1 Introduction
Industrial production of non-ferrous metal is the third major source of atmospheric trace metals worldwide, and the largest source of atmospheric As, Cd, Cu, and Zn (Cloquet et al., 2015; Pacyna and Pacyna, 2001). The anthropogenic activities might play a key role in modifying the zinc (Zn) biogeochemical cycling. The understanding of the Zn transfer processes between soils and plants should help us to fully assess the effects of anthropogenic activities on the metal cycling in the Earth's critical zone. Recent improvements in MC-ICP-MS (multiple collector inductively coupled plasma mass spectrometry) allow obtaining highly precise and accurate data for non-traditional stable zinc isotope compositions in plants and soils (e.g., Arnold et al., 2010a,b, 2015; Houben et al., 2014; Jouvin et al., 2009, 2012; Moynier et al., 2009; Smolders et al., 2013; Tang et al., 2012; Viers et al., 2007; von Blanckenburg et al., 2009; Weiss et al., 2005). Zinc isotopes can be used as powerful tools for a better understanding of the mechanisms of Zn uptake, transport, storage, and tolerance by plants. In addition, Zn isotopes contribute for tracing Zn sources and Zn transfer processes that control Zn biogeochemistry in the Earth's critical zone.
Over the past decade, Zn isotope fractionation has been measured during Zn uptake and translocation by higher plants to model fractionation mechanisms in Zn-deficient or Zn-sufficient conditions. Weiss et al. (2005) performed a pioneering study on rice, tomato and lettuce in hydroponic experiments. They demonstrated enrichment with heavy Zn isotopes in roots with respect to the nutrient solution and a plant species-dependent enrichment with light Zn isotopes in the shoots. Arnold et al. (2010a) complemented that study by demonstrating for rice the Zn complexation by phytosiderophores, favouring heavy Zn isotope enrichments, before uptake through the root membrane. This was subsequently modelled by Jouvin et al. (2012) as a zinc specific uptake process. The contribution of Zn-exudate complexes to Zn uptake in tomato seedlings under Zn-deficient conditions was recently suggested based on Zn isotope enrichments in a hydroponic study of Smolders et al. (2013). Viers et al. (2007) compared Zn fractionation intensity in tree and herb species, and identified a correlation between the extent of light Zn enrichment in the leaves, relative whole plant, and length of the plants. This hypothesis was confirmed by Moynier et al. (2009), who highlighted the increase in light isotope enrichment along the leaf length of bamboos. Fujii and Albarède (2012) complemented those studies by exploring the isotopic fractionation of Zn phosphates, which can be the species responsible for the enrichment with heavy Zn isotopes in the root system, and may also account for the high δ66Zn of herbaceous plants with respect to nutrient solutions.
Isotope fractionation in plants changes with Zn supply. At excess Zn supply, large Zn isotope fractionations observed in the plants were associated with a main Zn accumulation in roots, which suggested specific Zn tolerance strategies such as Zn2+ binding with high-affinity ligand at the root cell walls (Aucour et al., 2011), chelation by phytochelatines and vacuolar compartmentation (Caldelas et al., 2011), Zn transport across the plasma membrane by a combinations of ZIP transporters and efflux pumping (Tang et al., 2012).
Finally, Jouvin et al. (2012) and Deng et al. (2014) have investigated isotope fractionation during the potential competitive transport of Zn in the presence of other metals (like Cu, Ni and Cd). They have suggested distinct uptake strategies for Zn and Cu (predominant reduction mechanism for Cu acquisition), and potential competition between Zn and Ni during the uptake process.
Various studies have measured the change in Zn fractionation between nutrient solutions, roots and shoots under deficient to adequate Zn supply (Arnold et al., 2010a; Jouvin et al., 2009, 2012; Smolders et al., 2013; Viers et al., 2007; Weiss et al., 2005) but, as far as we know, much less studies have yet been made under toxic conditions (Aucour et al., 2011; Caldelas et al., 2011), especially on contaminated soils (Houben et al., 2014; Tang et al., 2012), where uptake and translocation mechanisms can be different compared to adequate Zn supply. As a result, the biogeochemical cycles of Zn are yet unclear for Zn-polluted sites. The main aim of this study is to investigate the Zn isotope fractionation between soil and plant parts under toxic Zn conditions to provide a better insight into the soil–plant interactions and plant tolerance strategies in Zn-excess conditions. Two plants were grown on three soil samples collected from two different Zn contaminated sites. One plant species was ryegrass (Lolium multiflorum L.) belonging to the grasses, which are often considered to be pioneers and suitable plants for covering contaminated substrate (Arienzo et al., 2004). Moreover, because of its high capacity for the accumulation of toxic substances and its tolerance against heavy metals, ryegrass is frequently selected as a bioindicator plant used for Zn biomonitoring (Houben and Sonnet, 2012). The second plant species was rape (Brassica napus L.) and was selected for its fast growth, elevated fully-harvestable biomass production and high energy potential. Rape is increasingly suggested for covering metal-contaminated soils while providing a valuable source of income (Houben et al., 2013). Three main questions will be addressed. (1) Does the plant cover preserve the isotopic fingerprints of the soils/soil solution across different soils? (2) To what extent does the plant species affect Zn isotope fractionation within plants? (3) Does Zn isotope fractionation in plant parts evidence specific tolerance strategy (in passive or active mode) developed by the plants in Zn-excess conditions?
2 Materials and methods
2.1 Soil sampling sites
The Liège Province, in southeastern Belgium, was the main centre of metallurgical activities in the country. In this area, soils were largely affected by heavy-metal (Zn, Pb, Cd) contamination due to atmospheric fallouts and industrial tailings originating from adjacent lead or zinc smelters. Three contrasting soils from two sampling sites were collected for the culture experiments.
