1 Introduction
FeII–III hydroxysalt green rusts (GR) were long ago recognised as transitory intermediate products during the wet corrosion of iron-base materials. For instance, Girard, entitling his thesis work in 1935 “de la constitution des rouilles ” [10], attributed a green compound forming in a sulphated medium to a hydrated magnetite. Thus, the preparation of GRs by oxidation of Fe(OH)2 was the predilection of corrosion scientists that essentially tried to simulate the process of oxidation of Fe species. It was used extensively for many years in several aqueous solutions containing the major anions Cl−,
2 Aerial oxidation of Fe(OH)2 within solution
2.1 Experimental
Ferrous hydroxide precipitated into solution by mixing a ferrous salt comprising anion
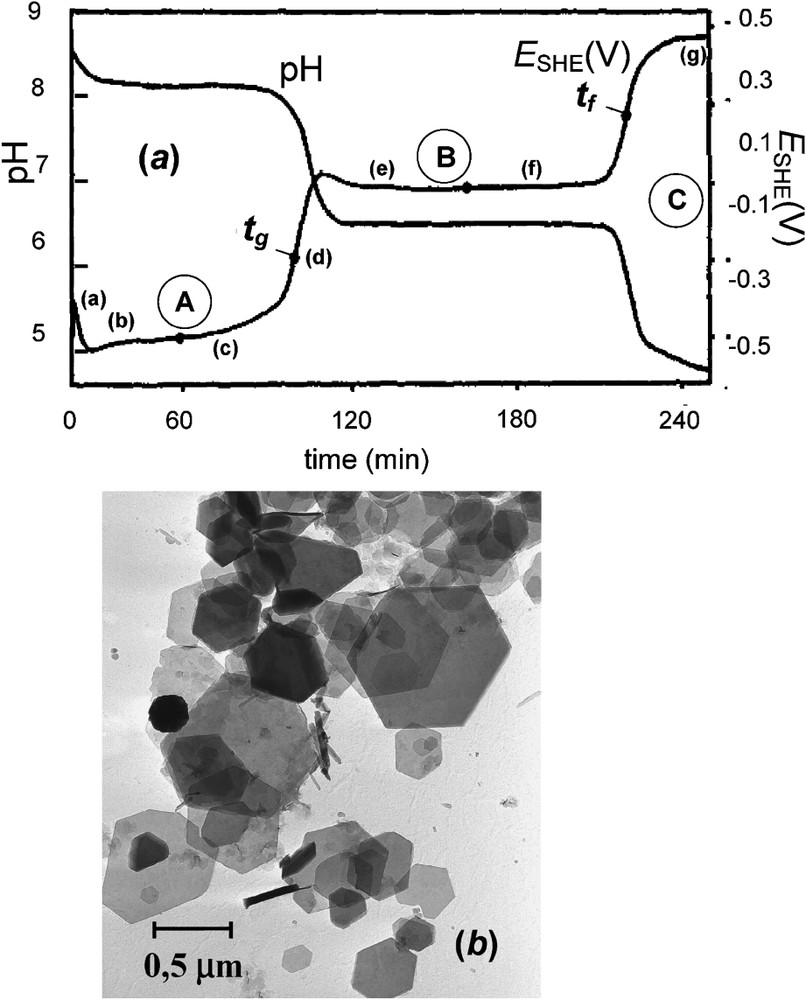
(a) Zero-current potential
(a) Zero-current potential
(a) Courbes du potentiel d'électrode à courant nul
(a) Courbes du potentiel d'électrode à courant nul
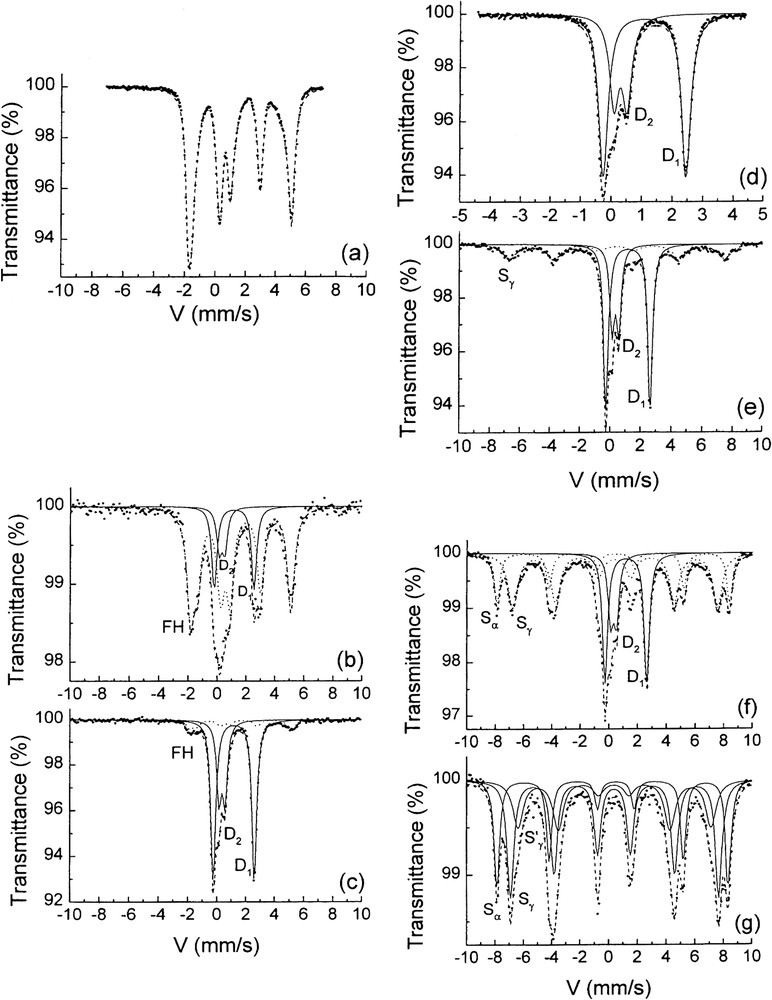
Transmission Mössbauer spectra measured at 15 K of the solid phases sampled at various times of the oxidation of ferrous hydroxide. Times (a)–(g) of sampling are indicated on the
