1 Introduction
The French phaseolus bean is an important economic cash and export crop in Egypt and other parts of the world. Medium heavy or light soils are preferred for the growth of this crop. Improvement of phaseolus bean production in the Mediterranean area requires selection of effective rhizobial strains and bean cultivars well adapted to the conditions of the semi-arid climate prevailing in this region [1].
In the Rhizobium-legume symbiosis, the process of N2 fixation is strongly related to the physiological state of the host plant. Therefore, a competitive and persistent rhizobial strain is not expected to express its full capacity for nitrogen fixation if limiting factors (e.g., salinity, unfavorable soil pH, nutrient deficiency, mineral toxicity, temperature extremes, insufficient or excessive soil moisture, inadequate photosynthesis, plant diseases, and grazing) impose limitations on the vigor of the host legume [2]. A given stress may also have more than one effect: e.g., salinity may act as a water stress, which affects the photosynthetic rate, or may affect nodule metabolism directly. The most problematic environments for rhizobia are marginal lands with low rainfall, extreme of temperatures, acidic soils of low nutrient status, and poor water-holding capacity [3].
George and Robert [4] examined six effective Rhizobium leguminosarum bv. phaseoli strains for nodulation competitiveness on bean (Phaseolus vulgaris L.) using all possible two-strain combinations of inoculum. Nodule occupancy was determined with strain-specific fluorescent antibodies. The strains were divided into three groups according to their overall competitive abilities on pole bean cv. Kentucky Wonder and bush bean cv. Bountiful. Strains TAL 182 and TAL 1472 were highly competitive (greater than 70% nodule occupancy); strains KIM-5, Viking 1, and CIAT 899 were moderately competitive (approximately 50% nodule occupancy); and strain CIAT 632 was poorly competitive (less than 5% nodule occupancy). The competitiveness of the six strains was similar on the two host cultivars.
Beattie [5] studied the quantitative comparison of the laboratory and field competitiveness of R. leguminosarum biovar phaseoli strains. They reported that the nodulation competitiveness of KIM5s and CE3 in the laboratory has provided information that helped in better understanding of competitiveness of both strains in the field.
You [6] found that Rhizobium etli strain TAL182 was a competitive strain for effective nodulation of beans.
Several authors applied molecular biology tools for analyzing the common bean rhizobia. Bernal and Graham [7] distinguished three clusters of bean rhizobia using phenotypic analysis and principal-component analysis of Box AIR-PCR banding patterns. They conducted the study on isolates from Mexico, and the northern and southern Andean regions, as well as isolates from southern Ecuador exhibiting significant genetic diversity. They indicated that differences in compatibility of host and Rhizobium could be a factor in the poor nodulation and N2 fixation in this crop. Acosta-Duran and Martinez-Romero [8] using PCR-RFLP patterns and sequences of 16S rRNA genes for fingerprinting of rhizobia from Gliricidia sepium reported the presence of Rhizobium etli and Rhizobium tropici in the nodules of this legume tree. PCR-mediated restriction fragment length polymorphism (RFLP) analysis of the 16S-23S rRNA was used to study the diversity of rhizobial populations isolated from common bean (Phaseolus vulgaris L.) in acid soils [9]. Aguilar [10] characterized the collection of rhizobial isolates from nodules of wild beans P. vulgaris var. aborigineus growing in virgin lands. The characterization was done on the basis of host range, growth, hybridization to a nifH probe, analysis of genes coding for 16S rRNA (16S rDNA), DNA fingerprinting, and plasmid profiles. Nodules in field-collected wild bean plants were largely dominated by rhizobia carrying the 16S rDNA allele of R. etli. Strain-specific and species-specific primers were used for rhizobial strain identification [11].
P. vulgaris is known to be a relatively permissive host, nodulating effectively with many rhizobial species [12]. In Kenya, the dominant types of Phaseolus-nodulating rhizobia differ between acidic and high-pH soils, with R. tropici dominating in the acidic soil [13]. The R. tropici was identified as the most acid-tolerant rhizobium species described to date [14]. It is therefore tempting to assume that R. tropici might generally be better adapted to acidic soils than other species of Phaseolus-nodulating rhizobia.
