1 Introduction
FeII–FeIII hydroxysalts are commonly called green rusts (GR), as first proposed in 1959 by Bernal et al. [3], because they were green compounds found during the process of corrosion of iron. However, they belong to a larger family of di- and trivalent cation compounds, the layered double hydroxides (LDH), and their originality is that the cations come from the same iron element. Therefore, they possess very reactive redox properties and specific precautions must be taken to study them carefully. The purpose of this article is to present the methods that are used to identify and characterise the iron compounds and more specifically the FeII–III hydroxysalts. Special interest will concern the speciation of iron because redox reactions constitute the major part of the studied phases. Not only the crystallographic structure of the compounds must be characterised, but also the degree of oxidation of iron must be distinguished from a quantitative viewpoint. While X-ray diffraction (XRD) studies help to determine the crystallographic structure, Mössbauer spectroscopy completes the speciation. In the first part, the methods and characteristics of iron compounds will be reviewed, with emphasis on synthetic GRs. Then, specific issues relative to synthetic products will be treated before displaying results obtained with the oxyhydroxycarbonate GR mineral, named recently ‘fougerite’ (IMA 2003-057). Three methods for synthesising GRs in the laboratory will be presented separately in following articles: (i) coprecitation of FeII and FeIII ions in solutions containing specific anions [25], (ii) oxidation of Fe(OH)2 in presence of anions [7,11,17,22], (iii) reduction of ferric oxyhydroxides by dissimilatory iron reducing bacteria [4,18].
2 Speciation of Fe and characterisation of iron compounds
A review of all X-ray diffraction patterns encountered in redox reactions involving iron compounds is not intended. We limit ourselves only to the GRs. In contrast, typical Mössbauer spectra of phases usually encountered during the cycle of iron will be presented, with special emphasis on ferrous hydroxide, GRs, ferric oxyhydroxides and magnetite.
2.1 X-ray diffraction
XRD was first extensively utilised by Bernal et al. [3], who recognised two types of patterns, one called green rust one, GR1, and the other one green rust two, GR2. Within the frame of LDH, their crystal structure is described as brucite-like layers
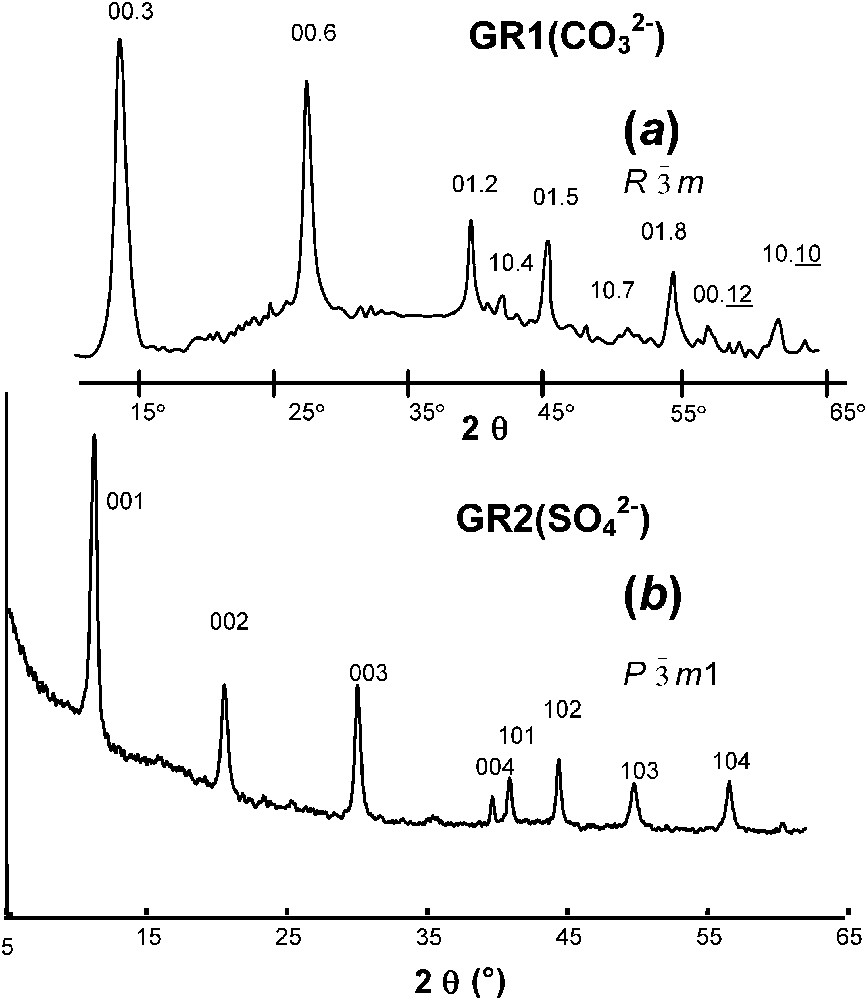
XRD patterns of GR1 versus GR2 (radiation Co K
Clichés de diffraction de rayons X de rouilles vertes 1 et 2 (rayonnement Co K
2.2 Mössbauer spectroscopy
The recoilless emission and absorption of γ-rays in solids was discovered by R. Mössbauer in 1958, who was rewarded by the Nobel Prize. The obtained information concerns the nuclear levels of the probed elements that are the object of the hyperfine interactions between nuclei and surrounding electric charges, electrons and ions as well. It strongly resembles nuclear magnetic resonance (NMR), but is limited to elements where exist adequate radioactive sources, e.g., Fe, Sn, Sb and rare earths. Fe constitutes about 90% of studies where the source is
The centre of gravity of a spectral component determines the isomer shift δ expressed in mm s−1 with respect to a reference, e.g., that of metallic α iron measured at room temperature; it is due to the Coulombic interaction between the valence s electrons and the nucleus considered as a point charge. The distance Δ in mm s−1 between the two peaks within a doublet characterises the quadrupole splitting due to the interaction between the nuclear quadrupole moment and the electric-field gradient at the resonant nucleus, which comes from the shape of the electron shells and the symmetry of the ion distribution within the lattice as well. Finally, the magnetic sextet comes from the coupling between the hyperfine field H, expressed in kOe, and the magnetic moment of the nucleus in the often encountered case where the quadrupole shift ε can be considered only as a perturbation of the Zeeman splitting. Therefore, all spectral components are characterised by
Fig. 2 exhibits Mössbauer spectra measured at 77 K for the principal ferric oxyhydroxides that are encountered in Nature or as corrosion end-products of iron. These are goethite α-FeOOH, akaganeite β-FeOOH, lepidocrocite γ-FeOOH, feroxyhyte δ-FeOOH, and ferrihydrite to which magnetite Fe3O4 is added. They are different for all phases, even though measuring spectra at several temperatures is sometimes necessary to strengthen the identification; for instance, a temperature chosen between those of the magnetic ordering of two phases can distinguish them unambiguously.