Transmission Mössbauer spectra measured at 15 K of the solid phases sampled at various times of the oxidation of ferrous hydroxide. Times (a)–(g) of sampling are indicated on the
Spectres Mössbauer par transmission mesurés à 15 K des phases solides prélevées à diverses périodes de l'oxydation de l'hydroxyde ferreux [6]. Les instants de prélèvement de (a) à (g) sont indiqués sur la courbe
Spectres Mössbauer par transmission mesurés à 15 K des phases solides prélevées à diverses périodes de l'oxydation de l'hydroxyde ferreux [6]. Les instants de prélèvement de (a) à (g) sont indiqués sur la courbe
2.2 Mass-balance diagram
2.2.1 Formation of green rust (
Interpretation of the
| (1a) |

Mass-balance diagram of iron species in a sulphate containing medium where GR2(
Diagramme bilan matière des espèces du fer dans un milieu contenant des ions sulfate où se forme GR2(
Then, stage B that elapses from point B (
| (1b) |
Then, during stage C, Fe2+ within solution gets oxidised into FeOOH (cf. Fig. 2g) for one third of it and 2/3 in solution; the solution gets acidic gradually (cf. Fig. 1a):
| (1c) |
As a whole, the initial Fe(OH)2 at point A becomes ferric oxyhydroxide α-FeOOH goethite and Fe3+ ions in acidic solution at point D according to:
| (1d) |
At D on tie-line EI, the mixture of FeOOH and Fe3+ is in the 8:1 proportion (Eq. (1d)).
