrations in lichen presented in Fig. 2 show that Ti/Al ratios ranged from 0.035 to 0.1, and bracket the silicate upper crust Ti/Al ratio [0.064, (Taylor and Mc Lennan, 1995)] regardless of the sampling year. Fe vs Al and Cr vs Al relationships (not shown) exhibit a restricted range of variation around the average upper crust value from 2001 to 2008, while Fe and Cr excesses were recorded in lichens sampled in 2009.
Median values for metals (μg/g)a and Enrichment Factors for lichens collected between 2001 and 2009.
| Year | 2001 | 2003 | 2006 | 2008 | 2009 |
| (n)b | 50 | 35 | 12 | 21 | 12 |
| Al | 829 | 622 | 590 | 678 | 1347 |
| (se) | 70 | 75 | 125 | 165 | 270 |
| Ti | 48 | 57 | 25 | 34 | 52 |
| (se) | 5 | 11 | 4 | 6.5 | 11.5 |
| Fe | 765 | 829 | 731 | 900 | 3720 |
| (se) | 85 | 145 | 85 | 215 | 1560 |
| Pb | 8.5 | 10 | 8.5 | 13 | 31 |
| (se) | 2 | 3.2 | 2.8 | 3.3 | 12.5 |
| Cd | 0.26 | 0.24 | 0.14 | 0.15 | 0.26 |
| (se) | 0.03 | 0.06 | 0.07 | 0.04 | 0.06 |
| Cu | 6.4 | 7.4 | 6.7 | 7.6 | 10.4 |
| (se) | 0.65 | 1.2 | 1.4 | 1.4 | 2.1 |
| Zn | 62 | 67 | 63 | 60 | 157 |
| (se) | 6.5 | 8 | 9.5 | 11 | 45 |
| Cr | 3.5 | 4.1 | 3.2 | 3.5 | 12.4 |
| (se) | 0.4 | 0.6 | 0.5 | 1.1 | 9.8 |
| Ni | 2.7 | 2 | 1.9 | 1.9 | 3.2 |
| (se) | 0.2 | 0.2 | 0.3 | 0.3 | 1 |
| 112 | 111 | 93 | 99.5 | 107 | |
| (se) | 11 | 11 | 6.7 | 5.5 | 7.8 |
| EF-Fe | 2.2 | 2.8 | 2.5 | 2.9 | 6.7 |
| (se) | 0.09 | 0.15 | 0.2 | 0.15 | 1.25 |
| EF-Pb | 47 | 57 | 56 | 47 | 105 |
| (se) | 5 | 9 | 8 | 18 | 13 |
| EF-Cd | 294 | 210 | 261 | 185 | 185 |
| (se) | 25 | 50 | 8 | 33 | 18 |
| EF-Cu | 25 | 33 | 40 | 38 | 34 |
| (se) | 1.4 | 1.8 | 4.5 | 2.7 | 2.7 |
| EF-Zn | 87 | 93 | 113 | 104 | 140 |
| (se) | 5.5 | 10 | 14 | 8.5 | 17 |
| EF-Cr | 9.8 | 12.3 | 12.2 | 11.8 | 25.5 |
| (se) | 1.9 | 1 | 1.6 | 1 | 10 |
| EF-Ni | 12.8 | 9.3 | 13.1 | 10.8 | 12 |
| (se) | 1.5 | 1.2 | 1.7 | 0.7 | 2 |
| EF-Hg | 504 | 694 | 1236 | 998 | 379 |
| (se) | 340 | 155 | 256 | 138 | 54 |
a Except for Hg, ng/g.
b Number of samples for all elements except Hg; n for Hg: 4, 9, 7, 16, 8 for 2001, 2003, 2006, 2008, 2009, respectively.