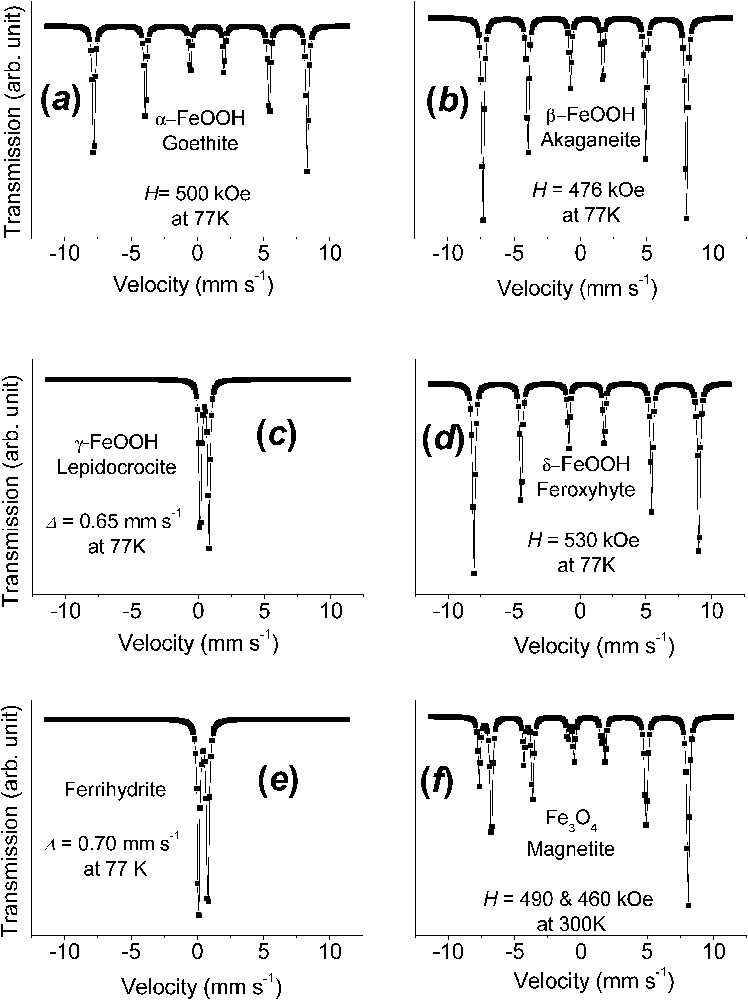
Mössbauer spectra measured at 77 K of the principal ferric oxyhydroxides, the common rusts and minerals, and of magnetite at 300 K: (a) α-FeOOH goethite; (b) β-FeOOH akaganeite; (c) γ-FeOOH lepidocrocite; (d) δ-FeOOH feroxyhyte; (e) ferrihydrite; (f) Fe3O4 magnetite.
Spectres Mössbauer mesurés à 77 K des principaux oxyhydroxydes ferriques, les rouilles ordinaires et les minéraux, et de la magnétite à 300 K : (a) α-FeOOH, la goethite ; (b) β-FeOOH, l'akaganéite ; (c) γ-FeOOH, la lépidocrocite ; (d) δ-FeOOH, la feroxyhyte ; (e) la ferrihydrite ; (f) Fe3O4, la magnétite.
