1 Introduction
Elemental toxicity in plants is a complex problem, the characteristics of which depend on the species, the element concentration, the form and the soil pH and composition. Soil contamination by heavy metals (HM) has become a critical environmental concern due to potential adverse ecological effects. Such toxic elements are considered as soil pollutants due to their widespread occurrence, and their acute and chronic toxicity. Pollution by metals such as zinc is very common. Zn concentrations in the range of 150 to 300 mg kg−1 have been measured in polluted soils [1,2].
Among different methods used to remediate polluted soils, phytoremediation appears as a cost-effective method to address recalcitrant environmental contamination [3–6]. Phytoremediation includes phytoextraction, phytodegradation, phytostabilisation, phytovolatilisation, and rhizofiltration. Phytoextraction is based on the ability of (hyper)accumulating plants to take up metals from soils, to concentrate them in the roots and to translocate them to the aerial parts [7].
Hyperaccumulation may lead to a possible valorization of pollutants. In that case, the plants are incinerated in order to extract the metals from the ashes and re-use them in metallurgy (phytomining). Ideally, the plants used for phytoextraction should be hyperaccumulators and yield a high biomass. The properties of these plants can be optimised by biotechnology [8].
The objective of this work is to produce variants or mutants of crop plants, regenerated from tissue culture and modified for the main factors controlling the uptake, transport, and accumulation of pollutants. The genotypes selected for HM content (hyper- or hypoaccumulators) would also provide a useful tool to achieve a better understanding of the mechanisms involved in this complex phenomenon [9]. They should finally help to select interesting varieties for phytoremediation of polluted fields or for improved food safety [10]. Rapeseed (Brassica napus L.) is known to be able to accumulate substantial amounts of metals; moreover, this plant has a high biomass, various genotypes are easily available and the plant belongs to the Brassicaceae [11], a family in which several hyperaccumulator species exist. The aim of our project was to select hyperaccumulator plants by the technique of transversal Thin Cell Layers (tTCLs) cultivated in vitro in the presence of heavy metals. The tTCLs technique has already been used successfully for the micropropagation of a variety of plants, including recalcitrant ones. Furthermore, this technique induced a fast and intensive neoformation process [12,13].
Although we have not found anything of the sort in the literature, we have assumed that exerting a selective pressure during the neoformation process from tTCLs may help to select plants with exceptional zinc tolerance and/or accumulating capacity. We are also going to study the morphological and physiological features of regenerated plants submitted to zinc excess. Previous studies have indicated that HM (particularly zinc) have effects on chlorophyll [14,15] and proline [16,17] contents in plants. Our present work deals with the effects of different concentrations of ZnSO4 applied during the regeneration process from tTCLs, on the size, biomass, growth, and endogenous Zn content of regenerated plants grown in greenhouse. The contents in chlorophyll, carotenoid, and proline, known as stress markers, will also be evaluated.
2 Material and methods
2.1 Plant material
In this study, rapeseed (Brassica napus var. Drakkar) was selected. This genotype was a pure, genetically fixed line and it was obtained by autofertilization. The seeds were disinfected in 5% (w/v) calcium hypochlorite for 30 min and washed three times with sterile distilled water during 10 min. They were sowed in test tubes on Murashige and Skoog's medium (MS) [18] containing 2% of sucrose and 0.65% agar. The treated seeds were incubated under a photoperiod of 12 h light/12 h dark and a thermoperiod of 22/20 °C (light/dark). Explants of hypocotyl and petiole from 15-day-old seedlings were excised transversally (0.5–1 mm in thickness) and disposed in Petri dishes containing MS medium supplemented with 1-naphthaleneacetic acid (NAA: 0.1 to 0.3 mg l−1), 6-benzylaminopurine (BAP: 1 to 3 mg l−1), sucrose (3%) and agar (0.65%). ZnSO4 (100 to 1000 μM) was dissolved in MES monohydrate 2-(N-morpholino)ethanesulfonic acid buffer (1 mM, pH: 5.8–5.9) and added to the medium. The frequency of shoot regeneration was determined after two weeks.
2.2 Plant regeneration and sampling of experimental specimens
The regenerated shoots were acclimatized under a photoperiod of 16 h light/8 h dark at . The surviving plants were transferred to pots containing a sterile soil and grown in a greenhouse. Each pot was watered twice a week, altering water and a modified Hoagland solution. After three weeks, the regenerants were soaked each day into 2000 μM of ZnSO4 for one week. The plants were finally harvested and subdivided in limbs (L), stems and petioles (S + P) and roots (R). These different parts of plants were thoroughly rinsed with distilled water, oven dried at 80 °C for 72 h and used for ZnSO4 analysis. The chlorophyll and carotenoid contents of the leaves were measured colorimetrically in 80% acetone. The proline content of the different organs fixed with liquid nitrogen was measured after the completion of the experiments. The chemical assays were repeated three times. At harvest, the fresh weight of different organs was measured. In addition, to determine dry weight, the tissues were heated at 70 °C for three days.
