1 Introduction
In soils, FeIII species are very frequently trapped in solid iron oxyhydroxides. In contrast, FeII species move much more rapidly because they are easily dissolved. Therefore, the understanding of the possible chemical reactions that occur at the solid/solution interface between FeII dissolved species and the various ferric oxyhydroxides is fundamental. It is now well established that dissimilatory iron reducing bacteria (DIRB) play a major role in the biogeochemical cycle of iron, because they are responsible for the reductive dissolution of ferric oxyhydroxides FeOOH. Once FeII dissolved species are produced by the bacteria, a reaction of coprecipitation between FeII species and the FeIII species remaining into FeOOH may occur and mixed FeII–FeIII compounds form. Such coprecipitation reactions between FeII and FeIII species were studied in abiotic conditions by several authors, e.g., [2,8,9,12,15–17]. It was namely shown that FeII species react easily with lepidocrocite (or ferrihydrite) to form either green rust [8,16] or magnetite [9,12,17] and that the adsorption of hydroxylated FeII species on the surface of the ferric oxyhydroxide FeOOH was an initial step that precedes the formation of magnetite [15,17]. In FeIII rich medium, the formation of magnetite Fe3O4 was favoured, but was sometimes preceded by the precipitation of green rusts [12,15]. It is now well established that in anoxic conditions green rusts are transformed either in a mixture of magnetite and siderite {Fe3O4, FeCO3}, when carbonate anions are present [3,5], or in a mixture of magnetite and ferrous hydroxide {Fe3O4, Fe(OH)2} [14] at pH higher than 9.
By using the experimental method used in the early work of Arden [2], reactions of coprecipitation were studied systematically by performing the titration of various FeII–FeIII solutions [14]. A mass-balance diagram was shown to be a powerful tool to study the various steps of the reaction and the properties of this diagram are explained here. The formation of hydroxycarbonate green rust and Al-substituted hydroxysulphate green rust by coprecipitation was also studied recently [1,5].
2 Experimental method
The samples were prepared by the coprecipitation of FeII and FeIII species in different kinds of aqueous solutions. This simple method, which consists to add a basic solution to a mixture that contains FeII and FeIII dissolved species, is well known for the synthesis of the other members of the layered double hydroxides (LDHs) family. Due to the fact that the divalent cation FeII can easily be oxidised, the evacuation of dissolved oxygen was ensured by a continuous N2 bubbling in the aqueous solution introduced in a gas tight reactor. The initial solution was prepared by dissolving in demineralised water appropriate amounts of FeII and FeIII salts. A basic solution was progressively added to the initial mixture by using a peristaltic pump at a constant flow rate of ∼0.5 ml min−1. The duration of the experiments was ∼5 h and the pH of the solution was monitored continuously. Depending on the type of samples that was synthesised, different methods were used that are summarised in Table 1. The suspensions were filtered under an inert atmosphere and the precipitates were analysed by several techniques: X-ray diffraction (XRD) at a wavelength , transmission Mössbauer spectroscopy (TMS) and transmission electron microscopy (TEM). Details concerning the method of analyses were given in a previous work [14]. The Mössbauer spectra were calibrated by using an iron foil at room temperature and were fitted with Lorentzian-shape lines. The concentrations of Fe and in the aqueous solution were measured by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). Titration with 1,10-phenantroline was used for ferrous iron determination.
Conditions of preparation of the samples synthesised by coprecipitation: hydroxysulphate green rust {GR()}, hydroxycarbonate green rust {GR()} and Al-substituted hydroxysulphate green rust {Al-GR()}
Conditions de préparation des échantillons synthétisés par coprécipitation : rouille verte sulfatée {GR()}, rouille verte carbonatée {GR()} et rouille verte sulfatée substituée aluminium {Al-GR()}
| Type of green rust | Salts and concentration of the initial solution | Base | ||
| GR() | 0; 0.1; 0.14; 0.17; 0.2; 0.25, 0.33; 0.5; 0.67, 1 | 0 | FeSO4⋅7 H2O | [NaOH] = 0.8 M |
| Fe2(SO4)3⋅5 H2O | ||||
| [Fe]=4×10−1 M | ||||
| GR() | 0, 0.33, 0.4, 0.5, 1 | 0 | FeSO4⋅7 H2O | [Na2CO3] = 0.57 M |
| Fe2(SO4)3⋅5 H2O | ||||
| [Fe]=6.7×10−2 M | ||||
| GR() | 0.25 | 0 | FeCl2⋅4 H2O | [Na2CO3] = 0.57 M |
| FeCl3⋅6 H2O | ||||
| [Fe]=6.7×10−2 M | ||||
| Al-GR() | 0.2; 0.185; 0.16; 0.1 | 0; 0.015; 0.04; 0.1 | FeSO4⋅7 H2O | [NaOH] = 0.8 M |
| Fe2(SO4)3⋅5 H2O | ||||
| Al2(SO4)3⋅5 H2O | ||||
| [Fe]+[Al]=4×10−1 M |
3 Properties of the mass-balance diagram
3.1 Construction of the diagram
If one considers the mass-balance of the previously described coprecipitation experiments, the nature and the relative abundances of the iron containing compounds that form are governed by two parameters: (i) the ferric molar fraction of the initial mixed FeII–FeIII solution, i.e. the ratio ; (ii) the hydroxylation rate that corresponds to the molar ratio where n(OH−) represents the number of moles of hydroxyl species added in solution, n(FeIII) and n(FeII) are the numbers of FeIII and FeII ions introduced into the initial solution. The value of the ratio R is well defined when the basic solution used in the experiment is a strong base such as NaOH.