The spectrum of α-FeOOH goethite, which is brownish ochre, is characterised by asymmetric lines of the magnetic sextets due to a field distribution, up to 500 kOe at 77 K, and negative quadrupole shift
A last phase often encountered as a corrosion product when the presence of oxygen is insufficient, but also common in Nature, is black magnetite and its more oxidised form is maghemite γ-Fe2O3. Mössbauer studies of magnetite is a topic by itself, since it has raised many questions. When at stoichiometry Fe3O4, the spectrum is constituted of two sextets of 491 and about 460 kOe at 300 K, respectively [6,28]. Its inverse spinel structure possesses two crystal sites A and B, which are tetrahedral and octahedral, respectively. At room temperature, the larger field that corresponds to the tetrahedral site A has half the intensity of the smaller field of B. This is due to electron hopping in the octahedral site between FeII and FeIII ions, which are in fact in some intermediate Fe2.5+ configuration above the Verwey temperature of 120 K for Fe3O4. Below it, e.g., 82 K, there exist five sextets: one FeIII in A site at 511 kOe, two FeIII in B site at 533 and 516 kOe and two FeII in B site at 473 and 374 kOe [6,28]. It often occurs also that the intensity ratio between A and B sextets is no longer equal to 2 because of vacant FeII ions in the octahedral sites. This ratio allows to determine the departure from the ideal formula Fe3O4 and maghemite γ-Fe2O3 is merely the same phase once all FeII ions have been removed; there exists only one sextet at 490 kOe at 77 K [28].
A typical spectrum of ferrous state is illustrated by that of ferrous hydroxide Fe(OH)2 (Fig. 3) [11,24]. At 78 K, it is a quadrupole doublet with large negative quadrupole splitting Δ of about
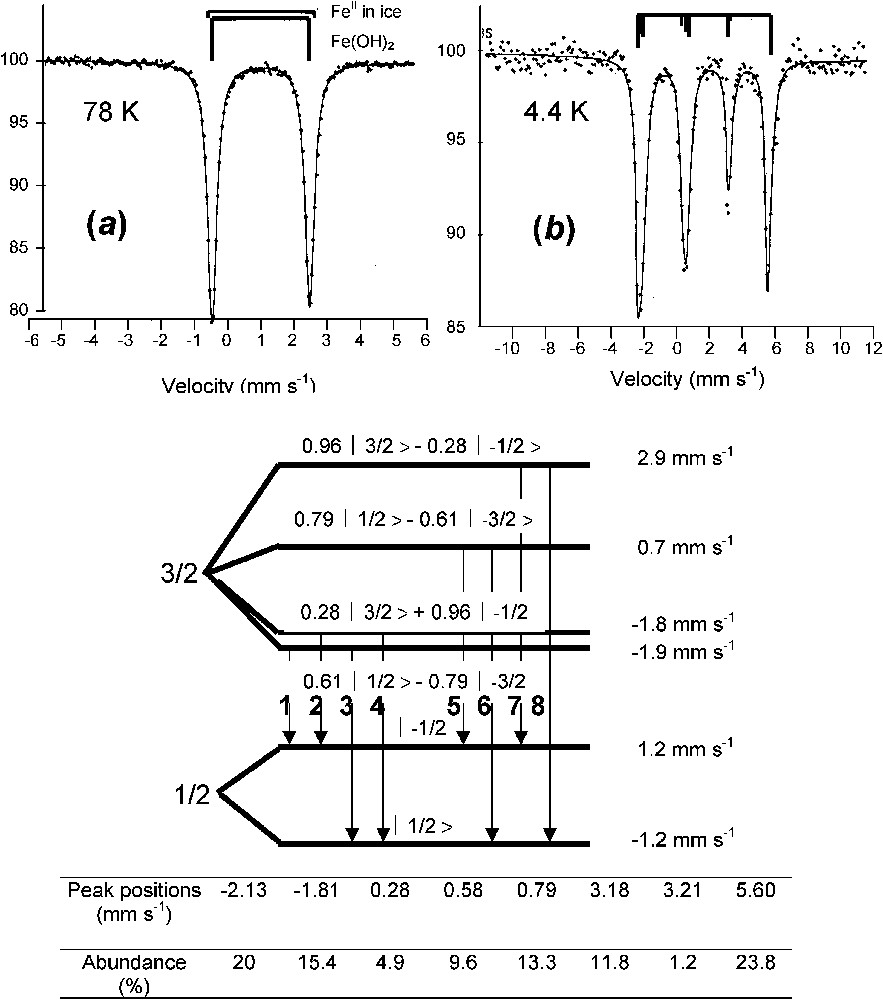
Mössbauer spectra of ferrous hydroxide Fe(OH)2 measured at (a) 78 K and (b) 4 K. The spectrum at 4 K comprises eight absorption peaks, an unusual situation where the quadrupole effect cannot be considered as only a perturbation of the magnetic Zeeman splitting [11,24].
Spectres Mössbauer de l'hydroxyde ferreux Fe(OH)2 mesuré (a) à 78 K et (b) à 4 K. Le spectre à 4 K comprend huit pics d'absorption, car l'effet quadripolaire n'est plus considéré comme perturbation de l'effet Zeeman nucléaire.