2.2.2 Formation of magnetite (
The direct oxidation of Fe(OH)2 at stoichiometry, i.e. initially at
| (2a) |
| (2b) |
2.2.3 Trigger value
It has been carefully established by coprecipitation of FeII and FeIII ions in the presence of
2.3 Products of oxidation within solution
2.3.1 Lepidocrocite and goethite for GR1(Cl−) and GR2(
The determination of the products of oxidation of GRs are of major importance for some technological issues involving corrosion and environmental science as well. It is also a topic by itself for explaining the genesis of minerals. Early works were devoted to the final products of oxidation of Fe(OH)2 when varying the initial ratio R={[OH−]/[Fetotal]}. This is illustrated for the chloride-containing medium where

End-products of the aerial oxidation of Fe(OH)2 in chloride containing medium with respect to the initial ratio
Produits finals de l'oxydation à l'air de Fe(OH)2 en milieu chloruré en fonction du rapport initial
2.3.2 Ferrihydrite and goethite for GR1(
The carbonate-containing medium behaves differently at
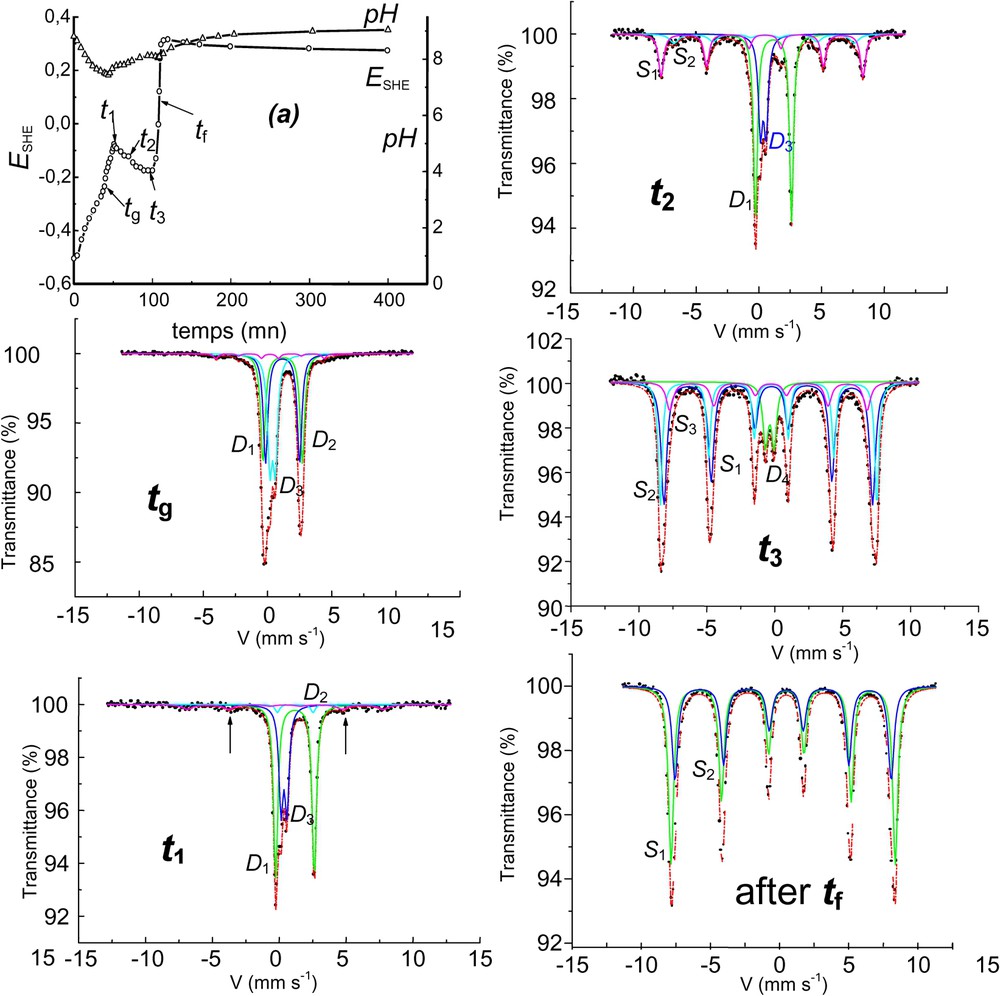
Aerial oxidation of Fe(OH)2 in the carbonate-containing solution and corresponding Mössbauer spectra showing the formation of ferrihydrite at the end of stage B before transforming into goethite. The solution is buffered by
Aerial oxidation of Fe(OH)2 in the carbonate-containing solution and corresponding Mössbauer spectra showing the formation of ferrihydrite at the end of stage B before transforming into goethite. The solution is buffered by
Oxydation à l'air de Fe(OH)2 en solution carbonatée et spectres Mössbauer afférents montrant la formation de ferrihydrite en fin d'étape B, avant transformation en goethite. La solution est tamponnée par les ions