It is convenient to represent in a mass-balance diagram [14] the ratio as a function of the ferric molar fraction of the various iron species that may form (Fig. 1), where n(OH−), n(FeIII) and n(FeII) represent here the number moles of OH−, FeIII and FeII consumed during the precipitation of 1 mol of iron of a given compound. As an example, the ferric oxyhydroxides of chemical formula FeOOH (or the ferric oxides Fe2O3) are represented by point in the diagram presented in Fig. 1, because the precipitation of 1 mol of FeIII into these compounds is described by the following chemical reactions:
| (1a) |
| (1b) |
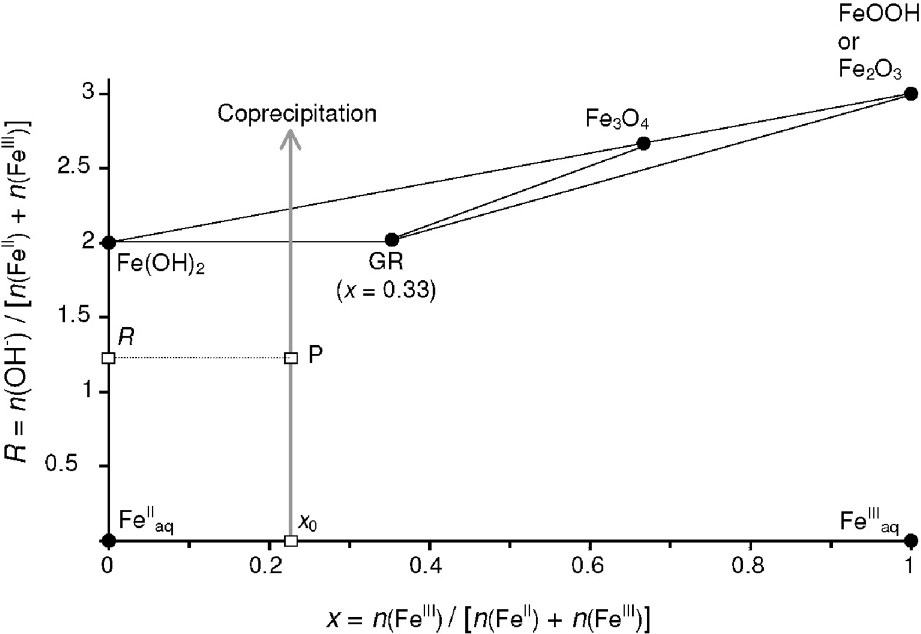
FeII–FeIII mass-balance diagram, showing the route of a coprecipitation experiment.
Diagramme bilan matière du système FeII–FeIII et représentation du chemin correspondant à une réaction de coprécipitation.
In a same manner, the position of the different solid compounds can easily be determined: ferrous hydroxide Fe(OH)2, green rust (GR) of general chemical formula , magnetite Fe3O4 and ferric oxide Fe2O3 correspond respectively to points , , and (Fig. 1). Non-stoichiometric magnetite Fe(3−x)O4 is situated on the tie-line that links point to point . The aqueous species and are represented by points and , respectively.
3.2 Interpreting the diagram
A coprecipitation experiment that is realised at a constant ferric molar fraction ratio corresponds to the vertical line shown in Fig. 1. At each instant of the experiment, i.e. for each point situated on the vertical line, the nature and the relative abundance of the different compounds that may precipitate can be determined graphically. Therefore, the following cases will be considered:
- – (a) a single compound forms if P is situated at a point that corresponds to a given compound, e.g., point that corresponds to GR in Fig. 1;
- – (b) a two-compound system forms if point P is situated on a tie-line that joins two compounds, e.g., the tie-line that joins Fe(OH)2 to GR in Fig. 1. In a general case, let us consider the formation of a mixture of two compounds α and β that correspond to the two points and presented in Fig. 2. The conservation of matter leads to:
(2)
where n(OH−), n(FeIII), n(OH−)[α], n(FeIII)[α], n(OH−)[β] and n(FeIII)[β] are the respective mole numbers of OH− and FeIII consumed during the formation of the whole mixture and their repartition in the α and β compounds. It can be demonstrated that Eqs. (2) and (3) are equivalent to the following equations:(3) (4) (5) It should be noted that if Mössbauer spectroscopy is performed for analysing the mixture, and occur to be the relative areas of the subspectra of phases α and β, respectively. The quantitative predictions made with the mass-balance diagram can be directly compared to quantitative analyses made with Mössbauer spectroscopy as already done in a previous work [16];
- – (c) a three-compound mixture forms if point P is situated inside a triangle of three compounds, e.g., Fe(OH)2, GR and Fe3O4 in Fig. 1. Let us again consider the general case of three compounds represented by the three points , and in Fig. 2. A similar demonstration to that followed for the two-compounds mixture will lead to the vectorial relation:
(9) The Fe molar fractions and can be determined graphically by using the parallel to the sides of triangle (, , ) as shown in Fig. 2 and the following equations are verified:
(12) (13) (14)
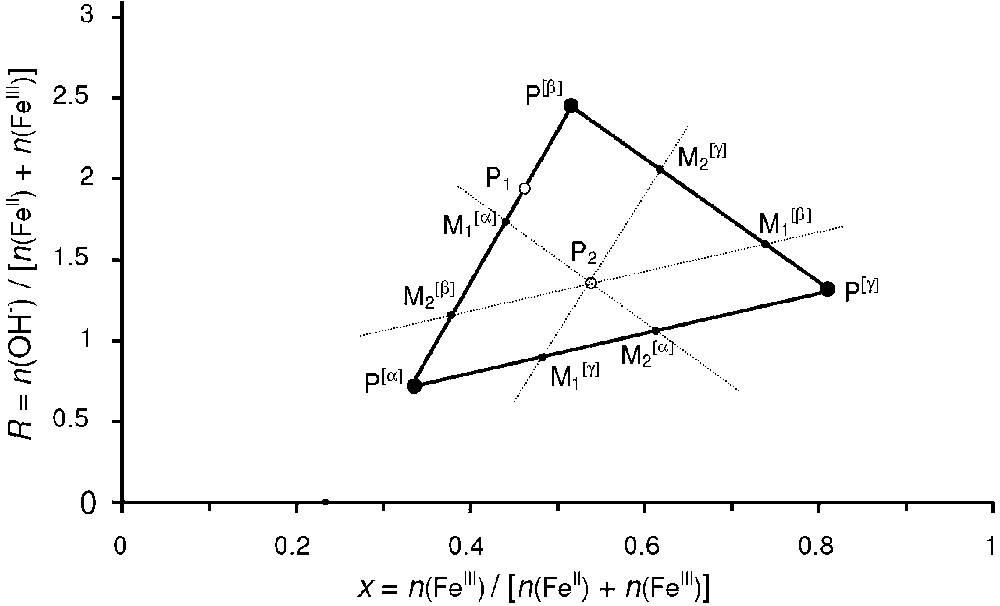
Graphical determination of the relative abundance of the compounds in the mass-balance diagram. For point P1, the two-compound system {α+β} forms and the relative quantities are given by Eqs. (7) and (8). For point P2, the three-compound system {α+β+γ} forms and the relative quantities are given by Eqs. (12), (13) and (14).