Spectra of green rusts GRs measured at 78 K are presented in Fig. 4 [12]. They all have common features (Table 1). One or two quadrupole doublets
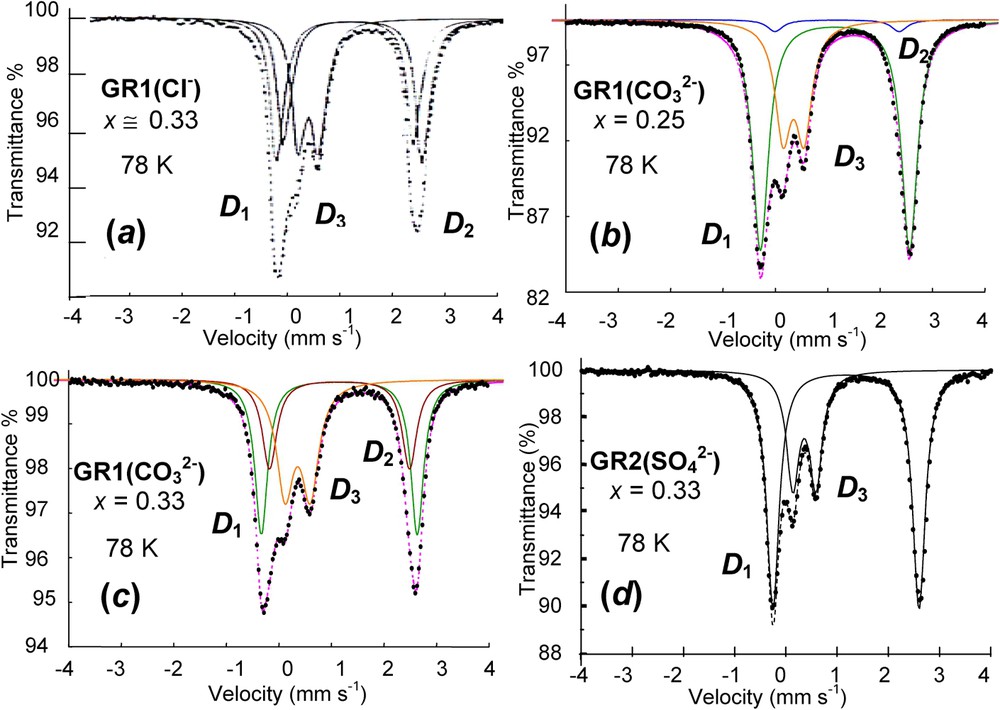
Mössbauer spectra measured at 78 K of four various green rusts: (a) GR1(Cl−) at
Mössbauer spectra measured at 78 K of four various green rusts: (a) GR1(Cl−) at
Spectres Mössbauer mesurés à 78 K de quatre diverses rouilles vertes : (a) GR1(Cl−) à
Spectres Mössbauer mesurés à 78 K de quatre diverses rouilles vertes : (a) GR1(Cl−) à
Hyperfine parameters of Mössbauer spectra of GRs measured at 78 K as illustrated in Fig. 4 and selected from the literature; δ (mm s−1): isomer shift (α-iron as reference at ambient); Δ (mm s−1): quadrupole splitting; RA (%): relative abundance
Paramètres hyperfins des spectres Mössbauer de GRs mesurés à 78 K et illustrés sur la Fig. 4 à partir d'une sélection trouvée dans la littérature ; δ (mm s−1) : déplacement isomérique (référence fer α à l'ambiante) ; Δ (mm s−1) : éclatement quadrupolaire ; RA (%) : abondance relative
| x | GR1(Cl−) | GR1(Cl−) |
|
|
|
||||||||||
| 0.25 | 0.33 | 0.25 | 0.33 | 0.25 | |||||||||||
| Ref. | Not shown | Fig. 4a | Fig. 4b | Fig. 4c | Fig. 4d | ||||||||||
| [11] | [20] | [12] | [4] | [26] | |||||||||||
| δΔ (mm s−1) | RA (%) | δΔ (mm s−1) | RA (%) | δΔ (mm s−1) | RA (%) | δΔ (mm s−1) | RA (%) | δΔ (mm s−1) | RA (%) | ||||||
|
|
1.26 | 2.88 | 48 | 1.27 | 2.89 | 37 | 1.28 | 2.97 | 62 | 1.27 | 2.93 | 51 | 1.27 | 2.88 | 66 |
|
|
1.25 | 2.60 | 24 | 1.25 | 2.60 | 32 | 1.28 | 2.55 | 12 | 1.28 | 2.64 | 15 | |||
|
|
0.47 | 0.41 | 24 | 0.47 | 0.41 | 31 | 0.47 | 0.43 | 26 | 0.47 | 0.42 | 34 | 0.47 | 0.44 | 34 |
| W | 1.37 | 3.36 | 4 |
2.3 Miscellaneous methods
Besides its deep green colour when synthesised and the celadon porcelain shades of fougerite in the field, GRs display thin hexagonal crystals as seen (Fig. 5) by transmission electron microscopy (TEM). Their size depends strongly upon the method of preparation from coprecipitation (
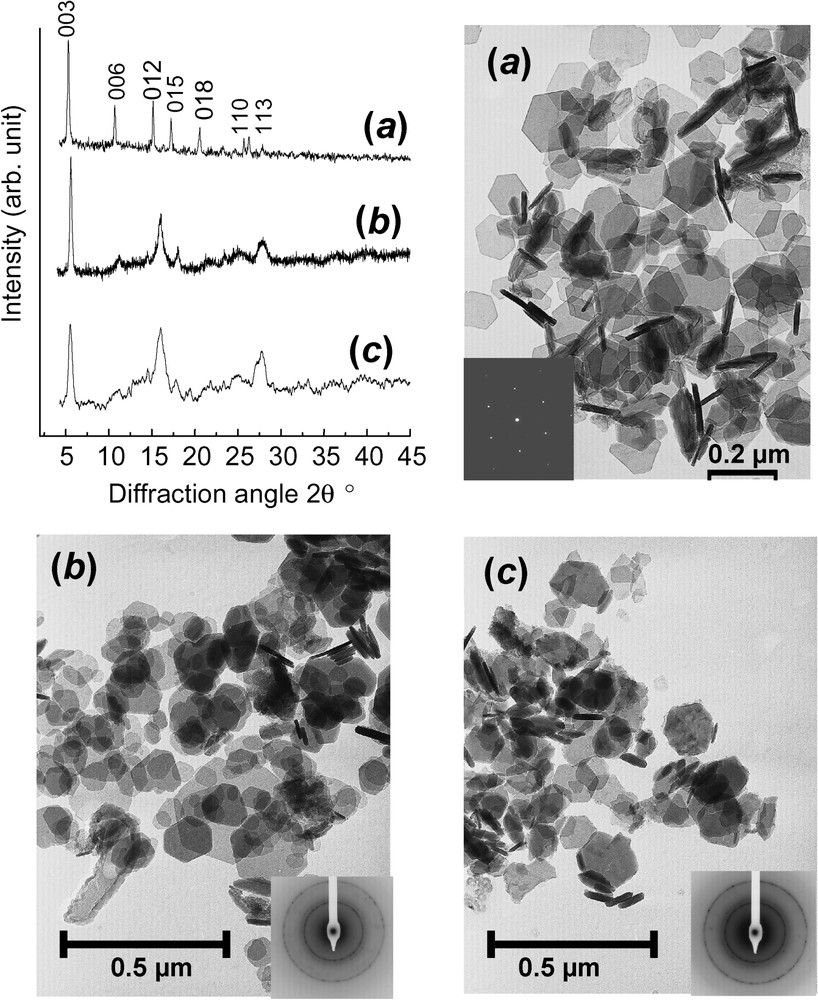
XRD patterns using Mo K
XRD patterns using Mo K
Clichés de diffraction X utilisant le rayonnement Mo K
Clichés de diffraction X utilisant le rayonnement Mo K