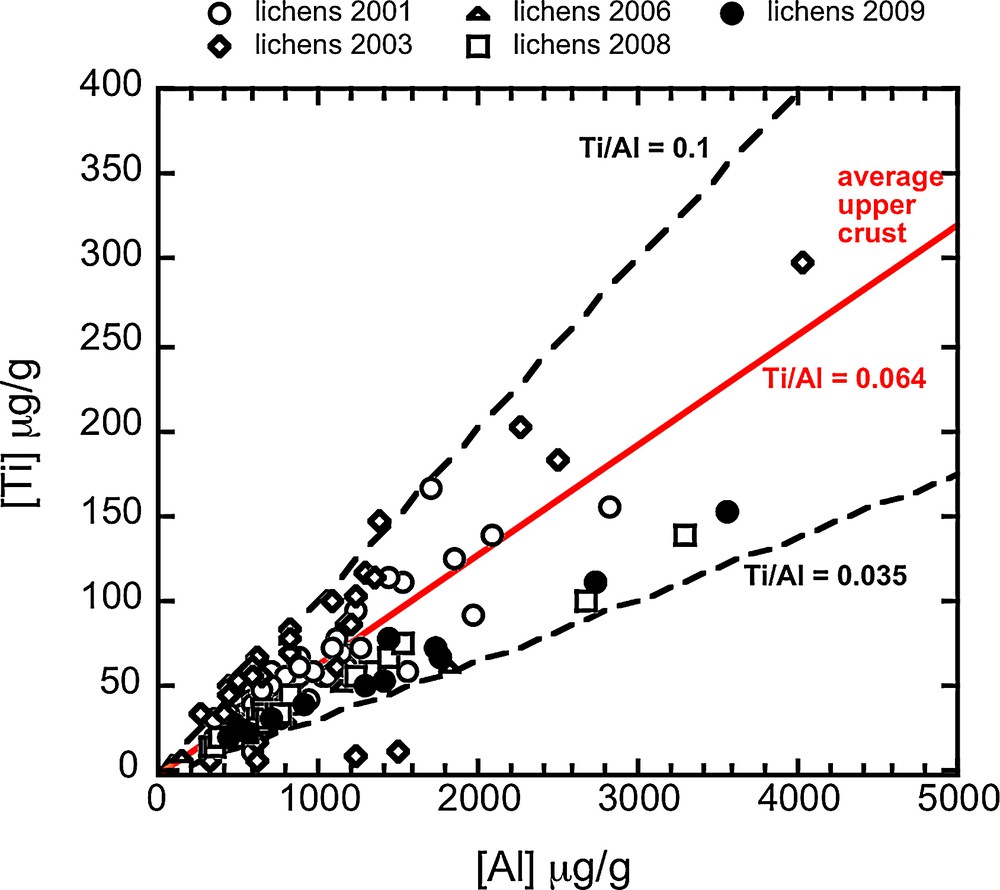
(Color online.) (A) Ti (B) Fe, and (C) Cr vs Al concentration measured in lichens. Relative proportions of Ti and Al are similar to those of the silicate upper crust, reflecting the presence of mineral aerosols in lichens. Relative proportions of Fe–Cr and Al are also similar to those of the silicate upper crust for lichen collected in urban, suburban and rural zones (2001–2008), but significant Fe and Cr excesses are found in lichens collected in the industrial zone (2009).
The enrichment factors remained relatively constant from 2001 to 2008, but a large increase in Fe, Pb, Zn and Cr was again observed in the 2009 lichens. In 2009, the lichens were exclusively collected in the industrial area located to the north of the Metz urban area. The contrasting elemental signatures and EF values highlight a significant contribution from local anthropogenic sources, mainly steel industries.
However, the anthropogenic contributions of Fe, Pb, Zn and Cr are two to three times higher in 2009 than in the other years, but are similar for Cd, Cu, Ni and Hg (Table 1). Rather than representing temporal trends in concentration, the dataset suggests that a spatial interpretation, linked to the location of the source of these metals, is appropriate. Median elemental concentrations in each area are reported in Table 2. Except for Hg and Ni contents, which are similar in all areas, spatial trends accompany the median elemental concentrations. An increase in metal concentrations can be observed from zone to zone: rural < suburban < urban < industrial.