Site 1 is called Prayon and belongs to the city of Trooz in the Liège Province. The two soils from this site were collected from a hill, which was intensively subjected to aerial dust and fumes emitted by the smoke stack from a former smelting plant dedicated to Zn production until 1974. The two soils were chosen according to their distinct substratum: a Givetian calcareous bedrock for the PC soil (for Prayon Calcareous soil) and an acid Famennian Shale bedrock for the PS soil (for Prayon Shale-derived soil). Due to the slight slope (20°), the chimney position and the main wind direction, the PC soil has been a preferential host of metal-bearing aerial fallouts. The Prayon site is intensively studied for its rich, diversified and specific flora associated with a spontaneous re-vegetation (Agrostis capillaris, Viola calaminaria, Noccaea caerulescens, Armeria maritima subsp. halleri). Site 2 is an industrial tailing located at Angleur, near the city of Liège, and between the Ourthe River and the Canal of the Ourthe. The slag heap built from waste deposits from a former Zn smelter that was operated from 1837 until 1905 by the “Société des mines et fonderies de zinc de la Vieille-Montagne” (for more details, see Ganne et al., 2006). This specific substrate favoured the establishment of adapted metallophyte flora (A. maritima subsp. halleri and A. capillaris). However, several parts of the slag-derived soil are still bare and one such bare soil was AB sampling soil site (for Angleur's Bare slag heap soil). In the present study, only soil samples from the surface layers (∼0–7 cm, Table 1) are considered. After collection on each site, several kilograms of soils were homogenized and air-dried in the laboratory, ready for the culture experiments.
Chemical characteristics of the three soils used in the culture experiment. The soils originate from the Prayon site (PC and PS soils, for Prayon Calcareous soil and Prayon Shale-derived soil, respectively) and from the Angleur site (AB for Angleur's Bare slag heap soil).
| Soil | Sampling depth | Znbulk soila | Corgb | pHc | Exchangeable cationsd | CEC (pH 7)d | ||||
| Ca2+ | K+ | Mg2+ | Na+ | |||||||
| cm | g·kg−1 | % | cmolc·kg−1 | |||||||
| PC | 0–7 | 74.8 | 1.18 | 11.05 | 6.40 | 4.65 | 0.32 | 0.25 | 0.18 | 22.65 |
| PS | 0–6 | 7.4 | 0.33 | 21.65 | 4.78 | 16.77 | 0.45 | 1.38 | 0.12 | 37.96 |
| AB | 0–7 | 54.8 | 0.05 | 4.05 | 7.34 | 5.72 | 0.22 | 0.17 | 0.01 | 11.43 |
a Total concentration was determined by ICP–AES after an acid dissolution method (concentrated HNO3 and HCl), detection limit = 0.03 mg·kg−1.
b Organic carbon content was determined according to the Walkley and Black method (Page et al., 1982).
c pH in solid:water suspension (1:5).
d Exchangeable cation (Ca, K, Mg, Na and Zn) concentrations and cationic exchange capacity (CEC) determined using a percolation column (Metson method), NH4–Ac at pH 7 (Page et al., 1982).
2.2 Plant species and culture experiment
For the culture experiment, two plant species were chosen on the basis of their high and fast biomass productivity, their suitability to the experiment conditions (previously tested by Chaignon and Hinsinger, 2003; Lambrechts et al., 2011), and their distinct physiological mechanisms: Brassica napus L. cv Adelie (rape) and Lolium multiflorum L. cv Meribel (ryegrass). L. multiflorum is a metal-tolerant monocotyledonous plant species, while B. napus belongs to the dicotyledonous species and is well known to be tolerant for some abiotic stresses, like high metal contents in soils (Houben et al., 2013; Marchiol et al., 2004). For L. multiflorum, no stem is observed, whereas a short stem is present for B. napus but was not isolated; only roots and shoots (including stems of B. napus) account for the chemical analyses in the present study.
The culture experiment was performed with a homemade cropping device whose design was adapted from Kruyts (2002) and Niebes et al. (1993) (Fig. 1), which allows us to sample roots with almost no soil contamination. Two PVC cylinders composed the cropping device: the plant compartment (internal diameter of 80 mm) and the soil compartment (internal diameter of 90 mm). The plants were germinated and grown on a polyamide mesh (mesh size: 20 μm) situated at the bottom of the plant compartment cylinder. The soil compartment lied on a cap covered by a second polyamide mesh (20 μm). All the apparatus parts were acid-washed (pH 3, HCl) and thoroughly rinsed with deionized water before assembly.
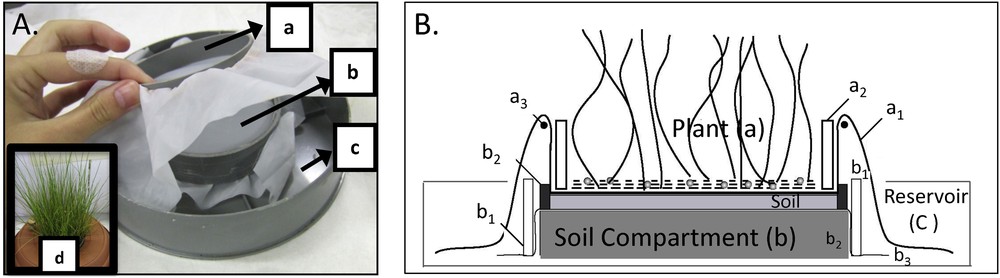
(Colour online.) Picture (A) and cartoon (B) of the culture experiment device with (a) the plant compartment composed of (a1) a polyamide mesh (mesh size = 20 μm) fixed to (a2) the cylinder (i.d. 80 mm) by (a3) an elastic; (b) the soil compartment composed of (b1) a cylinder (i.d. 90 mm) containing (b2) two supports whose one covered by (b3) a polyamide mesh (20 μm) and filled by the soil; (c) the reservoir filled by the nutrient solution; and (d) the cover to limit evaporation of the nutrient solution with central opening for the plant.