3 Structure of FeII–III hydroxysalt green rusts
Models of crystal structures of GRs and fougerite must be consistent with both XRD and Mössbauer spectroscopy. Starting from cation layer stacking determined by XRD in Fe(OH)2 and GRs, cation and anion distributions in layers and interlayers can been derived [12].
3.1 Stacking sequences from XRD (Fig. 6)
GRs are issued from Fe(OH)2 by intercalating anions that are balanced by FeIII inside brucite-like layers while replacing FeII. If
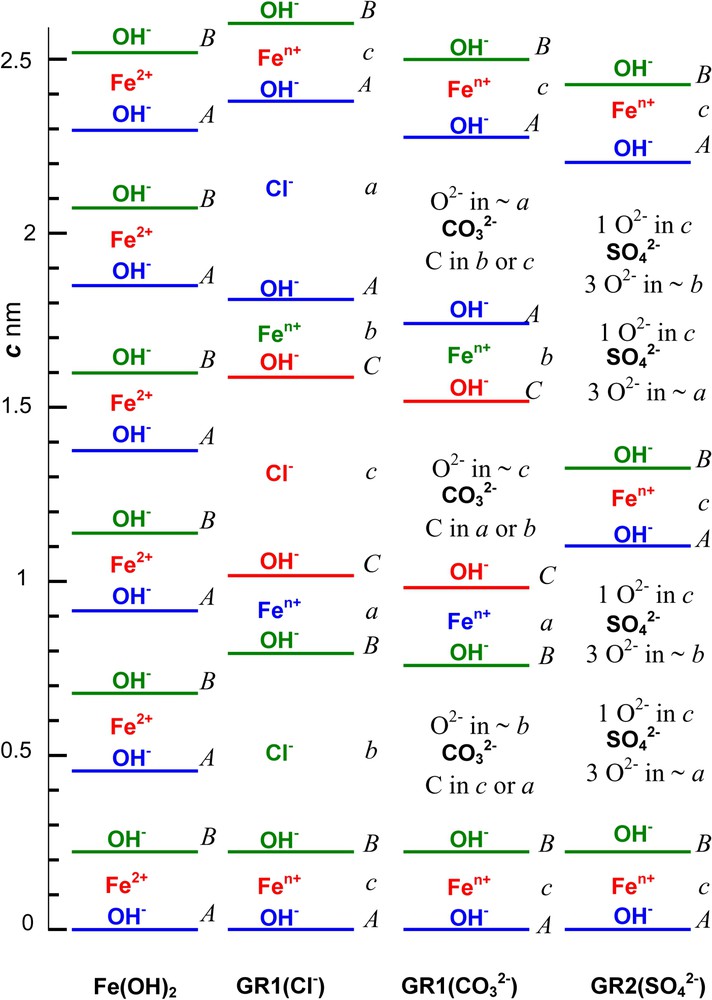
Stacking sequence along the 3-fold axis at scale of Fe cation layers, OH− ion layers and anion interlayers in compounds: (a) Fe(OH)2; (b) GR1(Cl−); (c)
Séquence d'empilement représentée à l'échelle le long de l'axe ternaire des couches cationiques de fer, des couches d'ions OH− et d'intercouches anioniques dans les composés (a) Fe(OH)2 ; (b) GR1(Cl−) ; (c)
Séquence d'empilement représentée à l'échelle le long de l'axe ternaire des couches cationiques de fer, des couches d'ions OH− et d'intercouches anioniques dans les composés (a) Fe(OH)2 ; (b) GR1(Cl−) ; (c)
Within a
3.2 Cation and anion distributions from Mössbauer spectroscopy
The models we propose concern only the GR1s since the Rietveld analysis has completely settled the structure of GR2 [26]. It is consistent with the Mössbauer spectrum, since the order of anions generates only one type of FeIII site (Fig. 4, Table 1). Two GR1 are treated: those of hydroxychloride GR1(Cl−) and hydroxycarbonate
Let us first take the example of GR1(Cl−) for
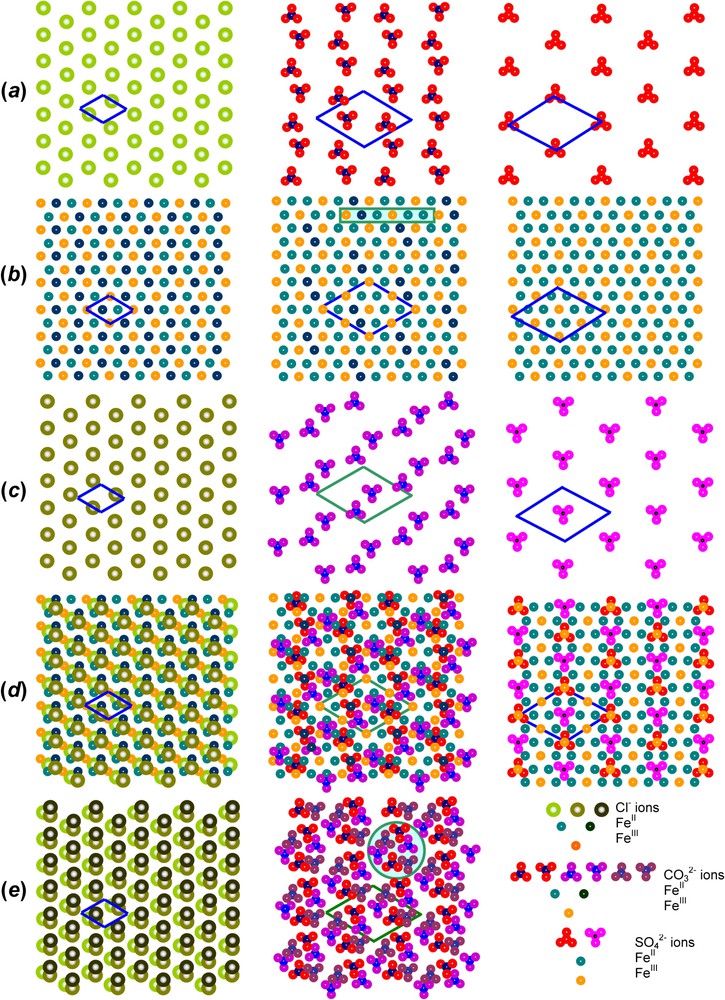
(001) Projections perpendicular to the c axis of the GR structure of, from left to right, GR1(Cl−),
(001) Projections perpendicular to the c axis of the GR structure of, from left to right, GR1(Cl−),
Projections (001) perpendiculaires à l'axe c de la structure GR, de gauche à droite, GR1(Cl−),
Projections (001) perpendiculaires à l'axe c de la structure GR, de gauche à droite, GR1(Cl−),
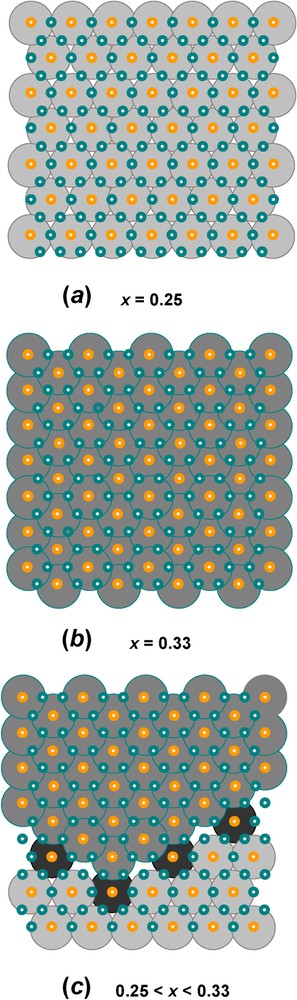
Domains with long-range order of FeIII cations in the hexagonal pavement of brucite-like layers found in FeII–III hydroxysalt green rusts: (a) long-range order at x=0.25 with periodicity