The aim of this work was to evaluate the performance of two phaseolus rhizobia strains in two soils collected from bean growing areas in Egypt.
2 Material and methods
2.1 Soils
Soils were collected from bean growing fields. Clayey soil from Menofeuia at the Delta of river Nile and Silty loam soil from Ismailia, east of the Nile Delta. These two regions are the main sites for phaseolus bean cultivation in Egypt.
2.2 Bean cultivars
Bean seeds cvs Bronco and Giza 6 were obtained from certified germoplasm collection at the Vegetable Research Institute, Agricultural Research Center (ARC), Ministry of Agriculture, Egypt.
2.3 Strains
Two rhizobial strains were used as inoculants. Strain Ph. 163 is R. etli strain obtained from Laboratory of Microbiology and Molecular Biology, University Mohamed-V–Agdal, Faculty of Sciences, Rabat, Morocco. The other strain CE3 was obtained from the International Institute of Genetics and Biophysics (IIGB), Naples, Italy.
2.4 Planting and inoculation
The experiments were carried out in a green house, at New Borg El-Arab farm, Mubarak City For Scientific Research and Technology Applications, in plastic pots 50 cm in diameter filled with 15 kg of soil. The planting took place in late summer (early September). To prepare rhizobial inoculants, yeast mannitol extract medium was used [15]. Broth cultures in early stationary growth phase were diluted 1 to 4 with one-fifth strength of the respective fresh medium. Diluted broth (110 ml) was injected aseptically into sealed polyethylene bags containing 150 g (wet weight) of sterilized peat [15] and mixed manually.
The treatments were as follows: uninoculated plants, fertilized plants (60 kg N/acre), inoculation with Ph. 163 strain, and CE3 strain each strain separately, as well as a mixed inoculant containing Ph. 163+CE3 strains.
Nitrogen fertilization (+N treatment) was applied as split equal doses at 7, 14 and 28 days after planting.
After 50 days after planting several measurements were carried out to assess nodulation, plant growth and N2 fixation. Nodule number and dry weight were determined as an indication of inoculation response; shoot and Root dry weight as an indication of growth response, and N-uptake as an indication of nitrogen fixation efficiency. The results of the experiment were statistically analyzed using Duncan's multiple-range test [16].
2.5 Determination of nodule occupancy
The nodule occupancy by inoculant rhizobial strains was assessed using FA technique [17] and molecular biological techniques REP-PCR fingerprinting [18].
2.6 Preparation of strain specific Fluorescent Antibody (FAs)
Fluorescent antibodies were prepared by injecting antigens of strains Ph. 163 and CE3 into white rabbits. The antisera were collected and the titer determination was established. The antibodies were conjugation with FTIC. The method of FA preparation was carried out according to [17]. Isolates from 20 nodules randomly selected for each treatment were stained with FAs. Then, the stained isolates were examined under a Zeiss universal microscope equipped for epifluorescence and phase contrast. Incident illumination was from an HBO-50 W/Ac light source, with a fluorescence isothiocynate filter back. A strong positive reaction was indicated by brilliant yellow green fluorescence of the smears on a dark purple background. No cells would be visible (i.e., no fluorescence) if the specific strain was not present on the smear.
2.7 Application of REP-PCR for nodule fingerprinting
REP-PCR fingerprinting patterns from bacterial total genomic DNA were generated using the BOX1 primer. The same isolates from the 20 nodules used typed by FA technique were used for DNA isolation and REP-PCR fingerprinting according to [18].
All REP-PCR patterns of nodules isolates were compared to the patterns generated from standard strains CE3 and Ph. 163 as reference pattern for nodule formation by these strains.