The experiment was conducted on 36 plant devices including three different substrates (three soil samples), two plant species, one nutrient solution and three replicates by treatment (control experiments have been performed in parallel, using clean sand as soil and deionized water as nutritive solution; due to the small amounts of dry plant parts in these control tests, elemental compositions have been measured—not reported here, but no isotopic ratio).
The plant devices were placed in a growth chamber where the following parameters were controlled: temperature (20 °C), humidity (around 90%) and light intensity (16-h photoperiod and mean light varying from 120 to 180 μmol m−2·s−1, except during the seven first days of the hydroponic period where darkness was maintained). Two main growth periods were distinguished: the germination period (7 days) followed by the plant–soil contact period (14 days).
2.2.1 First step: germination phase
In each plant compartment (on a surface area of 46.6 cm2, Fig. 1), 0.8 g of B. napus seeds or 1.5 g of L. multiflorum seeds were sown, i.e. about 350 seeds and 100 seeds per pot, respectively. Those high proportions of seeds per pot have ensured root mat development, which consequently will exacerbate the interactions at the root–soil interface. The germination rate was around 90%. The first three days, the seeds were watered by soaking the polyamide mesh with deionised water. During the next four days, seedlings were fed with a Zn-free nutrient solution: KCl (0.05 mM), K2SO4 (0.25 mM), MgCl2 (0.05 mM), MgSO4 (0.05 mM), NaH2PO4 (0.05 mM), FeNaEDTA (0.08 mM), H3BO3 (0.08 mM), MnCl2.4H2O (0.008 mM), CuSO4.5H2O (0.0008 mM), NH4MoO24.4H2O (0.0056 mM), CaCl2.2H2O (0.5 mM), CaSO4.2H2O (0.5 mM), NH4Cl (1 mM) and (NH4)2SO4 (0.5 mM).
2.2.2 Second step: plant–soil contact
After drying and sieving (<2 mm) the soil substrates (according to the common practice described in Baize, 2006), each soil compartment was filled with ∼5 g of soil from one of the three origins. The soil compartment was then covered with a cap opened in the middle to allow the growth of the plant but preventing the evaporation of the plant nutrient solution (Fig. 1). The liquid reservoirs were filled with a Zn-free nutrient solution, to water the plants by soaking the meshes. The volume of added nutrient solution was measured strictly for a precise estimation of the transpiration volume by plants during the plant–soil contact period (taking into account the residual water volume and the evaporated volume based on the experimental devices without plants). Three replicates of each treatment were conducted, defining six different combinations of plant species and soil samples (2 species × 1 nutrient solution × 3 substrates × 3 replicates). After two weeks, the plants were isolated from the soil; the separation was facilitated by the use of meshes preventing the adhesion of soil particles to the roots. Plant shoots were also separated from the root parts. Roots (and shoots) were repeatedly rinsed for 5 min with deionised water in an ultrasonic bath. Then, shoots and roots were dried (60 °C for 72 h), weighed and crushed in an agate mortar prior to analysis.
2.3 Soil and plant sample preparations–elemental analyses
After sampling, soil samples were air-dried and sieved (2 mm). Following the methodology of Degryse et al. (2003), the soil Zn was extracted with CaCl2 to estimate the isotope composition of the mobile fraction, i.e. the soil solution. This extract mimics the soil solution because the inorganic composition of the extract is similar to that of most soil solutions (Degryse et al., 2003). This method implies shaking of soil aliquots (2.5 g) in centrifuge tubes with 0.01 M CaCl2 (25 ml) for 24 h in a lateral shaker. The samples were subsequently centrifuged (13,400 rpm for 5 min) and supernatants were filtered through 0.45-μm polycarbonate filters. The final solutions of CaCl2 extracts were acidified before the ICP-AES analyses (Thermo Jarrell Ash Iris Advantage), which were performed at UCL (Belgium): Zn (and other metal) concentrations () were measured (Table 1). Zinc concentrations in shoots, roots—as well as in the initial seeds—were also determined by ICP-AES after acid digestion of plant part powders in concentrated HNO3 at 95 °C; the solution was subsequently evaporated to dryness and re-dissolved in a mixture of concentrated HNO3 and HCl (1:3 ratio) at room temperature. Careful attention was paid to potential risks of Zn contamination by the reagents used for chemical preparations; procedural blanks were always used.
2.4 Zn chemical purification and isotopic analysis
Whereas elemental analyses were performed on all the soil and plant parts, three replicates of each treatment (from the different combinations of plant species and soil samples) were carefully mixed and homogenized into a single composite sample for the isotopic analyses (after precise determination of Zn concentration and dry biomass, aliquots of equivalent Zn amounts of the three replicates were considered).