Fig. 6 allows comparing the reaction path in the case of the carbonated medium with those of chloride and sulphate media. The two first stages are similar and correspond to the same reactions: stage A is the oxidation of Fe(OH)2 into the GR at

Mass-balance diagram comprising FeII–III hydroxycarbonate green rust GR1(
Mass-balance diagram comprising FeII–III hydroxycarbonate green rust GR1(
Diagramme bilan matière comportant l'hydroxycarbonate ferreux–ferrique GR1(
Diagramme bilan matière comportant l'hydroxycarbonate ferreux–ferrique GR1(
Stage A: Fe(OH)2 gets oxidised by incorporating carbonate ions:
| (3a) |
Stage B: GR1(
| (3b) |
Stage C:
| (3c) |
As a whole, Fe(OH)2 gets oxidised into α-FeOOH with oxygen by incorporating the excess of
| (3d) |
Note that for strong acids, e.g., for Cl− or
3 Deprotonation of green rusts
3.1 Fast or dry oxidation
The fast oxidation of GRs by hydrogen peroxide or an aerial oxidation of previously dried GRs gives rise to the fully deprotonated ‘ferric GR’. For instance, in the case of GR1(Cl−), instead of getting the XRD pattern of lepidocrocite or ferrihydrite as for the aerial oxidation in solution, the oxidised product has a pattern quite similar to that initially observed and only lattice parameters are slightly different with blurred and broadened lines [19]; however, its Mössbauer spectrum displays only ferric sextets at 12 K and no ferrous quadrupole doublet at 78 K. Thus, its formula is
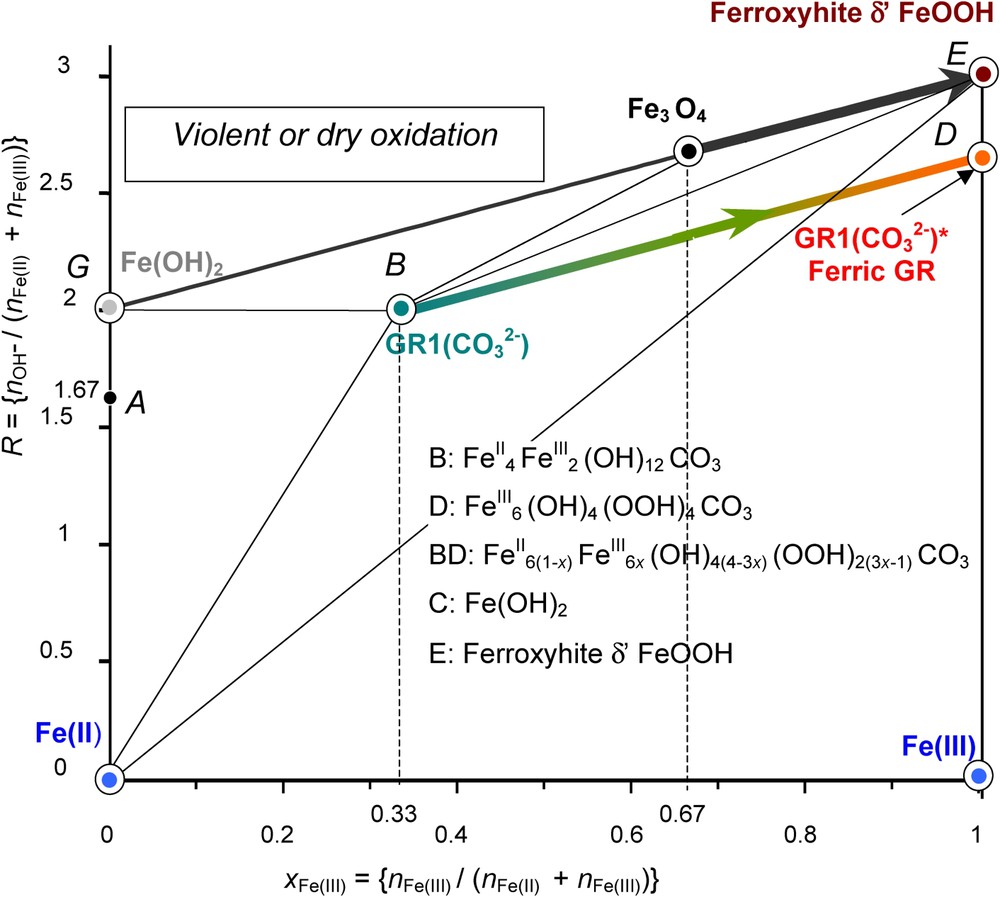
Mass-balance diagram comprising FeII–III hydroxycarbonate green rust GR1(
Mass-balance diagram comprising FeII–III hydroxycarbonate green rust GR1(
Diagramme bilan matière comportant l'hydroxycarbonate ferreux–ferrique GR1(
Diagramme bilan matière comportant l'hydroxycarbonate ferreux–ferrique GR1(