Median values for metals (μg/g)a and Enrichment Factors for lichens collected between 2001 and 2009 in the various zones.
| Year | Urban | Suburban | Rural | Industrial |
| (n)b | 19 | 32 | 31 | 8 |
| Al | 893 | 795 | 593 | 1580 |
| (se) | 628 | 803 | 282 | 979 |
| Ti | 55 | 45 | 33 | 68 |
| (se) | 49 | 61 | 22 | 41 |
| Fe | 910 | 841 | 591 | 5783 |
| (se) | 653 | 807 | 267 | 5515 |
| Pb | 17 | 14 | 5 | 43 |
| (se) | 26 | 10 | 6 | 44 |
| Cd | 0.5 | 0.2 | 0.2 | 0.4 |
| (se) | 0.5 | 0.1 | 0.1 | 0.2 |
| Cu | 11 | 7 | 5 | 14 |
| (se) | 9 | 5 | 1 | 8 |
| Zn | 82 | 65 | 50 | 199 |
| (se) | 41 | 25 | 20 | 170 |
| Cr | 4 | 5 | 3 | 20 |
| (se) | 2 | 3 | 1 | 37 |
| Ni | 3 | 2 | 1 | 5 |
| (se) | 1 | 1 | 1 | 3 |
| Hg | 97 | 108 | 97 | 107 |
| (se) | 24 | 21 | 34 | 24 |
| EF-Fe | 2 | 3 | 2 | 9 |
| (se) | 0.4 | 1 | 1 | 4 |
| EF-Pb | 74 | 55 | 40 | 123 |
| (se) | 86 | 34 | 35 | 32 |
| EF-Cd | 209 | 177 | 202 | |
| (se) | 341 | 94 | 204 | 55 |
| EF-Cu | 29 | 25 | 30 | |
| (se) | 17 | 8 | 15 | 7 |
| EF-Zn | 96 | 97 | 89 | 163 |
| (se) | 31 | 35 | 58 | 42 |
| EF-Cr | 9 | 12 | 11 | 29 |
| (se) | 2 | 5 | 6 | 38 |
| EF-Ni | 12 | 12 | 11 | 13 |
| (se) | 4 | 5 | 5 | 4 |
| EF-Hg | 724 | 1060 | 379 | |
| (se) | 424 | 555 | 1229 | 142 |
a Except for Hg, ng/g.
b Number of samples for all elements except Hg; n for Hg: 10, 17, 11, 7 for urban, suburban, rural, industrial, respectively.
Enrichment factors (Table 2) illustrate different spatial trends depending on the element considered. The industrial area had the highest EFs for Fe, Pb, Zn and Cr; the urban area presented the highest Cd and Cu EFs; the rural area presented the lowest Fe, Pb, Cd, Cu, Zn, Ni values; and the EFs in the suburban area presented intermediate values or values similar to those recorded in urban and rural areas. In contrast with these metals, the Hg EF was higher in the rural area than in the industrial area.
In Fig. 3, a large variation in the Pb EF (from 11 to 260) is observed over the entire geographical area with time. The median Pb EF remained in a narrow range between 2001 and 2008 (47–57), while a higher Pb EF (105) was observed in 2009 in the industrial area. Furthermore, the median Pb EF values presented in Fig. 4 indicate a decrease from urban (80) to suburban (55) and rural (40) areas.