Domaines avec ordre à grande distance des cations FeIII dans le pavage hexagonal des couches de type brucitique que l'on trouve dans les rouilles vertes, hydroxysels ferreux–ferriques : (a) ordre à grande distance pour=0,25 de périodicité
Domaines avec ordre à grande distance des cations FeIII dans le pavage hexagonal des couches de type brucitique que l'on trouve dans les rouilles vertes, hydroxysels ferreux–ferriques : (a) ordre à grande distance pour=0,25 de périodicité Lire la suite
Let us take now for example, the case of GR1(
Finally, if comparing all cases of GR1(Cl−), GR1(
4 Deprotonation of green rusts
Aerial oxidation of GRs within the aqueous solution as observed during the corrosion process of iron has been extensively studied and will be developed later on. However, one most interesting phenomenon is the difference that is observed if the GR sample has been dried before slow aerial oxidation giving
Mössbauer spectra are fitted with Voigt profiles. They demonstrate that all Fe cations are ferric since only quadrupole splitting with low Δ value or magnetic sextets are displayed (ferrous states give rise to octets) (Fig. 9, Table 2). Measurements for
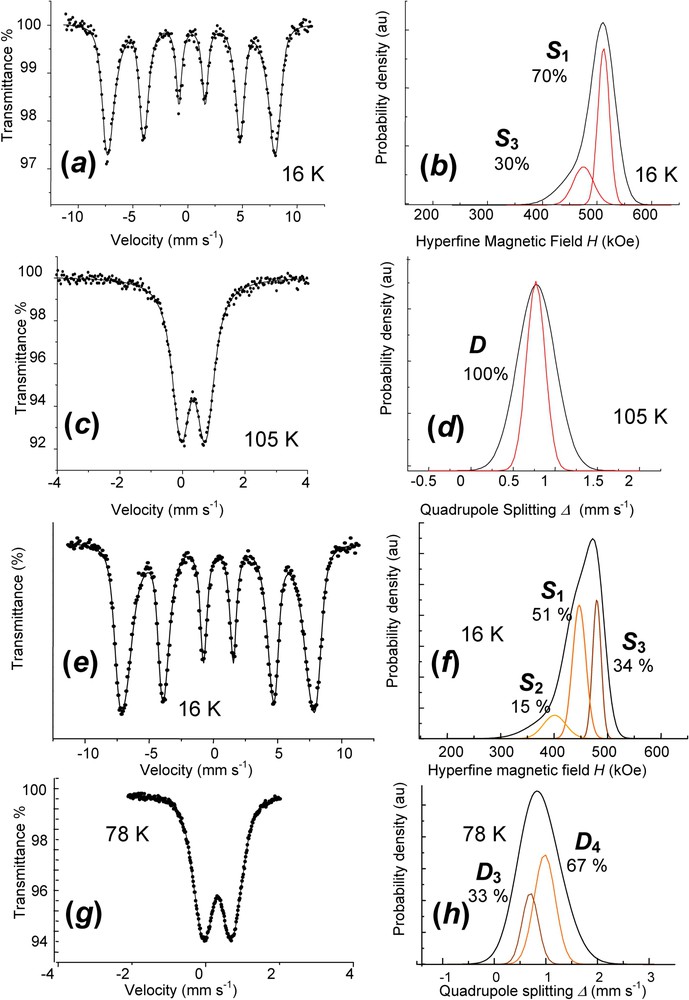
Mössbauer spectra and Voigt profile analysis of fully deprotonated ferric
Mössbauer spectra and Voigt profile analysis of fully deprotonated ferric
Spectres Mössbauer avec leur analyse en utilisant des profils de Voigt pour
Spectres Mössbauer avec leur analyse en utilisant des profils de Voigt pour
Mössbauer parameters for FeII–III hydroxycarbonate green rust and its ferric forms from Voigt profile analysis or classical Lorentzian shape lines (Fig. 9)
Paramètres Mössbauer pour la rouille verte, hydroxycarbonate FeII–III et ses formes ferriques à partir d'analyse de profil de Voigt ou raies classiques de forme lorenztienne (Fig. 9)
| Sample | Temp. | δ (mm s−1) | RA (%) | |||||
|
|
12 K |
|
1.18 | 3.00 | 50 | |||
|
|
1.18 | 2.59 | 17 | |||||
|
|
0.50 | 0.45 | 33 | |||||
|
|
16 K |
|
0.48 | ∼0.00 | 477 | 21 | 70 | |
| dry oxidation |
|
0.48 | ∼0.00 | 440 | 37 | 30 | ||
| 105 K | D | 0.47 | 0.77 | 0.22 | 100 | |||
|
|
16 K |
|
0.48 | ∼0.00 | 447 | 25 | 51 | |
| fast oxidation | 401 | |||||||
|
|
0.48 | ∼0.00 | 42 | 15 | ||||