The same type of phenomenon occurs if oxidising violently Fe(OH)2 with H2O2. A phase named δ-FeOOH forms. Its mineral counterpart is feroxyhyte [4]. In both cases, the original crystal lattice is conserved; each FeII(OH)6 octahedron becomes a FeIII(OOH)3 octahedron while the FeII gets oxidised. Reactions are quite similar as shown in the mass-balance diagram (Fig. 7). Starting at point G
| (4) |
For its part, the fast oxidation of GR1(
| (5) |
3.2 Voltammetry
Voltammetric curves have been drawn to form GR1(
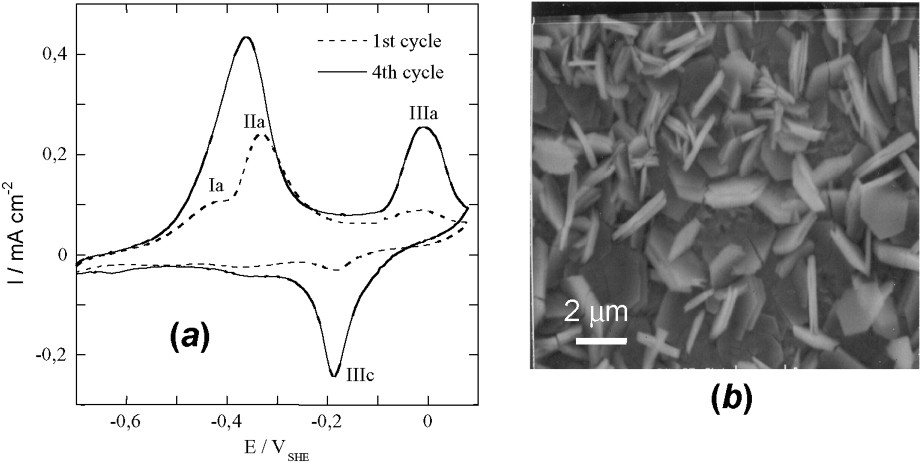
(a) Voltammograms obtained on iron disc at 10 mV s−1 in 0.4 M NaHCO3 solution at 25 °C and pH=9.6. (b) SEM image of the orange ferric compound resulting from the oxidation by air of electrochemically formed GR2(
(a) Voltampérogrammes obtenus avec un disque de fer à 10 mV s−1 dans une solution 0,4 M NaHCO3 à 25 °C et pH=9,6. (b) Image au microscope à balayage du composé ferrique orange résultant de l'oxydation à l'air de GR2(
4 Reduction of FeOOH
The mass-balance diagram was very useful for illustrating the three reaction stages that evolve during the oxidation processes of Fe(OH)2 as discussed previously for aerial oxidation, i.e. by following initially (stages A and B) a straight line of slope 1 (Figs. 3 and 6). Similarly, a fast oxidation as accomplished by using H2O2 gave rise to a deprotonation of GR, the ‘ferric GR∗’ and the pathway followed also a line of slope 1 from B to D as drawn for the carbonated medium (Fig. 7). How to materialise the pathways when starting from the ferric oxyhydroxides? From point E
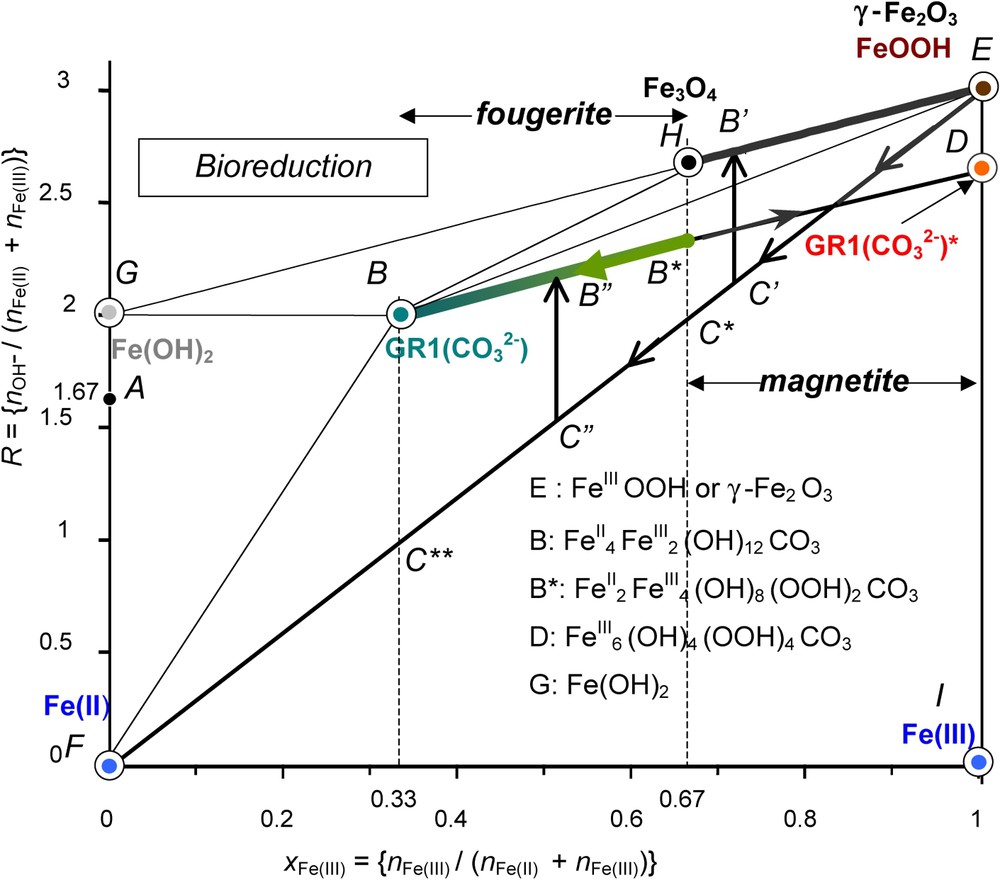
Mass-balance diagram including FeII–III hydroxycarbonate green rust GR1(
Mass-balance diagram including FeII–III hydroxycarbonate green rust GR1(
Diagramme bilan matière de l'hydroxycarbonate ferreux–ferrique GR1(