3 Results
The results (Table 1) show that Bronco cultivar gave positive response to inoculation in terms of nodule numbers and dry weight in clayey soil. This response was accompanied by significant increase in shoot dry weight. Strain 163 alone or in mixture with other strain induced more shoot dry weight accumulation and N-uptake than strain CE3.
Response of bean (Bronco cultivar) to rhizobial inoculation in clayey soil
| Treatments | Nodule number | Nodule dry weight (mg/plant) | Shoot dry weight (g/plant) | Root dry weight (g/plant) | N% | N-uptake (mg/plant) |
| Uninoc. | 0 c∗ | 0 c |
|
|
|
27.06 |
| +N∗∗ | 0 c | 0 c |
|
|
|
40.80 |
| Ph. 163 |
|
|
|
|
|
152.0 |
| CE3 |
|
|
|
|
|
90.0 |
| Mix. |
|
|
|
|
|
180.3 |
∗ Values labeled with the same letter are not significantly different using Duncan's multiple-range test.
∗∗ Fertilized with nitrogen.
In silty loam soil (Table 2), Bronco cultivar showed positive nodulation response to the inoculation with CE3. Good nodulation by indigenous rhizobia was observed. The CE3 inoculation gave the highest shoot dry weight accumulation.
Response of bean (Bronco cultivar) to rhizobial inoculation in silty loam soil
| Treatments | Nodule number | Nodule dry weight (mg/plant) | Shoot dry weight (g/plant) | Root dry weight (g/plant) | N% | N-uptake (mg/plant) |
| Uninoc. |
|
|
|
|
|
135.2 |
| +N∗∗ |
|
|
|
|
|
307.0 |
| Ph. 163 |
|
|
|
|
|
109.5 |
| CE3 |
|
|
|
|
|
286.2 |
| Mix. |
|
|
|
|
|
151.2 |
∗ Values labeled with the same letter are not significantly different using Duncan's multiple-range test.
∗∗ Fertilized with nitrogen.
The response of Giza 6 cultivar to rhizobial inoculation in clayey soil is presented in Table 3. The results show a positive response to inoculation in terms of nodule numbers and dry weight. This response was also positive in dry matter and biomass accumulation by the host plant. Inoculant strain CE3 enhanced plant growth and N-uptake better than Ph. 163.
Response of bean (Giza 6 cultivar) to rhizobial inoculation in clayey soil
| Treatments | Nodule number | Nodule dry weight (mg/plant) | Shoot dry weight (g/plant) | Root dry weight (g/plant) | N% | N-uptake (mg/plant) |
| Uninoc. |
|
|
|
|
|
48.9 |
| +N∗∗ |
|
nd |
|
|
|
238.3 |
| Ph. 163 |
|
|
|
|
|
108.7 |
| CE3 |
|
|
|
|
|
205.0 |
| Mix. |
|
|
|
|
|
214.9 |
∗ Values labeled with the same letter are not significantly different using Duncan's multiple-range test.
∗∗ Fertilized with Nitrogen.
In silty loam soil, Giza 6 cultivar showed a positive nodulation response with strain Ph. 163 (Table 4). The highest biomass accumulation in all inoculated treatments was reported with strain Ph. 163. However, it was significantly less than observed with full nitrogen doses treatment. The mixed inoculant strains were not always as good as single strain inoculants.
Response of bean (Giza 6 cultivar) to rhizobial inoculation in Silty loam soil
| Treatments | Nodule number | Nodule dry weight (mg/plant) | Shoot dry weight (g/plant) | Root dry weight (g/plant) | N% | N-uptake (mg/plant) |
| Uninoc. |
|
|
|
|
|
153.9 |
| +N∗∗ |
|
|
|
|
|
315.1 |
| Ph. 163 |
|
|
|
|
|
199.8 |
| CE3 |
|
|
|
|
|
113.4 |
| Mix. |
|
|
|
|
|
95.8 |
∗ Values labeled with the same letter are not significantly different using Duncan's multiple-range test.
∗∗ Fertilized with nitrogen.