|
|
0.48 | ∼0.00 | 480 | 16 | 34 | |||
| 77 K |
|
0.47 | 0.88 | 0.41 | 67 | |||
|
|
0.47 | 0.60 | 0.30 | 33 |
Finally, a continuous deprotonation can be observed for the overall range of x between 0.33 and 1. Samples of
Obtained values were 0.33, 0.50, 0.63, 0.78 and 1 and the corresponding spectra measured at 78 K are displayed in Fig. 10 (Table 3). The reference for
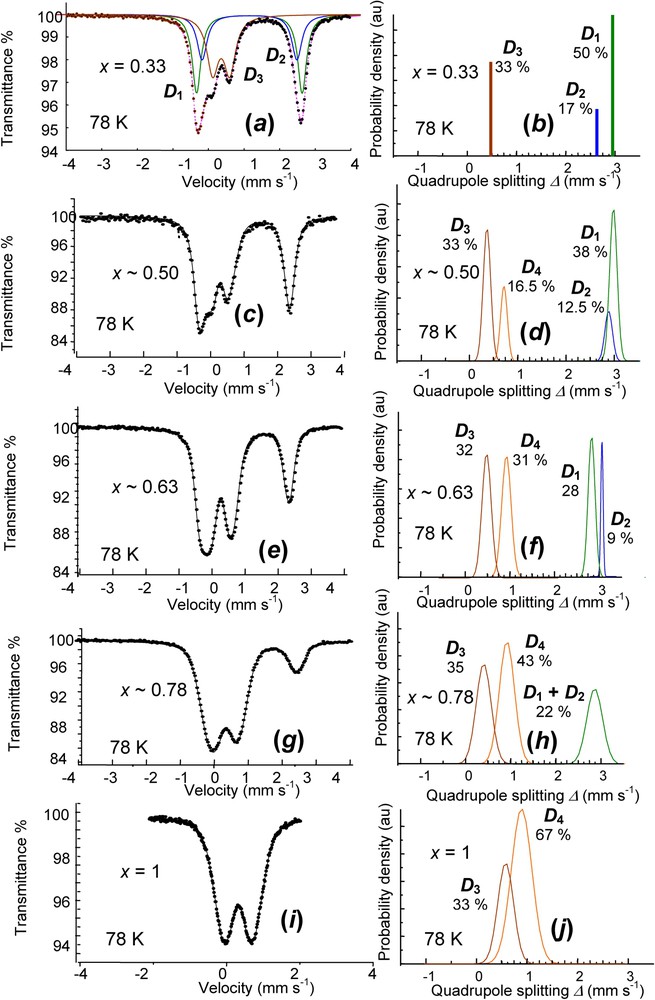
Mössbauer spectra measured at 78 K of a set of partially deprotonated hydroxycarbonate green rust samples using H2O2 for values of
Spectres Mössbauer mesurés à 78 K d'un ensemble d'échantillons de rouille verte hydroxycarbonate partiellement déprotonée en utilisant H2O2 pour des valeurs de
Hyperfine parameters of a set of partially deprotonated hydroxycarbonate green rust samples for values of
Paramètres hyperfins d'un ensemble d'échantillons d'hydroxycarbonate partiellement déprotonés pour des valeurs de
| Quadrupole doublets | δ (mm s−1) | RA (%) | |||
| x=0.33 | |||||
| FeII |
|
1.25 | 2.92 | 0 | 50 |
|
|
1.25 | 2.63 | 0 | 17 | |
| FeIII |
|
0.48 | 0.47 | 0 | 33 |
| x∼0.50 | |||||
| FeII |
|
1.21 | 2.98 | 0.14 | 38 |
|
|
1.21 | 2.72 | 0.16 | 12.5 | |
| FeIII |
|
0.49 | 0.40 | 0.15 | 33 |
|
|
0.49 | 0.70 | 0.28 | 16.5 | |
| x∼0.63 | |||||
| FeII |
|
1.24 | 2.80 | 0.15 | 28 |
|
|
1.24 | 3.05 | 0.05 | 9 | |
| FeIII |
|
0.48 | 0.49 | 0.20 | 32 |
|
|
0.48 | 0.90 | 0.21 | 31 | |
| x∼0.78 | |||||
| FeII |
|
1.21 | 2.89 | 0.31 | 22 |
| FeIII |
|
0.47 | 0.45 | 0.32 | 35 |
|
|
0.47 | 0.95 | 0.34 | 43 | |
| x=1 | |||||
| FeIII |
|
0.47 | 0.60 | 0.30 | 33 |
|
|
0.47 | 0.88 | 0.41 | 67 |
5 Cation substitution in green rusts
Studies were devoted to the substitution in GRs of FeII and FeIII by di- and trivalent cations proving that Mössbauer spectroscopy is the most proficient method. For instance, replacing FeII by NiII allowed us to stabilise GR(Cl−) to measure carefully lattice parameters [21]. An XAS study for understanding the structure of fougerite and the {[FeII]/[FeIII]} variation suggested that peak intensities could vary if some FeII were substituted by MgII ions, even reaching pyroaurite [23] and Mössbauer studies were equally successful by replacing FeII with MgII or