2.3 Zinc analysis
After digestion of 200 mg of dried plant material by a mixture of HNO3, HF and H2O2 (4 ml/1 ml/3 ml) until dryness at 100 °C, followed by 3 h at 80 °C in 4 ml HNO3, the tissue Zn content was measured by Electrothermal Atomic Absorption Spectrometry using a SIMAA 6100 Atomic Absorption Spectrometer (PerkinElmer) and the programme recommended by the manufacturer.
2.4 Chlorophyll and carotenoid analyses
The pigments were extracted from leaf discs in 80% acetone. The chlorophyll and carotenoid contents were determined by spectrophotometry according to the procedure described by Lichtenthaler [19] and using the following equations for the determination of the concentration of total chlorophyll and carotenoid in the leaf pigments:
2.5 Determination of proline content
Proline was estimated spectrophotometrically according to the methods described by Bates et al. [20], on the one hand, and Lee and Takahashi [21], on the other hand.
2.6 Statistical analysis
All our experiments were done in triplicate and repeated at least once. The data were compared by variance analysis (ANOVA) and the differences among means (5% level of significance) were tested by Duncan's Multiple Range Test using the software StatGraphics Plus 5.1.
3 Results
3.1 Caulogenesis induction
The best medium for shoot regeneration from different tTCL sources of Brassica napus L. was the MS medium supplemented with 3% sucrose, 3 mg l−1 BAP and 0.3 mg l−1 NAA in the conditions of our study. The size of the tTCL explants of Brassica napus L. was significantly increased after 5–6 days of culture. The growth was greater in the explants isolated from the hypocotyl than in those taken from the petiole. After 8–10 days, a small amount of light-green callus was observed on the subepidermal surface (Fig. 1A). When regeneration from tTCL explants occurred, these explants appeared to be green in colour and forming a crown of buds (Fig. 1B); then shoot buds could be detected after 15–20 days. Bud neoformation was usually associated with an intense rhizogenesis (Fig. 1C). Roots were formed on both regenerating and non-regenerating shoot bud explants. Shoot-regeneration ability was strongly influenced by the donor-organ: the evaluation of the regeneration potential of the plants revealed that tTCLs from hypocotyls had a higher frequency of shoot regeneration than those from petioles in the presence of ZnSO4 (Fig. 2). For tTCLs from both hypocotyl and petiole, shoot regeneration was not significantly affected by ZnSO4 concentrations up to 100 μM. At higher Zn concentrations, the shoot regeneration frequency decreased; however, some plants succeeded to continue to grow at Zn concentrations up to 500 μM (Fig. 2). After being transferred into test tubes, all the buds obtained in the presence of the different concentrations of ZnSO4 exhibited a spontaneous rooting ability and a rapid development (Fig. 3A and B). Just after rooting, the plantlets transferred to pots in greenhouse exhibited a normal development (Fig. 3C).

Shoot neoformation from Brassica napus (var Drakkar) tTCLs cultured on MS + 3 mg l−1 BAP + 0.3 mg l−1 ANA + 30 g l−1 sucrose + 100 μM of ZnSO4. (A) A light-green callus proliferation and the development of young shoot buds occurred on tTCLs originating from petioles after 8 days of culture (bar: 1 mm). (B) A crown of buds and multiple shoots formed on tTCLs originating from hypocotyls after two weeks (bar: 1.25 mm, bd = bud). (C) Multiple shoots formed on tTCLs from hypocotyls and intense rhizogenesis after two weeks of culture on MS + 3 mg l−1 BAP + 0.3 mg l−1 ANA + 30 g l−1 sucrose + 250 μM of ZnSO4 (bar: 10 mm, R = roots).