Détermination graphique des abondances relatives de chaque phase dans le diagramme bilan matière. Pour le point P1, le système biphasé {α+β} se forme et les quantités relatives de chaque phase sont données par les Éqs. (7) et (8). Pour le point P2, le système triphasé {α+β+γ} se forme et les quantités relatives de chaque phase sont données par les Éqs. (12), (13) et (14).
4 Mechanism of formation of hydroxysulphate green rust
4.1 Analyses of the pH curves
For 10 different values of the ferric molar fraction x, titration curves were presented in a previous work [14] and three typical curves are shown in Fig. 3. The curves are characterised by three plateaus separated by two equivalent points E1 and E2. For , a slow down of the pH curves is observed around a critical point called E* and the jump of pH is always preceded by a small peak between and 1.95. A direct visualisation of the compounds that form during the titration is presented in Fig. 4 where the R values that correspond to equivalent points E1, E2 and E* are indicated in the mass-balance diagram. One observes that the () values of points E1 are situated on a line . This indicates that the compound that forms during the first plateau is a sulphated ferric basic salt, i.e. a ferric oxyhydroxide where some of the OH− anions are substituted by anions. Schwertmanite of general chemical formula Fe16O16(OH)y(SO4)z⋅n H2O ( and ) [4], which is also presented in the mass-balance diagram, is an example of such a ferric basic salt. When , the () values of points E2 follow the line of the binary system {Fe(OH)2, GR(SO4)}. For , the points are situated in the triangle of the three-compound system {Fe(OH)2, GR(), Fe3O4}. The formation of these compounds was confirmed by using Mössbauer spectroscopy and the TMS spectra of two samples taken at points E2 of the titration curves are given in Fig. 5a and b. Hyperfine parameters and quantitative predictions made with the mass-balance diagram are given in Table 2. At , the Mössbauer spectrum of GR() is easily distinguished from the spectrum of Fe(OH)2, which is characterised by an octet, as already discussed [14]. There is a relative good agreement between the predicted and measured quantities for the mixture {GR(), Fe(OH)2}. It is not the case for the three-compound mixture and this may be attributed to the existence of non-stoichiometric magnetite.

Evolution of pH during the titration of different FeII–FeIII solutions.
Variation du pH au cours de la titration de différentes solutions mixtes FeII–FeIII.
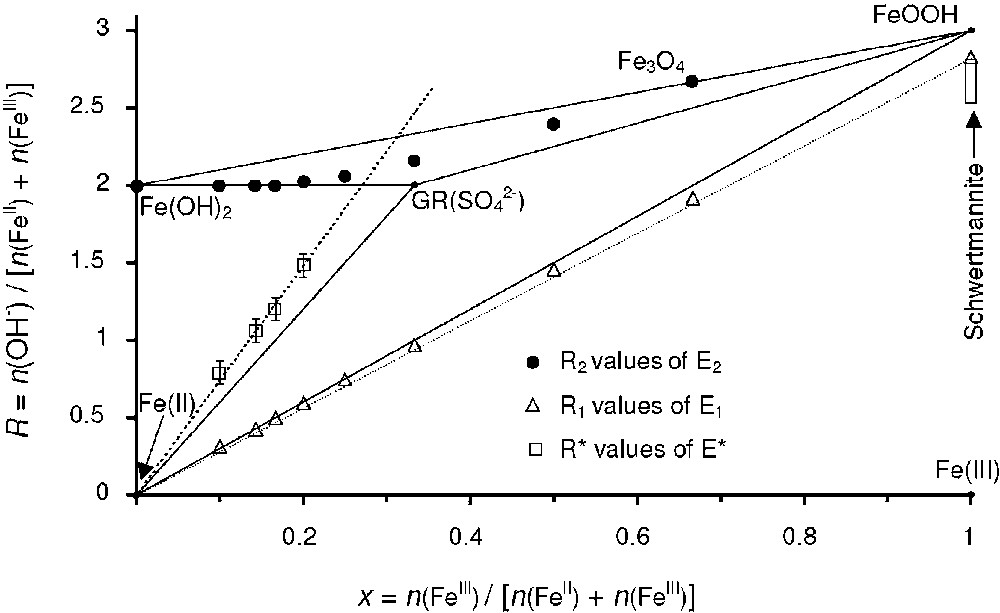
R values of equivalent points E1 and E2 and point E* presented in the mass-balance diagram.
Valeurs de R pour les différents points E1, E2 et E*, qui sont représentées dans le diagramme bilan matière.