FeIII with AlIII [12]. A continuous solid solution exists by substituting FeII by MII divalent cations or FeIII by MIII trivalent cations in GRs. However, the stoichiometry of {[MII]/[MIII]} = 2 is easier to reach with Fe than with all other cations, since diffusion in a solid phase is unnecessary and electron exchange simply does it.
6 FeII–III oxyhydroxycarbonate fougerite
A GR mineral sampled in the Gr horizon of a gley soil was first identified in 1996 in the forest of Fougères (Brittany) by Mössbauer spectroscopy and thus christened ‘fougerite’ (Fig. 11) [13,27]. However, the first Mössbauer spectrum of fougerite ever published was reported in 1985 by the Geological Survey of Denmark and Greenland [8], but it was attributed at that time to the clay minerals with which it was then mixed (Fig. 11c–f) [8,9]. Anyhow, all arguments lead now to the evidence that fougerite is the FeII–III oxyhydroxycarbonate, even though there is no direct identification yet of the inserted anions that lie in the interlayers. Following an old tradition [19], Fe(OH)(2+x) was for some time proposed essentially because of its variation of composition, whereas at that time all synthetic GRs had a well-established stoichiometry [13]. Since then, arguments have been devoted to cope with this challenge [12].

Mössbauer spectra of fougerite mineral compared to that of synthetic sample. (a) Synthetic
Mössbauer spectra of fougerite mineral compared to that of synthetic sample. (a) Synthetic
Spectres Mössbauer de minéral fougérite comparés, à celui d'un échantillon synthétique (a)
Spectres Mössbauer de minéral fougérite comparés, à celui d'un échantillon synthétique (a)
The GR mineral called fougerite is therefore based on the FeII–III hydroxycarbonate
7 Conclusion
XRD is the most reliable method for characterising crystalline compounds. However, amorphous or nanocrystals are hardly detectable and it is often badly quantitative. Therefore, XRD is of no help to detect fougerite in the field, because this mineral is competing with many well-crystallised phases. In contrast, Mössbauer spectroscopy of
All synthetic GRs have a stoichiometry for a ratio



Vous devez vous connecter pour continuer.
S'authentifier