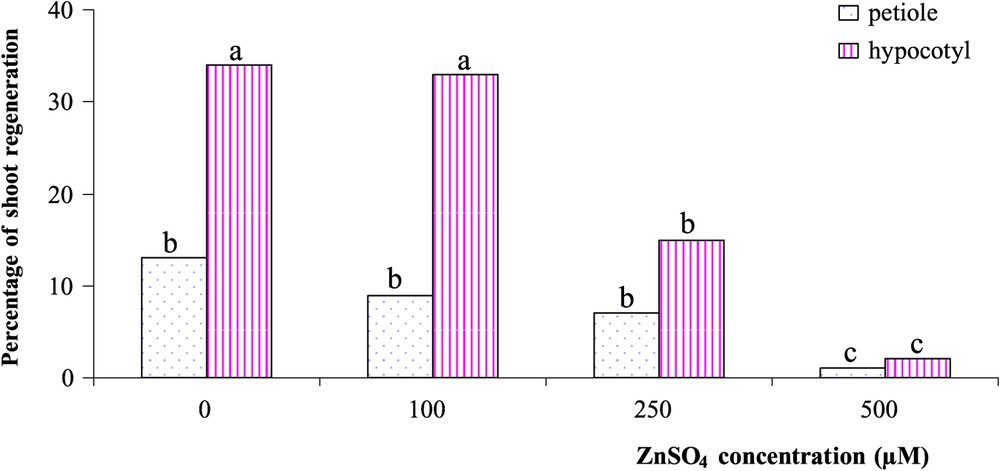
Effect of the concentration of ZnSO4 on the frequency of shoot regeneration with tTCLs from hypocotyl and petioles of Brassica napus (var Drakkar). The data were calculated from three replicated experiments, each with 50 tTCL explants. In each column, the values with different letters (a, b, c) are significantly different from one another according to ANOVA and the Duncan's test at the level of 5%.

Plants regenerated from hypocotyl tTCLs (A) in the presence of ZnSO4 (250 μM) (bar: 26 mm). (B) ZnSO4 (500 μM) transferred into test tubes on MS for development and rooting (bar: 23 mm). (C) Rooted plants regenerated from hypocotyl tTCLs in the presence of ZnSO4 (250 μM) transplanted in pots (bar: 10 cm).
3.2 Morphology and plant growth
The concentration of ZnSO4 applied during the neoformation process had a strong effect on the morphological characteristics of plants cultivated in greenhouse and treated with 2000 μM of ZnSO4. Plants regenerated in the presence of ZnSO4 at 100 and 250 μM exhibited a greater size combined with a great precocity in flowering, compared with those grown in the presence of larger Zn concentrations (Fig. 4).

Effects of ZnSO4 on the morphological characteristics of plants cultivated in greenhouse and treated with 2000 μM of zinc. Bar: 18 cm, C = control (Zn 0) = shoots regenerated in the absence of ZnSO4, Zn 100 = shoots regenerated in the presence of 100 μM of ZnSO4, Zn 250 = shoots regenerated in the presence of 250 μM of ZnSO4, Zn 500 = shoots regenerated in the presence of 500 μM of ZnSO4.
3.3 Zn accumulation
The results are presented in Table 1. The quantity of Zn accumulated in the stems and petioles of plants regenerated in vitro in the presence of 500 μM of ZnSO4 and treated in the greenhouse with 2000 μM of ZnSO4 was 177 μg g−1 DW. This value differed significantly from the Zn distribution in both control (61.6 μg g−1 DW) and the clone regenerated in the presence of 100 μM of ZnSO4 (70.7 μg g−1 DW).
The influence of ZnSO4 on different biochemical and growth parameters of regenerated plants
| ZnSO4 content during the neoformation process (μM) | Plant growth | Zn accumulation (μg g−1 DW) | Pigments limbs (mg g−1 FW) | Proline (mg g−1 DW) | ||||||||||||
| Fresh weight (g) | Dry weight (g) | Chlorophyll | Carotenoid | |||||||||||||
| L | R | L | R | L | R | L | R | |||||||||
| 0 | 48.56a | 44.85c | 15.97a | 8.98a | 6.12c | 2.61a | 109b | 61.6c | 82.4d | 0.558c | 0.27c | 0.83d | 84.5a | 1.44b | 1.59d | 1.0c |
| 100 | 39.17b | 70.74a | 14.26b | 6.04b | 21.18a | 2.225b | 88b | 70.7c | 296.2c | 0.89b | 0.35b | 1.24b | 72.9b | 2.16b | 3.33c | 1.13c |
| 250 | 30.49c | 58.63b | 10.88c | 4.34c | 9.28b | 1.815b | 86b | 108b | 331.4b | 0.83b | 0.33b | 1.16c | 68.4c | 1.69b | 4.12b | 1.71b |
| 500 | 14.99d | 42.67c | 8.76d | 1.30d | 5.83c | 1.26b | 134a | 177.1a | 398.1a | 1.066a | 0.73a | 1.80a | 45.3d | 5.37a | 7.78a | 3.34a |
3.4 Pigment content
Increasing the Zn concentration in the growth medium was responsible for an increase in the total chlorophyll content in the leaves of the regenerated plants (Table 1). By contrast, the carotenoid content of the leaves decreased with increasing zinc concentration during the neoformation process (Table 1).