Chemical treatments of soil and plant samples and of CaCl2 extracts were carried out under a class-100 laminar flow hood in a class-1000 cleaned room (at ULB). All the reagents used for sample treatments were distilled pro-analysis acids that were subsequently sub-boiled. The dilutions were performed with MilliQ water (18.2 MΩ·cm). Crushed soil and plant samples (about 2 mg) were previously dry ashed for 24 h at 450 °C in order to eliminate the organic matter. The samples for dry-ashing were dissolved by applying the tri-acid digestion technique (with concentrated 14 M HNO3, 24 M HF and re-dissolution in 6 M HCl) using a Teflon Savillex® beaker placed on a hot plate (120 °C) for evaporation until dryness. CaCl2 extracts were simply evaporated. Zn was then purified by a novel chromatographic separation technique on micro-columns loaded with 0.2 ml of AG1-X8 resin and filled with successive additions of acids (HCl, HNO3, HBr) (Mattielli et al., 2013). This method is especially adapted for the sample matrices characterized by high Zn concentrations compared to other cation abundances, allowing extremely low amounts of acids for elution and consequently low procedural blanks (≤2 ng of Zn). Upon separation, the eluate was dried down and digested with 100 μl of concentrated HNO3 to dissolve the potential co-eluted organics. A full recovery of Zn was quantitatively monitored to circumvent problems associated with potential isotopic fractionation on columns. The yield values were higher than 98 ± 5% (values obtained from Zn purification of 10 plant and five soil samples). The total procedural blanks average ∼7 ng.
The Zn isotopic ratios were measured on an Nu plasma I MC-ICP-MS in wet plasma mode (ULB, Belgium). Zn (and Cu, used for the doping technique) isotopic compositions were measured by static multi-collection. A single analysis consisted of a measurement of 60 ratios, i.e. three blocks of 20 cycles with an integration interval of 10 s. On-peak baseline measurement with 30-s integration time prior to each analysis was done on a 0.05 M HNO3 acid blank, which is then subtracted online during the analytical run of all the samples/standards. Nickel contributions were systematically corrected by monitoring mass 62 (62Ni). Mass discrimination effects were corrected by using simultaneous external normalization (Cu-doping method) and standard-sample bracketing with in-house JMC Zn–Cu standard solutions (previously calibrated against the JMC 3-0749L Zn and NIST SRM 976 Cu reference standard solutions—for complementary information, see Mattielli et al. (2009) and Petit et al. (2008)). Every sample was run between two standards and was analysed at least in triplicate; the Zn isotopic composition was expressed in δ66Zn relative to our in-house standard solution (Eq. (1))2.
During the period of data acquisition, repeated measurements of in-house Zn and Cu standards show a mean δ66Zn value of 0.00 ± 0.02‰ (2SD) (n = 117). In addition, repeated measurements of the Lyon JMC 3-0749L Zn standard solution batch used at ULB were performed and gave a mean δ66Zn value of +0.11 ± 0.03‰ (2SD) (n = 17) relative to our in-house solution (0.00 ± 0.02‰). Consequently, the δ66Zn results for the samples can then be converted relative to the JMC 3-0749L Zn by using the conventional conversion equation (Hoefs, 2008).
In order to assess the reproducibility, one sub-sample of soil horizons (AB) was digested in duplicate; the δ66Zn values were +0.20 ± 0.04‰ and +0.26 ± 0.02‰ (the latter is reported in Table 3). In addition, to validate the chemical method of Zn separation, Zn from two soils (independent study) was isolated by another chromatographic technique using AG-MP1 resin and 0.5 M HNO3 as an eluent (methodology described in Maréchal et al., 1999). The isotopic results obtained by the latter method (AG-MP1 resin) were −0.09 ± 0.01‰ and +0.23 ± 0.04‰, respectively for the first and second soil samples. The present method based on AG1-X8 resin gave the following δ66Zn values: −0.05 ± 0.02‰ and +0.23 ± 0.04‰, for the first and second soil samples, respectively. Finally, to control the reproducibility of the entire analytical method and check the accuracy of the isotopic measurements on the MC-ICP-MS, the BCR281 (ryegrass) reference material was analysed and gave a δ66Zn value of +0.38 ± 0.04‰ (n = 9), which is in good agreement with the previously published values (e.g., +0.40 ± 0.09‰ in Arnold et al., 2010b).
Zn isotopic compositions (‰) (± 2SD) of bulk soils from Prayon (PC & PS) and Angleur (AB) sites, CaCl2 extracts, plant parts (shoot and root) and the whole plant (calculated using the mass balance Eq. (2)) from the rape (B. napus) and ryegrass (L. multiflorum). The δ66Zn results relative to our in-house standards were converted into the JMC 3-0749L Zn standard using the conventional conversion equation (Hoefs, 2008).
| PC | PS | AB | |
| ‰ | |||
| δ66ZnJMC 3-0749L − δ66Znin-house JMC = +0.11 ± 0.03‰ (2SD) (n = 17) | δ66Zn reported relative to the JMC 3-0749L standard | ||
| Soil | |||
| δ66Znbulk soil | −0.05 ± 0.02 | −0.03 ± 0.01 | +0.26 ± 0.02 |
| −0.18 ± 0.02 | −0.15 ± 0.05 | +0.14 ± 0.01 | |
| –0.13 ± 0.04 | –0.12 ± 0.06 | –0.13 ± 0.03 | |
| Brassica napusL. (Rape) | |||
| δ66Znroot | +0.05 ± 0.01 | +0.05 ± 0.01 | +0.31 ± 0.05 |
| δ66Znshoot | −0.28 ± 0.05 | −0.34 ± 0.06 | +0.06 ± 0.04 |
| δ66Znwhole plant | −0.11 ± 0.07 | −0.13 ± 0.09 | +0.22 ± 0.11 |
| Δ66Znshoot–root | –0.23 ± 0.06 | –0.39 ± 0.07 | –0.25 ± 0.09 |
| Δ66Znroot–bulk soil | +0.00 ± 0.03 | +0.08 ± 0.02 | +0.05 ± 0.07 |
| +0.13 ± 0.03 | +0.20 ± 0.06 | +0.17 ± 0.06 | |
| Lolium multiflorumL. (Ryegrass) | |||
| δ66Znroot | −0.05 ± 0.03 | −0.10 ± 0.04 | +0.32 ± 0.04 |
| δ66Znshoot | −0.25 ± 0.04 | −0.14 ± 0.03 | +0.17 ± 0.01 |
| δ66Znwhole plant | −0.10 ± 0.06 | −0.11 ± 0.04 | +0.28 ± 0.11 |
| Δ66Znshoot–root | –0.20 ± 0.07 | –0.04 ± 0.07 | –0.15 ± 0.05 |
| Δ66Znroot–bulk soil | +0.00 ± 0.05 | –0.07 ± 0.05 | +0.06 ± 0.06 |
| +0.13 ± 0.05 | +0.05 ± 0.09 | +0.18 ± 0.05 |
2.5 Statistical analysis
Zn concentration, dry biomass and transpiration volume data were systematically analysed using two-way ANOVAs, with the main factors being soils and plant species, followed by a multiple mean comparison test (Tukey's HSD test). The Spearman correlation coefficients (rs) were calculated to determine the relationships between the isotopic and the elemental compositions. All statistical analyses were carried out using SAS 9.1 (SAS Institute, Cary, NC, USA).