Diagramme bilan matière de l'hydroxycarbonate ferreux–ferrique GR1(
4.1 Bioreduction of ferric oxyhydroxides
The second pathway EF is followed during the dissimilatory reduction of FeOOH by bacteria (Fig. 9) [12]. The practical role of DIRB is to reduce ferric species from solid state into Fe2+ ions that are dissolved in circumneutral medium by enzymatic activity [20,21]. This produces a mixture of Fe2+ and FeOOH and the running point migrates from E to F (Fig. 9).
The precipitate that forms depends on the position at which the mixture of FeOOH and FeII is located before a coprecipitation occurs in relation with the pH of the solution through the value of R. From E to C∗ where
| (6) |
4.2 The FeII–III hydroxycarbonate fougerite
Occurrences of GRs in the Gr horizon of a gley soil are consistent with the previous scheme. The mineral is formed by bacterial reduction of ferric oxyhydroxides [8]. Carbonate and FeII ions come from the consumption and decomposition of humic substances, and the respiration of bacteria. Route
Therefore, fougerite is
5
Monitored aerial oxidation of Fe(OH)2 was extensively utilised for preparing GRs, but also for determining their composition [5,6,14,17]. The preparation by coprecipitation of FeII and FeIII species has now supplanted it [2,22,23]. However, one major advantage of disposing of the
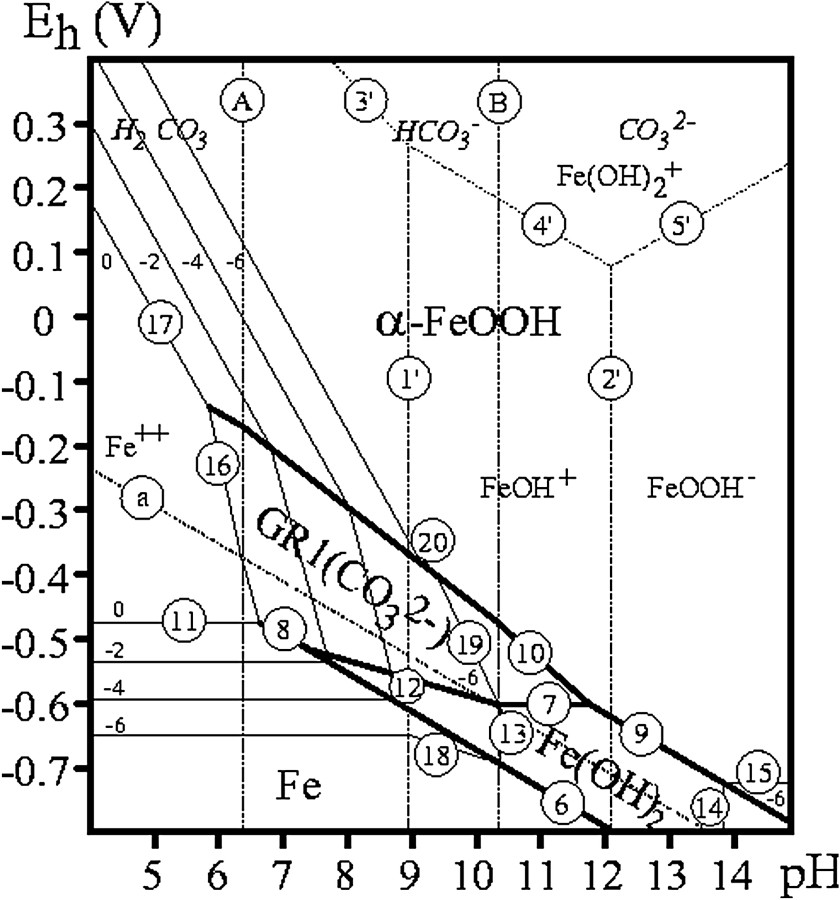


Diagrammes de Pourbaix
Equilibrium equations of Eh–pH Pourbaix diagram reactions for various green rusts: GR1(
Équations d'équilibre des réactions de diagrammes de Pourbaix
| Water/Eau | |
| (a) |
|
|
|
|
| Carbonate species/Espèces carbonatées | |
| (A) |
|
|
|
|
| (B) |
|
|
|
|
| Iron species/Espèces du fer | |
| (1) |
|
|
|
|
| (2) |
|
|
|
|
| (3) |
|
|
|
|
| (4) |
|
|
|
|
| (5) |
|
|
|
|
| (6) |
|
|
|
|