Competition for nodulation of the two bean cultivars by inoculant strains and native rhizobia was studied. The nodule occupancy by inoculant strains CE3 and Ph. 163 was assessed using REP-PCR fingerprinting and FA techniques. The REP-PCR fingerprinting was done using BOX1 primer.
The nodule occupancy by inoculant strains in silty loam and in clayey soils is presented in Tables 5 and 6 and Fig. 1. Inoculant strain Ph. 163 occupied 30–40% and 38–50 of nodules of cultivar Bronco in silty loam and clayey soil using REP-PCR and FA techniques respectively. Strain CE3 occupied 50% of nodules on the same cultivar in both soils. The mixed inocula resulted in higher proportions of nodules containing CE3 in silty loam soil and Ph. 163 in clayey soil. The native rhizobia occupied at least 50% of the nodules on this cultivar.
Percentages of nodule occupancy by the inoculant strains on bean cv. Bronco in two soils
| Treatments | Nodule occupancy | |||
| Silty loam | Clay | |||
| 163 | CE3 | 163 | CE3 | |
| Uninoc. | 0 (0) | 0 | 0 | 0 |
| Ph. 163 | 30 (40) | 0 (0) | 38 (50) | 0 (0) |
| CE3 | 0 (0) | 50 (40) | 0 (0) | 50 (50) |
| Mix. | 13 (30) | 40 (10) | 40 (30) | 10 (60) |
Percentages of nodule occupancy by the inoculant strains on bean cv. Giza (6) in two soils
| Treatments | Nodule occupancy | |||
| Silty loam | Clay | |||
| 163 | CE3 | 163 | CE3 | |
| Uninoc. | 0 | 0 | 0 | 0 |
| Ph. 163 | 30 (40) | 0 | 10 (0) | 0 |
| CE3 | 0 | 20 (10) | 0 | 60 (50) |
| Mix. | 20 (24) | 20 (20) | 20 (10) | 20 (50) |
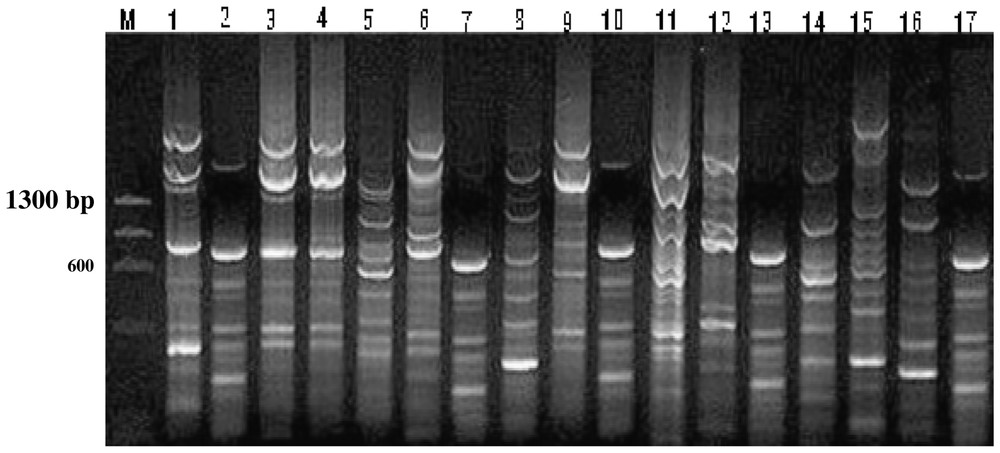
REP-PCR fingerprint patterns for rhizobia reference strains and environmental isolates. Lane M, DNA marker. Lanes 1 & 2, control strains Ph. 163 & CE3, respectively. Lanes 3–17, rhizobia environmental isolates from 1 to 15 respectively. It observes that the REP-PCR fingerprint patterns for isolates 1, 2, 4, and 7 (lanes 3, 4, 6, and 9) are very close each to other and to the rhizobia reference strains Ph. 163, while isolates 5, 8, 11 and 15 (lanes 7, 10, 13, and 17) are similar to strain CE3. However, REP-PCR patterns for isolates 3, 6, 9, 10, 12, 13, and 14 (lanes 5, 8, 11, 12, 14, 15, and 16) did not match similarity for any of the two reference strains. Masquer
REP-PCR fingerprint patterns for rhizobia reference strains and environmental isolates. Lane M, DNA marker. Lanes 1 & 2, control strains Ph. 163 & CE3, respectively. Lanes 3–17, rhizobia environmental isolates from 1 to 15 respectively. It observes that the REP-PCR ... Lire la suite
The native rhizobia were more competitive against the inoculant strains for nodulation of cultivar Giza 6 (Table 6). The inoculant strains 163 and CE3 were able to occupy between 10–30% of the total nodules on this cultivar, with exception of CE3, which occupied 50–60% in clayey soil.