3 Results
3.1 Plant growth and Zn concentrations
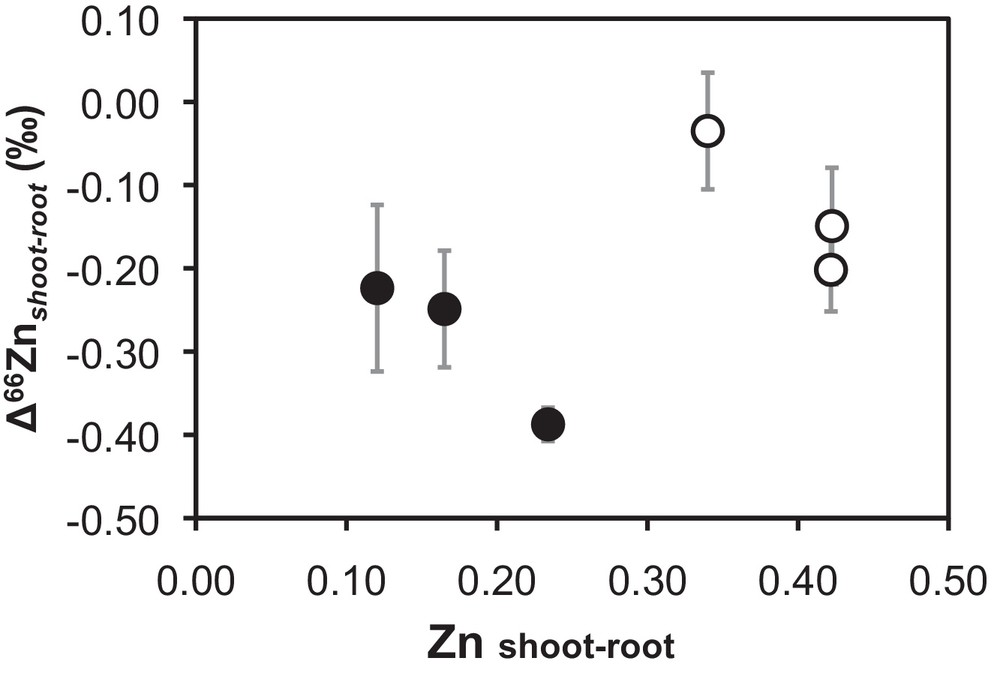
Zinc is a major constituent in the soil samples, with abundances ranging from 7.4 g·kg−1 in the shale-derived soil (PS) to 75 g·kg−1 in the calcareous soil (PC). The soil sample significantly affected (P < 0.001) the dry plant biomass. Plants growing on the least Zn contaminated soils (PS) contained also the lowest Zn concentrations and had the highest biomass productions, suggesting that toxic Zn supply is a growth-limiting factor (Tables 1 and 2). The L. multiflorum species has produced significantly more root biomass than B. napus. The transpiration volume/device was statistically unaffected by soil sample or plant species. Total plant Zn concentrations (i.e. weighted average of shoot and roots) are 4 to 14 times less concentrated than in the corresponding soils. The Zn concentrations in B. napus roots (5.0 to 18.9 g·kg−1) are significantly higher (P < 0.05) than in L. multiflorum roots (2.1 to 7.9 g·kg−1). The Zn concentrations in seeds of B. napus and L. multiflorum were measured and represent a Zn input of only 0.05 mg and 0.09 mg per device, respectively. These values are well below the total Zn in biomass after culture, i.e. ≥1.95 mg for B. napus and 1.65 mg in plant per device for L. multiflorum. Soil sample and plant species have a significant effect (P < 0.05) on Zn concentrations in the whole plant as well as in roots (Table 2). In contrast, the shoot to root Zn concentration ratios vary between 0.12 and 0.42 and are only affected by the plant species, but not by the soil sample.
Dry biomass (g), Zn concentration in shoots and roots (μg·g−1 dry matter ± SD, detection limit = 0.03 mg·kg−1), and transpiration volume (cm3) in rape and ryegrass cultivated on the three soils (PC and PS, Prayon Calcareous soil and Prayon Shale-derived soil, respectively; and AB, the Angleur's Bare slag heap soil). Different letters in superscript indicate the statistically significant differences between means (n = 3, P < 0.05).