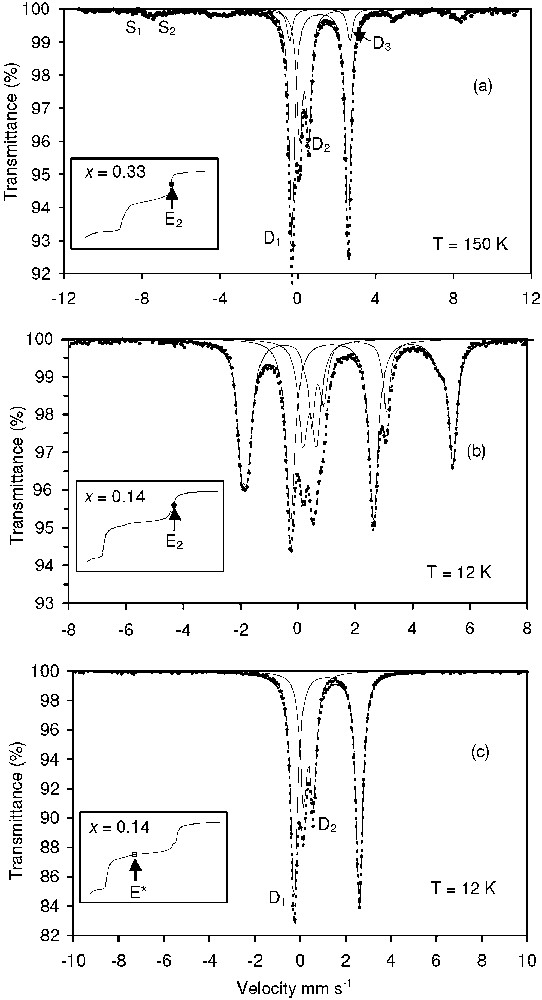
Mössbauer spectra of the solid compounds obtained at different points of the titration curves.
Spectres Mössbauer des composés solides obtenus en différents points des courbes de titration.
Hyperfine parameters of the Mössbauer spectra presented in Fig. 5
Paramètres hyperfins des spectres Mössbauer présentés sur la Fig. 5
| Spectra | Compounds | δ | Δ or | H | FWHM | RA (%) measured | RA (%) predicteda | |
| Fig. 5a | GR() | D1 | 1.29 | 2.87 | — | 0.32 | 53 | (D1 + D2) |
| 52 | ||||||||
| D2 | 0.48 | 0.46 | — | 0.32 | 28 | |||
| Fe(OH)2 | D3 | 1.28 | 3.09 | — | 0.32 | 8 | 24 | |
| Fe3O4 | S1 | 0.46 | 0 | 493 | 0.79 | 7 | (S1 + S2) | |
| 24 | ||||||||
| S2 | 0.86 | 0 | 468 | 0.69 | 4 | |||
| Fig. 5b | GR() | D1 | 1.32 | 2.88 | — | 0.37 | 34 | (D1 + D2) |
| 43 | ||||||||
| D2 | 0.55 | 0.47 | — | 0.37 | 18 | |||
| Fe(OH)2b | 1.3 | −3.1 | 166 | 0.34 | 48 | 57 | ||
| Fig. 5c | GR() | D1 | 1.32 | 2.86 | — | 0.34 | 66 | (D1 + D2) |
| D2 | 0.52 | 0.43 | — | 0.34 | 34 | 100 |
a Predicted by using the lever rules in the mass-balance diagram. Valeurs prédites en utilisant la règle des leviers dans le diagramme bilan matière.
b Simulation of this component by a mixture of quantum states according to Génin [6]. Simulation de cette composante à l'aide d'un mélange d‘état quantique d'après Génin [6].
The most interesting part of this analysis is the position of points E* observed on the pH curves when (Fig. 3). The pH values of the flat part of the second pH plateau, i.e. when , is very close to those measured during the titration of a standard FeII solution (not shown). It is therefore natural to propose that the first step of the second pH plateau, i.e. when , corresponds to the full transformation of the initially formed ferric oxyhydroxide into GR(). In the second step, i.e. when , the precipitation of the excess of present in solution leads to the formation of Fe(OH)2. The () of points E* presented in the mass-balance diagram in Fig. 4 increases roughly linearly according to where . This last linear relation is equivalent to a molar ratio n(OH−)/n(FeIII). In a previous work [16], it was demonstrated with Mössbauer spectroscopy that GR() forms in the absence of any others solid compounds when exactly 7 moles of OH− per mole of FeIII were added. When , the Mössbauer spectrum of the sample obtained in these conditions contains only one ferrous doublet D1 and one ferric doublet D2 (Fig. 5c). The measured ratio is in good agreement with the chemical formula . The fact that GR() forms when an excess of OH− species is added is in apparent contradiction with what is expected if one considers the previous chemical formula where the ratio n(OH−)/n(FeIII) is equal to 6. This means that a certain amount OH− species are consumed by another process that is concomitant to the formation of GR(). Another important experimental evidence that such a process occurs is the fact that the value observed for the second equivalent point of the pH titration curves of the ‘stoichiometric’ solution, i.e. when , overshoots clearly the expected value (Fig. 3). It can be seen on the same figure that such a phenomenon does not occur when and the measured value is in very good agreement with the value expected for the formation of stoichiometric magnetite.
4.2 Analyses of Fe and in aqueous solution
The different steps of the coprecipitation experiments were analysed by measuring the concentrations of Fe, FeII and that remain in the aqueous medium during the titration of the solution (Fig. 6). The grey dilution curve presented in Fig. 6a corresponds to the expected concentration assuming that these anions are not incorporated in any precipitates. In this case, the variation of concentration is only due to the increase of volume corresponding to the addition of the NaOH solution. Calculated concentration curves were done by using the following hypotheses: (i) a ferric oxyhydroxide FeOOH precipitates during the first pH plateau by consuming 3 mol of OH− per mole of FeIII and the reaction is therefore finished at point B of abscissa ; (ii) FeOOH reacts with to form GR() by consuming 2 mol of OH− per mole of iron and the reaction is accomplished at point C of abscissa ; (iii) GR(), which is unstable at pH ∼ 12 [16], is fully transformed into a mixture {Fe3O4, Fe(OH)2} at the end of the titration curve ().