The results reveal that both techniques were powerful in strain/nodule identification. The FA is more reliable in this type of studies as it can be performed on nodules without need to isolate rhizobia for pure DNA isolation. The results of nodule occupancy estimated by two techniques were not identical; however, the general trends of strains' comparative ability were similar.
4 Discussion
Phaseolus bean (P. vulgaris) is one of the major winter crops ranking directly after wheat, clover and bean in Egyptian agricultural. This legume is supposed to have symbiotic relation with specific rhizobia living in the soil. However, phaseolus beans in Egypt mostly fail to form nodules with indigenous rhizobia in the repeatedly cultivated soils or frequently with introduced rhizobia. This failure puzzled scientists, who suggested that it might be due to abiotic factors such as high available nitrogen, low available phosphorus, soil conditions particularly soil salinity or deficiency of micronutrients. Biotic factors limiting nodulation were also not excluded. These factors include competition rhizobial strains and/or predation of rhizobia by protozoa or phages [19]. Several researchers reported that the failure of nodulation and nitrogen fixation in phaseolus beans might be attributed to the possibility of loosing rhizobial plasmids carrying nod and nif genes [20,21]. This study was designed to address the performance of phaseolus bean rhizobia in soils from the major production sites in the Nile Delta. Two promising inocula strains were included in the study to assess their potential nodulation, N2 fixation and competitive ability in both soil types.
Our results show that the Bronco bean cultivar gave positive response to inoculation in both soils used in this study. This response was accompanied by significant increase in shoot dry weight. Strain 163 alone or in mixture with the other strain induced more shoot dry weight accumulation and N-uptake than strain CE3 in clayey soil. On the contrary, CE3 inoculation gave the highest shoot dry weight accumulation in silty loam soil.
Positive response to inoculation was found with Giza 6 cultivar grown in clayey soil. The inoculation induced increase in dry matter and biomass accumulation by plant. Inoculant strain CE3 enhanced plant growth and N-uptake more than strain Ph. 163. Strain CE3 in mixture with another strain Ph. 7 was found to enhance plant growth of two bean cultivars: Paultista and Samamtha [22].
In silty loam soil Giza 6 cultivar gave the highest biomass accumulation when inoculated with strain Ph. 163. However, this accumulation was significantly less than that with the full nitrogen dose treatment. Monibe [23] stated that the single inoculation of bean cultivar Giza 6 with two bean standard rhizobial strains and three local isolates alone was significantly lower than the fertilization with the full recommended dose of N fertilizer (60 kg acre−1). The fertilization with starter dose of nitrogen fertilizer (15 kg acre−1) with inoculation with any of the strains increased the shoot dry weight to the same level as the full-dose N fertilizer application with inoculation.
In this work, the mixed inoculant strains were not always as good as single strain inoculants in terms of biomass accumulation and N2 fixation. Monibe [24] found that single inoculation bean rhizobial strain; 182, performed better in terms of N2 fixation and N accumulation capacities as compared with the mixture of this strain with some other strains as dual inoculation. The value of both plant dry weight and N uptake was significantly higher in single strain than dual strain inocula.