| Soil | Dry biomass | Zn | Transpiration | ||||
| g | μg·g−1 | cm3 | |||||
| Roots | Shoots | Roots | Shoots | Whole plants | |||
| Brassica napus L. (Rape) | PC | 0.30 ± 0.02c,d | 0.82 ± 0.12c | 18900 ± 1100a | 2300 ± 400b | 6700 ± 400a | 322 ± 23a |
| PS | 0.33 ± 0.02c | 1.19 ± 0.07a | 5000 ± 300d | 1200 ± 40c,d | 2000 ± 60d | 568 ± 223a | |
| AB | 0.28 ± 0.01d | 1.00 ± 0.05b | 11400 ± 100b | 1900 ± 200b,c | 3900 ± 300c | 430 ± 144a | |
| Lolium multiflorum L. (Ryegrass) | PC | 0.85 ± 0.02b | 0.74 ± 0.03c | 7900 ± 400c | 3300 ± 500a | 5800 ± 70b | 467 ± 170a |
| PS | 1.06 ± 0.01a | 1.15 ± 0.02a | 2100 ± 100e | 700 ± 100d | 1400 ± 30d | 482 ± 143a | |
| AB | 0.88 ± 0.02b | 0.85 ± 0.02b,c | 5600 ± 100d | 2400 ± 300b | 4000 ± 20c | 361 ± 73a | |
| Significance of factors (DDL = 12) | |||||||
| Soils | <0.001 | <0.001 | <0.001 | 0.028 | <0.001 | 0.239 | |
| Species | <0.001 | 0.009 | <0.001 | <0.001 | <0.001 | 0.968 | |
| Soil X Species | <0.001 | 0.408 | <0.001 | 0.004 | 0.013 | 0.338 |
3.2 Zn isotopic compositions
Prayon soils (PC and PS) are characterized by similar δ66Zn values: −0.05 ± 0.02‰ (2SD) and −0.03 ± 0.01‰, respectively (δ66Zn results relative to the JMC 3-0749L Zn standard solution; knowing that the average δ66Zn value of the JMC 3-0749L standard gives +0.11 ± 0.03‰ (2SD) (n = 17) relative to our in-house solution (0.00 ± 0.02‰), meaning that δ66Znin-house for PC and PS soils are +0.06 ± 0.02‰ and +0.08 ± 0.01‰, respectively). In contrast, Angleur soil (AB) shows a higher δ66Zn of +0.26 ± 0.02‰ (Table 3). The CaCl2 extracts systematically display lower δ66Zn values compared to the bulk soils, indicating that the (surrogated) soil solutions are enriched in light Zn isotopes; factor 3 reaches an almost constant value of −0.13‰ for the three soils (Table 3). As generally observed in plants (e.g., Houben et al., 2014; Tang et al., 2012; Weiss et al., 2005), δ66Zn values in the shoots (δ66Znshoot from −0.34 ± 0.06‰ to +0.06 ± 0.01‰, Table 3) are systematically lower relative to the roots (δ66Znroot from −0.10 ± 0.04‰ to +0.32 ± 0.04‰). But the isotopic fractionations during Zn translocation from roots to shoots are not constant; they are larger in B. napus (Δ66Znshoot–root from −0.23 to −0.39‰) relative to L. multiflorum (Δ66Znshoot–root between −0.04 and −0.20‰). The δ66Zn values for the whole plant (Table 3) have been calculated by applying the mass balance equation:
| (2) |
4 Discussion
4.1 Zn isotopic signatures in soils, solutions and plants
Zinc isotopic signatures of the soils from the two distinct sites are typical values for contaminated soils (−0.05 ± 0.02‰ and −0.03 ± 0.01‰ for Prayon; +0.26 ± 0.02‰ for Angleur). In the literature, the published δ66Zn data range from −0.2 to +1.5‰ (Bigalke et al., 2010; Dolgopolova et al., 2006; Fekiacova et al., 2015; Sivry et al., 2008; Sonke et al., 2008; Tang et al., 2012). Sonke et al. (2008) have demonstrated a remarkably homogeneous δ66Zn value (+0.16 ± 0.20‰, n = 61 analyses for 10 mines) for ore-grade ZnS collected from various mining locations. Cloquet et al. (2008), Gioia et al. (2008) and Mattielli et al. (2009) have reported systematic light Zn isotope enrichments (relative to the initial ores) in aerial fallouts emitted by smoke stacks, which produce contaminated soils adjacent to smelters. Sivry et al. (2008) and Sonke et al. (2008) have demonstrated enrichments with heavy Zn isotopes in slag heaps, corresponding to solid residues of metallurgical process (smelting in blast furnace), complementary of aerial emissions. In agreement with those studies, the low positive δ66Zn values of contaminated Prayon soils (PS and PC) mainly reflect the aerial fallouts from the former Zn smelter, while the significantly higher positive δ66Zn values of the Angleur soil (AB) evidence the metallurgical scoria as the main Zn supply contributors in the soil.
The CaCl2 extracts, i.e. the soil solution surrogates, are systematically enriched in light Zn isotopes relative to the corresponding bulk soil. A constant fractionation between soil and related soil solution ( = −0.13‰) is found here irrespective of the soil sample. This confirms the initial observation of Arnold et al. (2010a,b) that Zn release from the soil solid phases to the liquid phase favours light Zn isotopes, which could reflect, according to Jouvin et al. (2009, 2012), a higher concentration of free Zn2+ ions in the soil solution.
The shoot Zn concentration ranges from 700 to 3300 mg Zn/kg. These values are at least one order above adequate Zn concentrations and reflect phytotoxicity due to Zn (Hamels et al., 2014). The Zn isotopic signature of the plants reflects that of the bulk soil. Within and among the plants, the isotope signatures differ. The Zn isotope signature in roots is isotopically heavier than in the corresponding soil solution (i.e. CaCl2 extracts). Relatively comparable magnitudes of Zn isotopic fractionation between roots and soil solutions are observed for the two sites and the two plants, which suggests a common Zn transfer mechanism from the various soils to plants, irrespective of the Zn availability. In agreement with Weiss et al. (2005) and Jouvin et al. (2012), the enrichment with heavy isotopes in the roots relative to solution reflects an adsorption of heavy Zn isotopes from the soil solution onto root cell walls. Alternatively, as proposed by Aucour et al. (2011), the positive isotope fractionation associated with the roots might indicate that Zn is bound to a high-affinity ligand, with cellular sequestration.