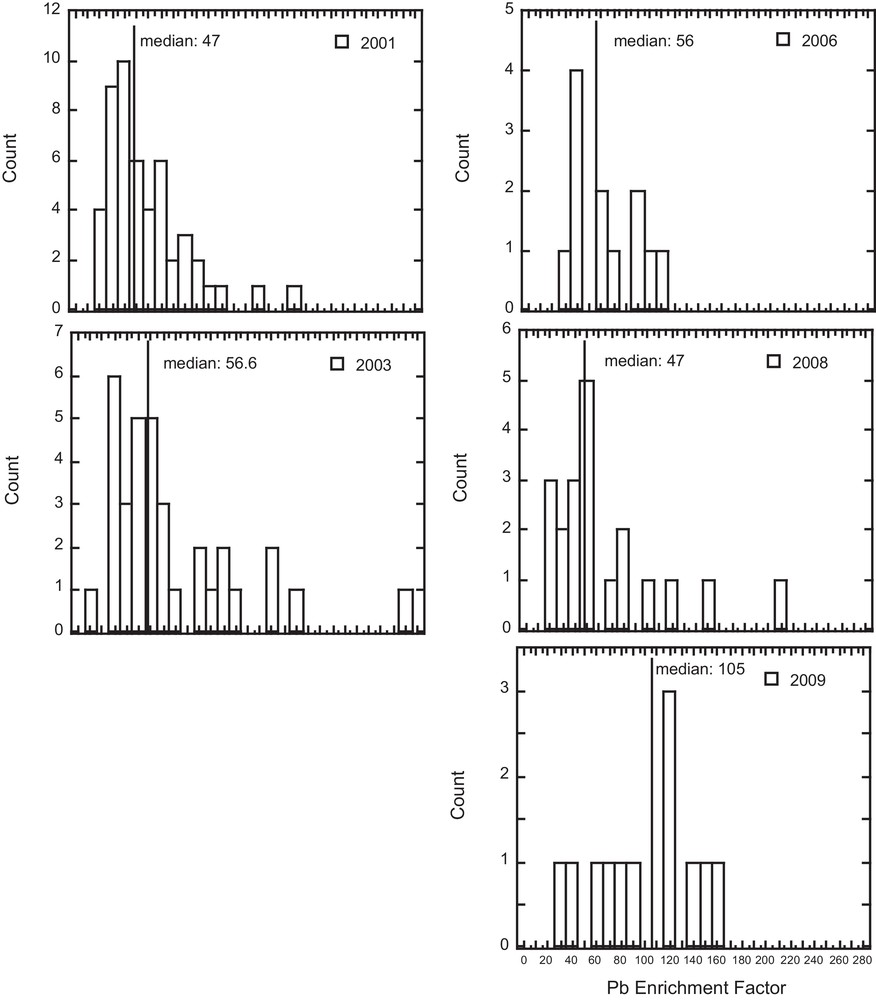
Lichen Pb Enrichment Factor (EF) histograms for years 2001, 2003, 2006, 2008 and 2009. The median values for years 2001 to 2008 (urban, suburban and rural zones) are similar (47 to 56), whereas the median value for 2009 (industrial zone) is significantly higher with a value of 105.
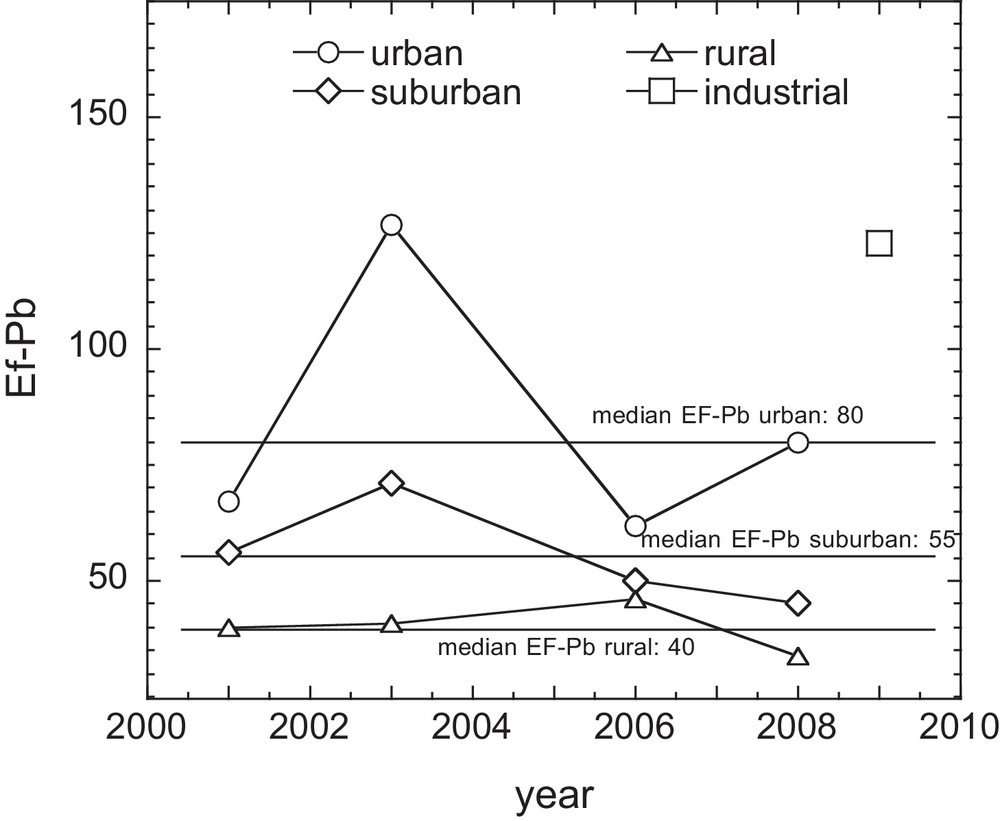
Temporal variation in lichen Pb Enrichment Factor (EF) median values from 2001 to 2008 (urban, suburban and rural zones) and the median EF for 2009 (industrial zone). EF values decrease systematically from industrial, urban, suburban to rural zones.