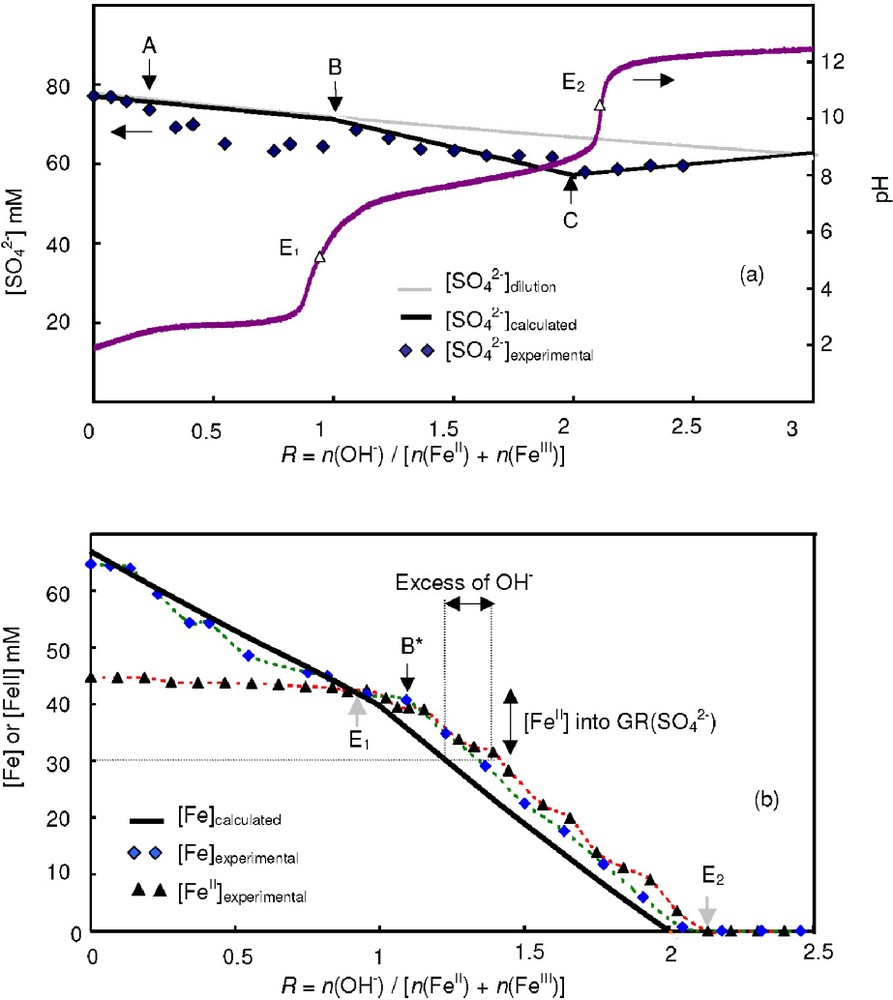
Evolution of the concentrations [], [Fe] and [FeII] in solution during the titration of the solution prepared for a ferric molar fraction x=0.33.
Variation des concentrations en solution [], [Fe] and [FeII] pour la titration correspondant à une valeur x=0.33 de la fraction molaire en ions FeIII.
The concentration of anions measured in solution () is clearly situated under the calculated curve between points A and B of Fig. 6a. This represents an experimental proof of the formation of a ferric sulphated basic salt, which begins when the flat part of the first pH plateau is reached. The decrease of the values is followed by an increase at and the experimental values reach again the calculated values around point B. This means that the initially formed ferric basic salt transforms into a ferric oxyhydroxide by releasing anions in solution around equivalent point E1. As expected, the values decrease during the formation of GR() between points E1 and E2 and then increase when GR() transforms into the mixture {Fe3O4, Fe(OH)2}.
The Fe concentrations measured in solution ([Fe]exp) are also situated slightly above the calculated values between points A and B confirming the formation of the ferric basic salt (Fig. 6b). By using both values and [Fe]exp measured during the titration of the reference FeIII solution (), the molar ratio of the ferric basic salt was calculated and it decreases between ∼0.6 to 0.3 for increasing values of R (). The evolution of the [Fe]exp values between points E1 and E2 is very interesting because the breakdown of the curves occurs at point B*, situated at (Fig. 6b). Then, the experimental curve decreases at a similar rate than the calculated curves, but stays always shifted to the right. As presented in Fig. 6b, this means that it is necessary to introduce an excess of OH− in solution in order to precipitate a given amount of FeII into GR(). This experimental result was confirmed by measuring the concentrations of FeII in solution. Between points E1 and E2, the curves corresponding to both [Fe]exp and [FeII]exp follow the same trends and are more or less superposed. Therefore, the concentration of FeIII cations present in the aqueous solution when GR() forms is negligible.