4.2 Species differences in Zn uptake, translocation and isotope fractionation
It is generally accepted that the uptake of Zn from soil has at least four main mechanisms (see also Aucour et al., 2011; Caldelas et al., 2011; Houben et al., 2014; Jouvin et al., 2012; Tang et al., 2012 and references herein): (1) root-induced soil acidification and mobilization of soil Zn (Houben et al., 2014; Loosemore et al., 2004); (2) Zn adsorption on root cell wall (Hall, 2002); (3) non-specific Zn transfer through the plasma membrane into the root symplast via low-affinity transporters (Hacisalihoglu et al., 2001), or (4) Zn specific uptake by either ZIP proteins or phytosiderophores released by the plant roots to transfer the Fe/Zn complexes through the membrane (Claus et al., 2013). The present growth media are far from the Fe- or Zn-deficiency conditions, hence the release of siderophores by L. multiflorum roots was probably negligible (Römheld and Marschner, 1990). The B. napus (dicotyledonous) has a larger total plant Zn concentration compared to L. multiflorum (monocotyledonous), mainly because of drastically higher Zn concentrations in its roots. According to Dufey and Braun (1986), Dufey et al. (2001) and Meychik and Yermakov (2001), the Cationic Exchange Capacity of Roots (CECR, directly related to the negative charge density on roots) in dicotyledonous species is higher than in monocotyledonous species, enhancing Zn adsorption on root cell walls.
The isotopic signatures of Zn in the whole plant were almost identical for both plant species in each of the soils; however, differences among species emerged when considering either root or shoot (Fig. 2). In agreement with previous studies (Arnold et al., 2010a; Jouvin et al., 2009; Viers et al., 2007; Weiss et al., 2005), shoots systematically display lower δ66Zn values compared to roots. As noticed by Caldelas et al. (2011), in Zn-excess conditions, roots systematically show positive δ66Zn values relative to the solution reflecting enrichment in heavy Zn isotopes, whilst shoots are isotopically lighter and display negative or similar δ66Zn values compared to the solution. There is more intense Zn isotope fractionation between roots and shoots in B. napus (Δ66Znshoot–root from −0.23 to −0.39‰) than in L. multiflorum (Δ66Znshoot–root from −0.04 to −0.20‰). Differences in Zn isotope fractionation between roots and shoots are correlated with the shoot–root Zn concentration ratios (Fig. 2), i.e. there is more enrichment in light Zn isotopes in shoots relative to roots as roots become more concentrated in Zn than shoots.
4.3 Zn translocation by plant transpiration flow
Isotope fractionation between roots and shoots might be the analogue of elemental fractionation, such as selective fractionation between strontium (Sr) and calcium (Ca) in plant parts, i.e. Sr accumulates in roots and Ca is more translocated to shoots, leading to a decreasing Sr:Ca ratio in plants with increasing distance from the roots (Drouet and Herbauts, 2008; Smolders and Merckx, 1993). Like Sr, in our experiment, heavy Zn isotopes accumulate in the root system and light Zn isotopes are preferentially transported further through the plant.
It is generally assumed that three main mechanisms are involved in the Zn transport from roots to shoots of plants: (1) exchange on the sorption sites of the plant tissues (adsorption on cell walls from the roots but also in the xylem vessel) and Zn loading into the xylem driven by heavy-metal ATPase transporting (Hanikenne et al., 2008); (2) diffusion (Zn transport on relatively short distance under concentration gradient) (Barberon and Geldner, 2014; Caldelas et al., 2011; Moynier et al., 2009; Weiss et al., 2005 and references herein); and (3) convection (mass flow transport in plant controlled by transpiration) (Barberon and Geldner, 2014; Lorenz et al., 1994). So far, the two first processes were essentially the only ones explored with the isotopic tool: as previously described by Jouvin et al. (2012), metal transport is carried out by the metal complexation with organic acids or amino acids or peptides produced by the plant. Those metals cross subsequently the cell membranes and circulate through the channels between cells and along the xylem and phloem by diffusion and ion exchange reactions, which induce Zn isotope fractionation. According to Rodushkin et al. (2004), Zn isotope fractionation occurring during diffusive transport would preferentially favour the transport of light Zn isotopes, and an increase of the fractionation extent with the diffusive distance. Several previous studies corroborated the hypothesis of long-distance diffusion process accounting for Zn isotope fractionation during transport in trees, palm trees, and herbaceous species (Viers et al., 2007), in bamboos compared to lentils (Moynier et al., 2009) and in Phragmites australis (Caldelas et al., 2011) in Zn-deficient or Zn-sufficient conditions. Those authors hypothesized a relationship between the fractionation extent and the length of the pathway or height of the plants. In the present work, in Zn-excess conditions all plant materials are very small, with a similar height of ∼10 cm, but the two species differ in shoot and root isotopic compositions and display large Δ66Znshoot–root (up to −0.39 ± 0.07‰). This suggests the control of Zn fractionation during translocation by another process, potentially species-dependent.