Variations in the Pb EF reflect spatial variations in source contributions rather than a temporal source variation. In order to better constrain the sources of Pb, we therefore determined the isotopic compositions of Pb in the lichens. Pb isotope measurements are reported in Table 3 and Fig. 5 as a function of the geographical area. For instance, 206Pb/207Pb ranged from 1.146 to 1.157 and each area is characterized by a distinct median Pb isotope ratio. Lead isotope ratios are aligned in the triple-isotope diagram (Fig. 5b), suggesting mixing between at least two end-members, one being more radiogenic than the other.
Median values for [Pb], EF-Pb and Pb isotopic ratios measured in lichens collected between 2001 and 2009 in the various zones.
| Year | Urban | Suburban | Rural | Industrial |
| (n)a | 16 | 26 | 25 | 8 |
| 206Pb/204Pb | 17.867 | 17.920 | 18.018 | 18.049 |
| (se) | 0.246 | 0.158 | 0.219 | 0.069 |
| 207Pb/204Pb | 15.590 | 15.600 | 15.605 | 15.612 |
| (se) | 0.018 | 0.020 | 0.022 | 0.012 |
| 208Pb/204Pb | 37.761 | 37.796 | 37.853 | 37.909 |
| (se) | 0.253 | 0.154 | 0.220 | 0.037 |
| 206Pb/207Pb | 1.1461 | 1.1496 | 1.1548 | 1.1566 |
| (se) | 0.0146 | 0.0092 | 0.0129 | 0.0039 |
| 208Pb/206Pb | 2.1131 | 2.1074 | 2.1016 | 2.1005 |
| (se) | 0.0153 | 0.0103 | 0.0141 | 0.0075 |
| (n)b | 19 | 32 | 31 | 8 |
| [Pb] mg/g | 17 | 14 | 5 | 43 |
| (se) | 26 | 10 | 6 | 44 |
| EF-Pb | 74 | 55 | 40 | 123 |
| (se) | 86 | 34 | 35 | 32 |
a Number of samples analyzed for isotopes.
b Number of samples analyzed for Pb concentrations.
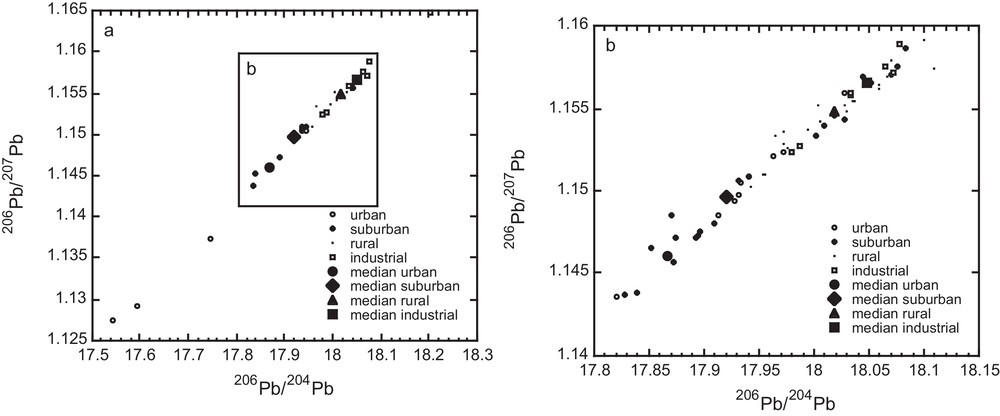
Lichen 206Pb/207Pb vs 206Pb/204Pb diagrams showing the distribution of all data divided into zones. The median isotope ratios of the individual zones, represented by the large symbols, have distinct values, as was observed for Pb EFs. The fact that the urban median value and industrial median value are found at opposite ends of the trend suggests distinct Pb sources for these zones.