4.3 Mode of formation of GR()
Analyses of the pH titration curves performed with the mass-balance diagram, Mössbauer spectroscopy and measurements of Fe and concentrations in solution show that an excess of OH− species is consumed while GR(), i.e. , is forming. This excess corresponds to an n(OH−)/n(FeIII) ratio of 7 instead of 6. It can be noticed that this amount corresponds also to the formation of the hypothetical mixture [FeOOH + 2 Fe(OH)2], which would contain no sulphate. In fact, once point E1 of the titration curves is reached, Fe(OH)2 does not form but the surface of the initially formed FeOOH solid acts as a hydroxylating ligand towards FeII ions in solution. Therefore, the mechanism of formation of GR() more likely involves a surface reaction between the ferric oxyhydroxide and octahedrally co-ordinated FeII cations. At pH ∼ 5 (point E1), partially hydroxylated ferrous species adsorbs on the FeOOH surface. At pH > 6–7, hydroxylation proceeds further and the formation of brucite like clusters by olation of [Fe(OH)2(OH2)4]0 complexes occurs [10,11]. As presented in Fig. 7, previously adsorbed (or anions coming from the solution) can get into the gap between the brucite like cluster and the FeOOH surface (Fig. 7b). This helps electrons to transfer from the ferrous cluster to the ferric interface through the interlayer. The hydroxylated solid-solution interface of bulk FeOOH can be consider as an FeIII(OH)3 layer and therefore the chemical reaction can be written as follows:
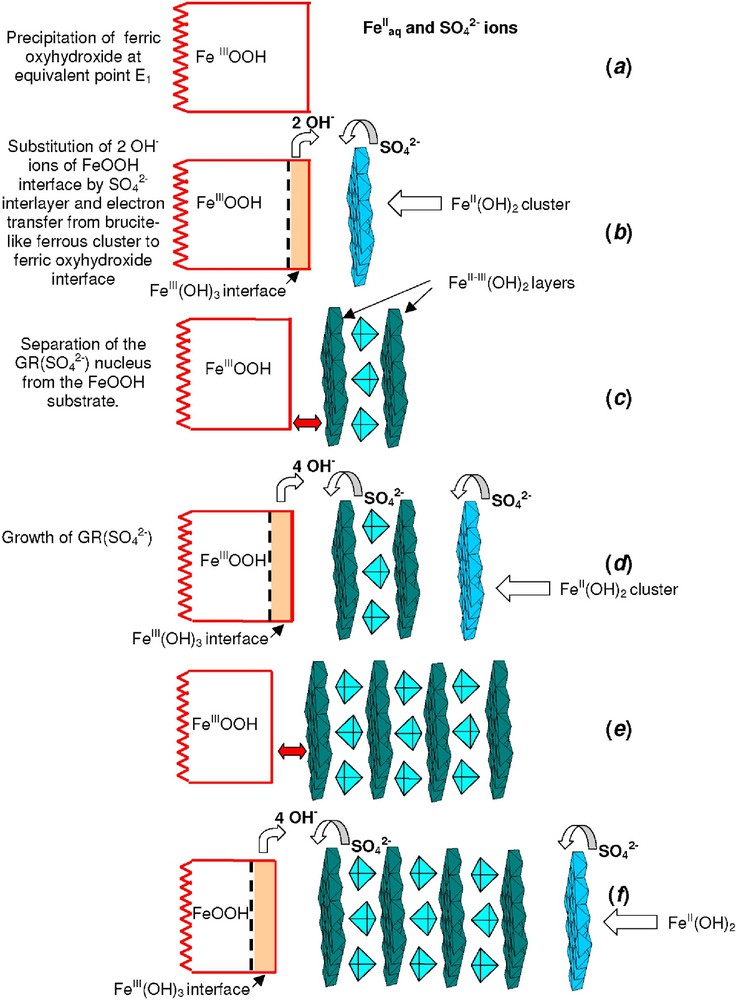
Mode of formation of hydroxysulphate green rust GR(). For the description of the different steps (a, b, c, ...) see the text.
Mode de formation de la rouille verte sulfatée. La description des différentes étapes (a, b, c, ...) sont décrites dans le texte.
The interlayer of anions between two brucite-like FeII–III(OH)2 layers is electrostatically balanced and an excess of OH− ion is released; a FeII–III(OH)2 cluster is now separated from the bulk substrate (Fig. 7c). Lateral growth of the clusters in the [100] direction proceeds much faster than in the [001] direction in order to obtain the flat hexagonal plates observed with TEM (Fig. 12). The process can be repeated if a new FeII(OH)2 cluster forms on the top of this first GR2() nucleus (Fig. 7d). Two interlayers may form simultaneously and the electron transfer is again accomplished from the FeII donor within the brucite-like cluster towards the FeIII acceptor of the oxyhydroxide interface (Fig. 7e). Therefore, it seems inappropriate to qualify the process as dissolution–reprecipitation. The GR2() crystal grows in situ at the expense of the FeOOH substrate by electron transfer.
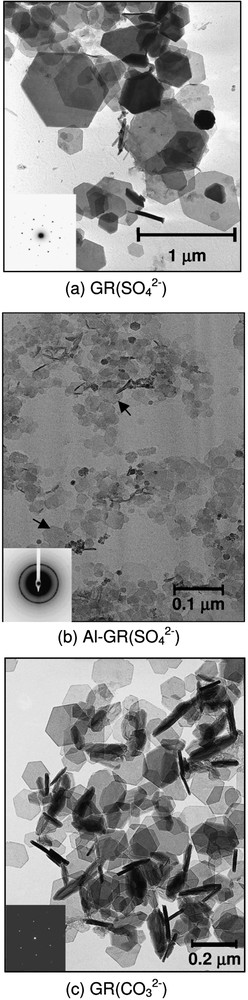
TEM images of hydroxysulphate green rust (a), Al-substituted hydroxysulphate green rust (b) and hydroxycarbonate green rust (c).
Images de microscopie électronique en transmission : (a) rouille verte sulfatée, (b) rouille verte sulfatée substituée aluminium, (c) rouille verte carbonatée.
5 Chemical composition of green rust
5.1 Hydroxysulphate green rust
FeII–FeIII solutions were precipitated by a NaOH solution at a constant ratio , as presented in Table 1. The Mössbauer spectra and hyperfine parameters obtained for three different values of the ferric molar fraction x are presented in Fig. 8 and Table 3, respectively. It appears that GR() precipitates in the quasi-absence of any other compound only when (Fig. 8b). Nevertheless, traces of Fe3O4 and/or FeOOH that correspond to a very low intensity sextet are present. When , the sample is a mixture of two compounds, i.e. {GR(), Fe(OH)2}, and a mixture of three compound when , i.e. {GR(), Fe3O4, FeOOH}. Whatever the sample is, a FeII:FeIII ratio of ∼1:2 is measured by using the relative areas of doublets D1 and D2 of GR(). Therefore, GR() has a well-defined stoichiometry that corresponds to the normalised chemical formula .

Evolution of the Mössbauer spectra as a function of the ferric molar fraction x for samples prepared in a sulphated aqueous medium at a constant hydroxylation level R=2.
Évolution des spectres Mössbauer en fonction de la fraction molaire en ions FeIII pour des échantillons préparés à un taux d'hydroxylation constant R=2.