The response to inoculation shows the presence of strain X cultivar X soil interaction (Table 7). This table helped to identify the suitable inocula strain to be used as inocula in each of the two major bean producing soil types. Strain Ph. 163 performs better with cultivar Bronco in clayey soil, whereas strain CE3 is more suitable in silty soil. For the Giza 6 cultivar, strain Ph. 163 is more suitable as inocula for bean in silty loam soil and strain CE3 in clayey soil. The strain X cultivar X soil interaction was always a matter of concern by BNF scientists [22]. To improve bean production, the inoculation strategy must be based on the selection of effective rhizobial strains and bean cultivars combination suitable for the conditions of cultivated areas. This type of information can be generated from the response to inoculation experiments like the one presented in this paper.
The best performing strain with the two cultivars in both soils
| Bean cultivars | Soils | |
| Silty loam | Clay | |
| Bronco | CE3 | 163 |
| Giza 6 | 163 | CE3 |
The proportion of nodules formed on a particular host legume is usually influenced by the competitive ability of the inocula strains and the indigenous rhizobia in the soil [25].
The outcome of the competition of rhizobial strains for nodulation of the host was reported to have a significant effect on either the nodulation success [18] and or N2 fixation efficiency [25].
Until 1968, the lack of reliable techniques to study the competitive relations between rhizobial strains hindered the unraveling the puzzle of success and failure of nodulation by inoculant rhizobia under certain conditions. In 1968 the fluorescent antibody technique was introduced as a powerful technique to study rhizobia in soil. Later on, this technique was employed for the studies of competitive relations between rhizobial strains [26–30]. With the advancement of science, the identification of bacterial strains using molecular biology technique improved to great extent. Among these techniques REP-PCR fingerprinting was suggested for study of rhizobia [31]. These techniques helped the competition studies between rhizobial strains, which were thought for a long time to affect the symbiotic performance of the rhizobia/legume symbiotic system [11,32,33].
In this work we assessed the competition between inoculant and native strains using widely acceptable techniques, namely FA and REP-PCR fingerprinting, to evaluate the response to inoculation by the promising inocula strains.
The results show that inoculant strains Ph. 163 competed for 30–40% of the nodule sites of cultivar Bronco in silty loam soil. Similar trends were found with the same cultivar in both soils where CE3 occupied 50% of the nodules. The native bean nodulating rhizobia occupied at least 50% of nodule on Bronco and up to 60% on Giza 6. The inoculant strains were able to occupy 10–30% of the nodules on the last cultivar, with the exception of CE3, which occupied up to 60% nodules in clayey soil.
The results reveal that both techniques were powerful in strain/nodule identification. The FA is more reliable in this type of studies, as it can be performed on nodules without need to isolate rhizobia in pure culture. However, this study showed that the results from both techniques have a comparative similarity.
The results show the role of the competitive relation between the strains, which limits the nodulation by the introduced inocula strains. This has been reported previously for several legume/rhizobia symbiotic system [27,28]. George and Robert [4] found that the proportion of competing strains in the inoculum influenced nodule occupancy; statistical comparisons between competitiveness indices showed that the relative competitiveness of bean rhizobia strains KIM5s and CE3 remained constant when the two strains were applied in a constant ratio over a range of inoculum concentrations, from 10(3) to 10(7) cells per seed, and when they were applied in various ratios to six P. vulgaris cultivars. Furthermore, the relative competitiveness of KIM5s and CE3 in the laboratory did not differ significantly from their relative competitiveness at the three field sites studied. Thus, a study of the basis for nodulation competitiveness of KIM5s and CE3 in the laboratory has the potential to provide an understanding of competitiveness both in the laboratory and in the field.
Therefore, we suggest to use the studied strains as commercial inocula for phaseolus bean.
Acknowledgements
This study is part of PhIMED project, supported by INCO/EU funds (grant/IC18 CT98 0313). The authors are grateful to project team.