4 Discussion
Lead is mainly derived from anthropogenic sources and has been widely used as a reference for tracing the numerous anthropogenic sources in environmental reservoirs (Komarek et al., 2008).
Potential sources of Pb can be identified from the various isotopic end-members in a tri-isotopic diagram (208Pb/206Pb vs 206Pb/207Pb; Fig. 6a and b). The known end-members might include, for instance, gasoline (Roy and Negrel, 2001; Véron et al., 1999), local industry (mainly steel industries; see Monna et al., 1997; Véron et al., 1999), average French industrial Pb (based on measurements from urban waste incinerator effluents; see Carignan et al., 2005) and geogenic end-members (Elbaz-Poulichet et al., 1984). The median industrial Pb ratios (Fig. 5) are found on the opposite side of the plot to the urban median, suggesting that the Pb found in suburban and rural areas mainly originates from a mixture of urban and industrial Pb. From Fig. 6a and b, it can be seen that the data can be explained by mixing between at least three end-members. The Pb isotopic ratios do not lie along a two-component mixing line. Two of these end-members are represented by local industrial Pb and gasoline Pb, and the third corresponds to the regional industrial Pb. The data are scattered between these three end-members, suggesting that natural Pb did not contribute significantly to the overall Pb in lichens. This is supported by the high Pb EF recorded in the lichens, which indicates that at least 95% of the Pb measured in lichens is of anthropogenic origin and that deposition of geogenic Pb is currently low.
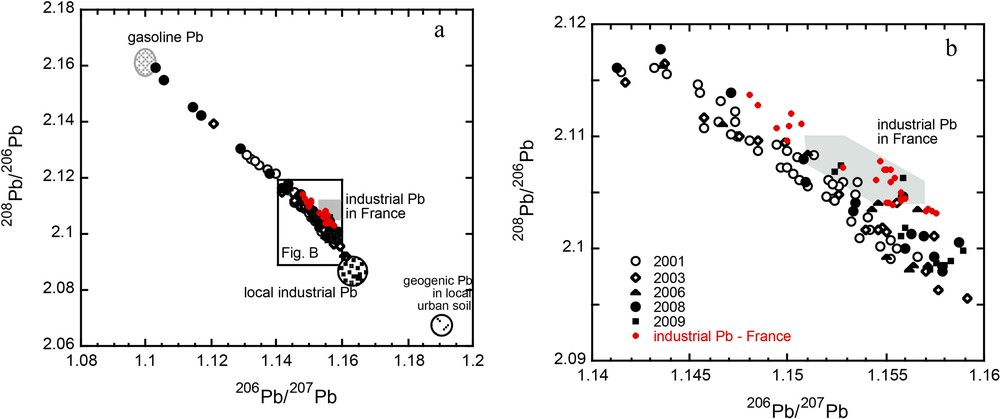
(Color online.) Lichen 208Pb/206Pb vs 206Pb/207Pb. For comparison, the fields for gasoline Pb in France, mean industrial Pb in France, local industrial Pb, and geogenic Pb in local urban soils are reported. The distribution of the data suggests contributions from at least three main sources of Pb. Data for industrial Pb in France are from dust collected from various municipal waste incinerators (Carignan et al., 2005).
Some of the lichens collected in 2008 showed a significant contribution from gasoline Pb. This finding was previously reported in Cloquet et al. (2006a) and Lahd Geage et al. (2008a), and most likely originates from re-suspension of road particles.