| (7) Carbonate-containing medium/Milieu contenant des carbonates | |
| (a) |
|
| |
|
| (b) |
|
| |
|
| (7) Chloride-containing medium/Milieu contenant des chlorures | |
|
|
|
|
|
|
| (7) Sulphate-containing medium/Milieu contenant des sulfates | |
|
|
|
|
|
|
| (8) Carbonate-containing medium/Milieu contenant des carbonates | |
|
|
|
|
|
|
| (8) Chloride-containing medium/Milieu contenant des chlorures | |
|
|
|
|
|
|
| (8) Sulphate-containing medium/Milieu contenant des sulfates | |
|
|
|
|
|
|
| (9) |
|
| (10) Carbonate-containing medium/Milieu contenant des carbonates | |
| (a) |
|
| |
|
| (b) |
|
| |
|
| (c) |
|
| |
|
| (10) Chloride-containing medium/Milieu contenant des chlorures | |
|
|
|
|
|
|
| (10) Sulphate-containing medium/Milieu contenant des sulfates | |
|
|
|
|
|
|
| (11) |
|
|
|
|
| (12) |
|
|
|
|
| (13) |
|
|
|
|
| (14) |
|
|
|
|
| (15) |
|
| (16) Carbonate-containing medium/Milieu contenant des carbonates | |
| (a) |
|
| |
|
| (b) |
|
| |
|
| (16) Chloride-containing medium/Milieu contenant des chlorures | |
|
|
|
|
|
|
| (16) Sulphate-containing medium/Milieu contenant des sulfates | |
|
|
|
|
|
|
| (17) |
|
| (18) |
|
|
|
|
| (19) Carbonate-containing medium/Milieu contenant des carbonates | |
|
|
|
|
|
|
| (19) Chloride-containing medium/Milieu contenant des chlorures | |
|
|
|
|
|
|
| (19) Sulphate-containing medium/Milieu contenant des sulfates | |
|
|
|
|
|
|
| (20) |
|
6 Conclusion
The aerial oxidation of Fe(OH)2 in the presence of anions, e.g., Cl−,
Other ways of oxidation, e.g., fast oxidation by H2O2, dry oxidation or voltammetry give rise to a deprotonation of the GR in situ, which can be complete, giving the ‘ferric GR’ that keeps essentially the initial structure. Biotic reduction of ferric oxyhydroxides by DIRB leads also to partially deprotonated GR1(