Hyperfine parameters of the Mössbauer spectra presented in Fig. 8
Paramètres hyperfins des spectres Mössbauer présentés sur la Fig. 8
| Spectra | Compounds | δ | Δ or | H | FWHM | RA (%) | |
| Fig. 8a | GR() | D1 | 1.32 | 2.88 | — | 0.37 | 63.5 |
| D2 | 0.55 | 0.47 | — | 0.37 | 32 | ||
| Fe(OH)2 | 1.3 | −3.1 | 166 | 0.34 | 4.5 | ||
| Fig. 8b | GR() | D1 | 1.35 | 2.88 | — | 0.37 | 60 |
| D2 | 0.49 | 0.5 | — | 0.37 | 35 | ||
| S | 0.69 | 0 | 474 | 0.34 | 5 | ||
| Fig. 8c | GR() | D1 | 1.32 | 2.86 | — | 0.55 | 40 |
| D2 | 0.52 | 0.43 | — | 0.55 | 22 | ||
| S1 | 0.46 | 0 | 529 | 0.63 | 4 | ||
| Fe3O4 | S2 | 0.5 | 0 | 519 | 0.55 | 5 | |
| S3 | 0.79 | −0.59 | 490 | 0.55 | 5 | ||
| FeOOH | S4 | 0.5 | −0.23 | 510 | 0.71 | 24 |
5.2 Hydroxycarbonate green rust
FeII–FeIII solutions were precipitated by a Na2CO3 solution at a constant molar ratio , as presented in Table 1. The samples were filtered and analysed immediately after the synthesis in order to avoid the transformation of GR() in a mixture of magnetite and siderite. On the contrary to what was observed for GR(), typical spectra of GR() that contain three doublets were obtained for both values and (Fig. 9a and b and Table 4). As predicted by using structural models [7], the area ratio between doublets D1:D2:D3 is close to for the sample prepared at . Doublet D3 was attributed to FeII species that has anions in their neighbourhood and a decrease of the relative area of doublet D3 is effectively observed for the green rust sample that contains less carbonate anions, i.e. when (Fig. 8a).When , the Mössbauer spectrum corresponds to a mixture of green rust and magnetite {GR(), Fe3O4} and the FeII:FeIII ratio of GR() is close to 2:1. The relative abundance of Fe3O4 that is given in Table 4, i.e. 23%, is in good agreement with the quantity predicted by using the lever rule in the mass-balance diagram, i.e. 21%. Finally, GR() has a variable chemical composition that corresponds to the normalised chemical formula where .
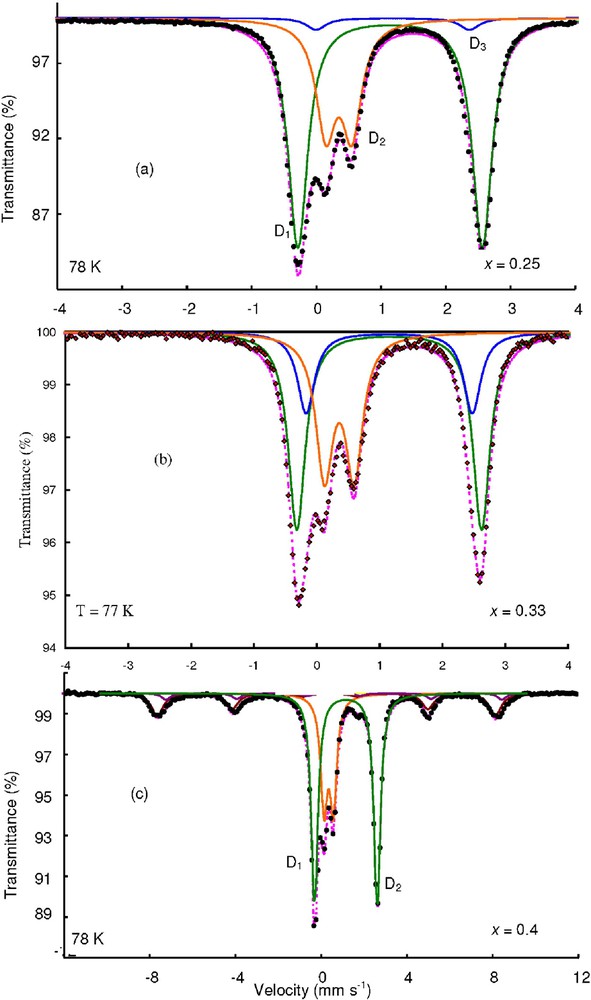
Evolution of the Mössbauer spectra as a function of the ferric molar fraction x for samples prepared in a carbonated aqueous medium.
Évolution des spectres Mössbauer en fonction de la fraction molaire en ions FeIII pour des échantillons préparée en milieu carbonaté.
Hyperfine parameters of the Mössbauer spectra presented in Fig. 9
Paramètres hyperfins des spectres Mössbauer présentés sur la Fig. 9
| Spectra | Compounds | δ | Δ or | H | FWHM | RA (%) | |
| Fig. 9a | GR() | D1 | 1.25 | 2.84 | – | 0.36 | 65 |
| D2 | 0.48 | 0.39 | – | 0.36 | 28 | ||
| D3 | 1.25 | 2.39 | – | 0.36 | 7 | ||
| Fig. 9b | GR() | D1 | 1.28 | 2.87 | – | 0.34 | 48 |
| D2 | 0.47 | 0.43 | – | 0.34 | 34 | ||
| S | 1.28 | 2.55 | 0.34 | 19 | |||
| Fig. 9c | GR() | D1 | 1.3 | 2.9 | – | 0.38 | 52 |
| D2 | 0.5 | 0.47 | – | 0.38 | 25 | ||
| Fe3O4 | S1 | 0.43 | 0 | 490 | 0.63 | 17 | |
| S2 | 0.5 | 0 | 482 | 0.55 | 6 |
5.3 Comparison with other layered double hydroxides
It has been often reported that most of the members of the MII–MIII layered double hydroxides (LDH) family of general chemical composition [13] have an upper limit of composition . This limit is generally attributed to the fact that if , MIII cations become nearest neighbours in the brucite-like layer and this is hardly possible due to electrostatic repulsion. If this last rule is respected for , the cation layer is characterised by an ordered array, where all MIII cations are surrounded by six MII cations. It appears that green rusts obey the same rule than the other LDHs.