Temporal differences in the Pb isotope ratios reported for lichens in each zone are best explained in terms of changes in the mixing proportions rather than source variations. This interpretation is also supported by a mixing diagram that reports Pb isotope ratios vs Al/Pb (Fig. 7a). In this figure, the end-members are defined by their Pb isotope ratios and their Pb concentration, and mixing between gasoline Pb and local industrial sources is again inferred. Once more, the geogenic Pb contribution is insignificant. The third component contributing to the isotopic compositions of the lichen is the atmospheric baseline Pb derived from industrial emissions in France. This end-member is relatively well defined by the Pb isotopes, but is more complex in terms of concentration. This is because of the scattered distribution of such a diffuse source. It might seem surprising to observe a greater contribution from long-range atmospheric deposition than from the geogenic source. However, the relatively high level of industrial emissions of Pb compared to natural emissions easily explains the difference in terms of their contributions to the atmosphere. From Fig. 7b, it is also clear that the contributions from each source have changed through time. Some of the 2001 lichens exhibit a Pb isotope ratio (206Pb/207Pb) of between 1.13 and 1.14 and an Al/Pb ratio of about 100, pointing towards the gasoline Pb field.
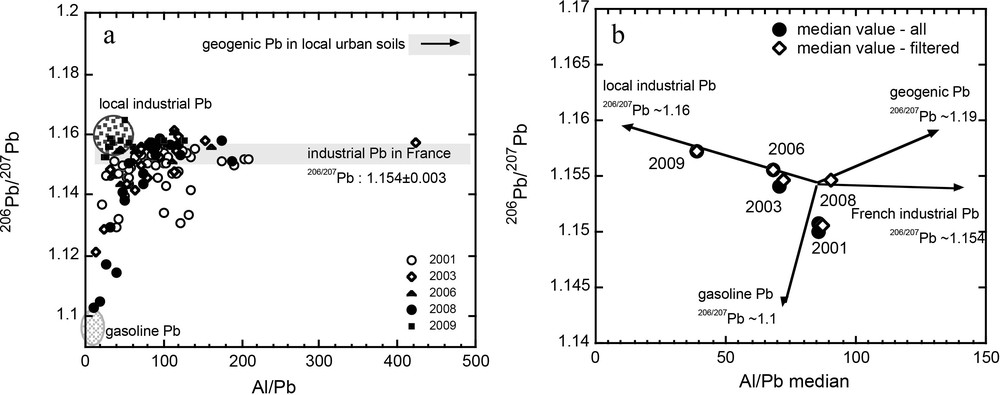
Lichen 206Pb/207Pb vs (a) Al/Pb and (b) median Al/Pb (all data and filtered data – see Fig. 6 caption). a. The fields for gasoline Pb in France, mean industrial Pb in France, local industrial Pb, and geogenic Pb in local urban soils are reported. b. Arrows point towards these 4 fields. “Filtered”: 206Pb/207Pb values below 1.14 were excluded in order to eliminate the major input of gasoline Pb sources.
The change in source contribution can also be observed by reporting the median value for lichen through time. In Fig. 7b, the median value and filtered median value are reported. The 206Pb/207Pb data have been filtered to eliminate the major input of gasoline Pb, which has a ratio below 1.14. Our decision to filter the data was based on the removal of lead-additives from gasoline in the early 2000s. No large difference between both total and filtered values can be observed for 2008. The data from the different years plot within a relatively restricted area with the exception of the 2009 data, which exhibits a lower median Al/Pb ratio. This narrow range in the data supports the hypothesis that variations are explained by changes in source contribution rather than by changes in source origin (the removal/addition of sources). The 2009 data were sampled close to sites of industrial activity that represent the local industrial Pb. Based on the end-members defined above, it can be seen that the 2001 lichens recorded a larger contribution from the gasoline Pb source. This is probably because the phasing out of leaded gasoline started at that time. The 2008 data are similar to the 2001 ones and can be explained by the re-suspension of old gasoline particles, as described earlier. However, it is not clear why such particles were not re-suspended prior to 2008. One explanation might be that the various activities in the zone were more intense between 2006 and 2008, but there is no information available to us to confirm such a hypothesis. Once the phasing out of leaded gasoline had been completed, the influence of this end-member became less important and the contribution from local industrial Pb became more visible. During the same period, no significant changes in the French industrial Pb and geogenic Pb emissions would have been expected. As a result of this change in source contributions, the median values for Pb isotope ratios and Al/Pb evolved towards the local industrial Pb end-member in 2003 and 2006. Next, the median value for the 2008 filtered data moved towards higher Al/Pb for a broadly similar 206Pb/207Pb isotope ratio, i.e., in the direction of geogenic Pb and French industrial Pb. As discussed above, the latter more likely explains this change. This shift reflects the lesser impact of local industrial Pb relative to long-range atmospheric deposition. This might be explained by reduced industrial activity, mainly in the steel industry, in the study area – see Petit et al. (2015) for references.