By attempting to confirm or refute the diffusion mechanism hypothesis, three simple diffusion models can be tested. A first simple calculation based on a one-dimensional diffusion model was conducted. If the plant is considered as a solid in contact with a liquid solution characterized by a constant Zn concentration, Zn will diffuse from the solution to the inside of the plant. A diffusion front will steadily advance inward the plant over a distance that can be roughly estimated to be the square root of D × t, where D is the diffusion coefficient with a value of 5·10−6 cm2·s−1 (approximation of the diffusion coefficient for Zn2+ in a free solution; Rodushkin et al., 2004) and t is the time. In the present study, the experiment duration t of 14 days corresponds to a distance reached by the diffusion front of only 2.46 cm, which is incompatible with the observed fractionation throughout the plant standing ±10 cm height. Second, assuming Δ66Zn = δ66Zntop − δ66Znentrance, the plant isotopic fractionation due to diffusion process can be also estimated by the following equation, according to Moynier et al. (2009):
| (3) |
The shoot–root Zn isotope fractionation exhibits a marked strong negative correlation with transpiration volume per dry biomass unit across species and soil samples (Fig. 3). This suggests a transpiration-controlled isotope discrimination. In disagreement with Aucour et al. (2011), who postulated that advection with transpiration stream does not fractionate the Zn isotopes, our results show higher Δ66Znshoot–root when the transpiration volume per biomass unit increases. Active transport (through carrier proteins) is associated with plants in metal-deficient conditions (or low metal concentrations) (Barberon and Geldner, 2014), while passive transport (transpiration flow) is generally observed in plant–soil systems displaying high metal levels (Hopmans and Bristow, 2002). Along the same lines, diffusion processes in soil or in the rhizosphere may control Zn uptake under deficient conditions, whereas mass flow may control the flux of Zn from soil to plants under high Zn supply (Degryse et al., 2009). Consequently, the passive Zn translocation from roots to leaves (or shoots) follows the transpiration path (a convection mechanism) within the xylem (Broadley et al., 2007; Page and Feller, 2005; Page et al., 2006a,b), and the effect of protein carriers would be negligible compared to the transpiration flow. The passive transport hypothesis by mass flow of water was also postulated by Henriet et al. (2006) for Si transport in banana at high Si concentrations.

Shoot–root Zn isotopic fractionation (Δ66Znshoot–root ‰ ± 2SD) vs. transpiration volume per plant biomass (transpiration/total dry biomass, cm3·g−1) for the two plant species, B. napus (rape) (black dots) and L. multiflorum (ryegrass) (white dots), cultivated on the three soils (PC, PS and AB).
It is puzzling how the transpiration flux on a plant dry weight affects the isotope discrimination between roots and shoots as shown in Fig. 3. The observed trend is weighted by the differences between both plants. Both plants have similar total plant Zn (μg Zn/plant) when compared pairwise per soil and have even similar shoot:root total Zn partition, albeit the B. napus plants have much smaller roots with higher Zn concentrations than L. multiflorum. As a result, the total flux of Zn from soil to plants is almost identical for each plant (per soil); however, there is more water flux on a total plant weight basis for rape than for ryegrass, i.e. a higher water flow across roots (weight based) for B. napus compared to L. multiflorum. One might suggest that Zn forms control fractionation along the soil–root–xylem path: free Zn could be transported faster towards the shoots than Zn-organic complexes that preferentially bind heavy Zn isotopes (Jouvin et al., 2009, 2012). As water flow increases per unit root weight, so increases the isotope fractionation. As a result, in the context of a highly contaminated soil–plant system, our data suggest that the translocation of Zn is mainly driven by the plant transpiration flow implying a convection mechanism rather than a diffusive process. During the translocation from roots to shoots, heavy isotopes are preferentially adsorbed on negatively charged sites of the cell walls from the surrounding xylem tissues, whereas light isotopes remain preferentially in the xylem sap; the proportion of light isotopes in the xylem sap increasing with increasing the transpiration volume/dry biomass ratio.
5 Conclusions
Former Zn smelters impact the isotopic fingerprint of Zn, i.e. fallout enriched soils contain lighter Zn isotopes, whereas slag-amended soils or slag heaps are characterized by heavier Zn isotopes. Soil solutions are enriched in light Zn isotopes. The isotope composition in two plants grown on the metal-contaminated soils reflects that of the soils and the compositions of both plants are similar when considering whole plant Zn. There is significant root–shoot Zn fractionation and this is species-dependent: the shoots of rape are more depleted in heavy Zn isotopes compared to those of Lolium multiflorum L. (ryegrass), and this goes along with higher Zn concentration in the roots of Brassica napus L. (rape), which, in turn, may be related to the higher cation exchange capacity of Zn in the roots. Root:shoot fractionation is more pronounced as the transpiration per unit of plant weight increases. This Zn isotope fractionation indicates a passive mode of Zn transport that is favoured in Zn-excess conditions. In a passive mode, the uptake will be driven by water absorption at the root level, and the Zn transport into the xylem sap will be mainly controlled by the transpiration flux. This mechanism does not totally rule out diffusion and adsorption mechanisms for accounting a part of the Zn transport and the related Zn isotope fractionation.
Acknowledgements
The authors thank the “Fonds de la Recherche Scientifique” (F.R.S.–FNRS, Belgium) for its financial support (FRFC conventions Nos. 2.4619.08 & 2.4599.11). We are grateful to Patrick Populaire for his help during the culture experiment, Claudine Givron for her precious help during soil analyses and Jeroen De Jong for the maintenance of the MC-ICP-MS. We warmly thank also Natagora, and especially Pasacal Hauteclair and the management committee of “Ile-aux-Corsaires” for giving us the opportunity to study the soil sampling sites. We acknowledge Sylvain Pichat for his careful review and constructive comments.
2 Isotopic measurements are expressed in ‰, relative to the standard solution, where RZn is the 66Zn/64Zn isotopic ratio of the sample (sample) and the bracketing standards (std1 and std2):
| (1) |
3 Zn fractionation factor (Δ66Zn, illustrated in Fig. 2) between two compartments 1 and 2 (Δ66Zn1–2), is calculated by difference in δ66Zn values: Δ66Zn1–2 = δ66Zn1 − δ66Zn2.