6 Synthesis of Al-substituted hydroxysulphate green rust and crystals morphology
Al-Substituted hydroxysulphate green rust {Al-GR()} samples were prepared in excess of FeII, i.e. x(FeII) = 0.8, by adding a NaOH solution at a ratio . The chemical formula of the expected compound is , with . The substitution of FeIII cations by AlIII cations is clearly demonstrated by using XRD and a progressive shift of the lines to higher angle is observed when the amount of AlIII increases (Fig. 10). This corresponds to lower cell parameters for Al-GR(). When , the values and are measured for Al-GR() in comparison with and for GR(). The cell parameters decrease because the ionic radius of AlIII, i.e. r(AlIII) = 0.53 Å, is lower than the ionic radius of FeIII, i.e. r(FeIII) = 0.64 Å. The Mössbauer spectrum of Al-GR() is characterised by the presence of a new ferrous doublet D3 and an increase of the FeII:FeIII ratio equal to 4:1 for the compound (Fig. 11 and Table 5). As shown previously [1], the presence of two ferrous doublets is attributed to the fact that FeII cations have both Fe and Al atoms in their neighbourhood.
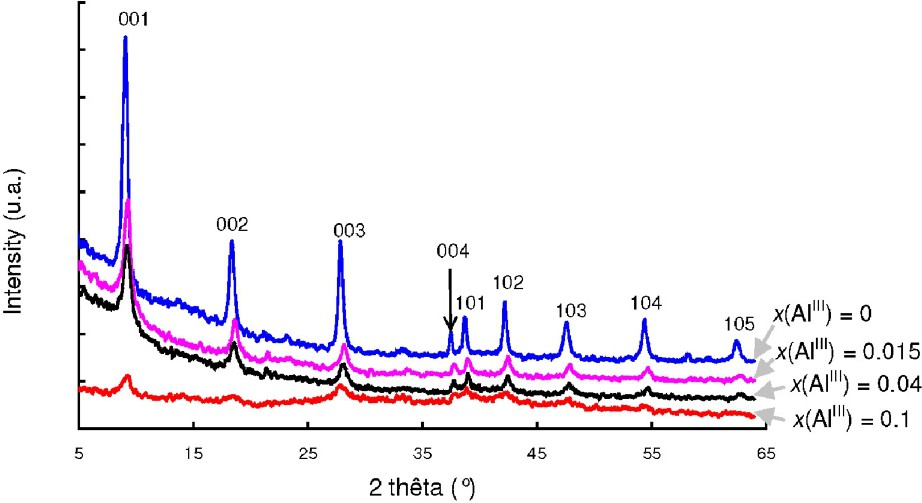
XRD patterns of Al-substituted hydroxysulphate green rust samples that contain increasing amount of aluminium.
Diffractogrammes de rayons X pour des échantillons de rouille verte sulfatée substituée aluminium.

Mössbauer spectra of hydroxysulphate green rust (a) and Al-substituted hydroxysulphate green rust (b).
(a) Spectre Mössbauer de la rouille verte sulfatée, (b) spectre Mössbauer de la rouille verte sulfatée substituée.
Hyperfine parameters of the Mössbauer spectra presented in Fig. 11
Paramètres hyperfins des spectres Mössbauer présentés sur la Fig. 11
| Spectra | Compounds | δ | Δ | FWHM | RA (%) | |
| Fig. 11a | GR() | D1 | 1.27 | 2.80 | 0.35 | 65 |
| D2 | 0.44 | 0.42 | 0.35 | 35 | ||
| Fig. 11b | Al-GR() | D1 | 1.27 | 2.84 | 0.34 | 56 |
| D2 | 0.47 | 0.49 | 0.34 | 20 | ||
| D3 | 1.27 | 2.44 | 0.34 | 24 |
A gradual increase of the width of the XRD lines is also observed in Fig. 10. As expected, the size of the hexagonal crystals of Al-GR(), i.e. ∼50 nm, is much lower than the crystal size of GR(), i.e. ∼500 nm (Fig. 12a and b). In a previous study [5], it has been shown that GR() crystals are much flatter than GR() crystals. Therefore, several GR() crystals that stay roughly perpendicularly to the grid are observed in Fig. 12c.
7 Conclusion
The coprecipitation of FeII and FeIII species was studied by using a mass-balance diagram. A direct visualisation of the nature and the relative quantities of the compounds that form is obtained by following simple geometrical rules. In sulphated aqueous medium, the formation of hydroxysulphate green rust {GR()} is accompanied by the consumption of an excess of OH− species if one considers the chemical formula . A mechanism of formation is proposed, where adsorbed octahedrally coordinated FeII cations and anions layers react at the surface of the ferric oxyhydroxide. GR() crystals grow in situ at the FeOOH/solution interface by electron transfer. Contrary to GR(), GR() has a variable chemical composition, i.e. is , where . The coprecipitation method was also used to synthesise Al-substituted hydroxysulphate green rust {Al-GR()}. Transmission electron microscopy images of green rust shows hexagonal plates and a decrease of crystal size in the order GR() > GR() > Al-GR(). The use of the mass-balance diagram is not limited to the study of precipitation reactions and all the chemical reactions that occur in the redox cycle of iron can be visualised.
Acknowledgements
The authors would like to thank Prof. Jean-Pierre Jolivet (‘Université Pierre-et-Marie-Curie’, Paris, France) for fruitful discussion, Dr Jaafard Ghanbaja (‘Université Henri-Poincaré’, Nancy, France) for performing the TEM experiments and Dr Isabelle Bihannic (Laboratoire ‘Environnement Minéralurgie’, INPL, Nancy, France) for the XRD patterns of Al-substituted green rusts.