The temporal variations observed in this study support the fact that atmospheric Pb isotopes tend to be homogenised through time. The atmospheric baseline Pb derived from industrial emissions across France became an important atmospheric pollutant source after the phasing out of leaded gasoline. This trend towards homogenisation, with a 206Pb/207Pb isotope ratio of 1.15–1.17, is similarly observed all around the world. It has been reported in lichen samples from the rest of Europe (Lahd Geagea et al., 2008b) and North America (Carignan and Gariepy, 1995), and has also been reported in other archives, such as tree rings (Stille et al., 2012), Antarctic snow (Van de Velde et al., 2005) and lake sediments (Aebischer et al., in prep.). The trend also highlights the importance of undertaking long-term monitoring studies using appropriate tools. The use of lichens and mosses is particularly important (Harmens et al., 2015) because they seem to be insensitive to climatic change at a regional scale in terms of their accumulation of metal from the atmosphere. If this is confirmed at a larger spatial and temporal scale, these organisms will become important proxies for atmospheric metal evolution in the future.
5 Conclusion
The results obtained in this study show that heavy metal inputs to the atmosphere did not systematically change during the decade of monitoring. This could imply that anthropogenic pressure remained constant in the atmosphere around the city over the 10-year period. However, when the data are sorted by area (urban, suburban, rural and industrial zones), a systematic change can be observed. Industrial areas are seen to have experienced higher metal fallout than urban, suburban and rural areas. Using Pb isotope ratios, the Pb atmospheric inputs can be explained by Pb derived from three sources: local industrial activity; average French industrial activity, via long-range transportation of particulates; and Pb in gasoline, mainly due to re-suspension of old particles. The natural Pb input signal is not clearly observed as it has been obscured by the large input of anthropogenic Pb to the atmosphere. Urban areas present a higher proportion of Pb from gasoline emissions whereas industrial areas are characterized by a large proportion of local industrial Pb. Overall, a change in source proportions is observed with time. In the early 2000s, higher proportions of Pb from gasoline were observed. The distributions then changed slightly, moving towards higher proportions from local industrial Pb and then finally towards a higher proportion of Pb from baseline French industrial Pb via long-range deposition. These conclusions support observations reported in other studies that suggest a trend towards a homogenisation of the heavy metal input to the atmosphere as traced by Pb. In this context, local sources can be easily identified and may strongly influence the atmospheric signal at a local scale. Lichens and other epiphytic organisms, such as mosses appear to be good proxies for atmospheric deposition at a local/regional scale and also very efficient at recording the urban/periurban atmospheric signal. Since the metal record does not seem to be affected through time or by local climate change in these organisms, the technique may prove itself invaluable in future evaluation of metal atmospheric inputs.
Acknowledgments
Julie Banse, Ludovic Fenet and Luc Marin are thanked for their help with lichen sampling and measurements. The SARM is acknowledged for the elemental measurements, with special thanks extended to Christine Blanchard and Delphine Yeghicheyan. Alice Williams is acknowledged for English revisions. The study benefited from EC2CO program supported by the INSU. This paper is dedicated to Caroline, Olivier and Jules. This is CRPG contribution number 2373.