3.5 Proline accumulation
A significant increase of the tissue content of proline was observed in the different parts of plants regenerated in vitro from tTCLs exposed to different concentrations of Zn during the neoformation process and after them being transferred into pots in the greenhouse (Table 1). Proline accumulation in the test plants varies as a function of Zn concentration.
4 Discussion and conclusion
Plants are known for their ability to resist under various types of stress (hydric deficiency, salt, heavy metals…). For plant physiologists, both the mechanisms of the effects of specific stress factors and the general consequences of the integrated effect of these factors are significant [22]. With regard to plant resistance to metallic stress, it was shown that this stress causes variations in the oxidative metabolism. Our data have shown that the exposure to ZnSO4 during the in vitro neoformation process from tTCLs affected significantly the zinc tolerance of plants. Many parameters were affected: morphology, growth, Zn accumulation, and the contents of total chlorophyll, carotenoid, and proline.
The toxic effect of HMs on plant growth is well known [23,24]. Our present findings have also revealed that the vegetative growth of plants regenerated in the presence of ZnSO4 was increased in the presence of 100 μM Zn, while it was decreased in the presence of higher Zn concentration (500 μM).
Plants regenerated in vitro from tTCLs in the presence of 500 μM ZnSO4 showed a tremendous capability to accumulate Zn in their stems and petioles. Such a Zn accumulation is significantly different from the Zn content and distribution in the non-Zn-treated controls and in the plants regenerated in the presence of 100 μM ZnSO4. This observation may be of particular importance for practical applications to phytoextraction.
The chlorophyll content is often used to assess the impact of environmental stresses in plants. The reason is that changes in pigment contents are linked to visual symptoms of plant illness and photosynthetic productivity [25]. Decreased chlorophyll contents have been observed in various plant species to be due to HM [26]. HMs (especially zinc) often inhibit metabolic processes by inhibiting the action of enzymes. Decreased chlorophyll contents associated with heavy metal stress may be the result of the inhibition of enzymes responsible for chlorophyll biosynthesis [27]. Here we have shown that increasing the Zn concentration in the growth medium tended to increase the content in total chlorophyll of the leaves of regenerated plants. Exposure to zinc during the in vitro neoformation process thus may cause an increase in the total chlorophyll content of plants acclimatized and transferred into the greenhouse. By contrast, the carotenoid content decreased in the leaves of plants regenerated in the presence of 500 μM Zn. As regards the effect of elevated concentration of HM on the synthesis and accumulation of photosynthetic pigments, contradictory data have been reported: some researchers reported decreased pigment content, whereas others found it unchanged or even increased [25,27].
Accumulation of free proline in response to HM exposure seems to be widespread among plants [28]. Proline increases the stress tolerance of plants through mechanisms such as osmoregulation, protection of enzymes against denaturing, and stabilization of protein synthesis [29]. An accumulation of free proline in response to Cu, Cd, and Zn was observed in non-tolerant and metal-tolerant Silene vulgaris (Moench) plants; the constitutive proline concentration in leaves was 5 to 6 times higher in the metal-tolerant than in the non-tolerant ecotype [30]. Our results have shown that a quantitative relationship exists between proline accumulation and zinc stress during the neoformation process, i.e. plants increased their proline content in response to zinc treatment. Proline accumulation in rapeseed regenerated in the presence of different concentration, of zinc is comparable to the response to water deficit [31], salinity [32], high-temperature [33] and low-temperature [34] stresses.
The results of the present work show that all plants regenerated in vitro from tTCLs in the presence of high Zn concentrations (250–500 μM) were able to accumulate significantly more Zn in their stems and petioles than non-Zn-treated controls and plants regenerated in the presence of low Zn concentrations (100 μM). These results are of significance, since this is the first report to our knowledge that plants regenerated in vitro from tTCLs may have an enhanced phytoextraction potential in the field. Moreover, it is noteworthy that Zn accumulation in our case occurred during a relatively short period (2 weeks in vitro and 4 weeks in the greenhouse).
The outcome of the study encourages us to undertake further researches in the use of in vitro-regenerated plants to create innovative lines of hyperaccumulator plants. Their aptitude to accumulate Zn will be compared with that of normal plants cultivated in the presence of different Zn concentrations. The present investigation may also be extended to other new treatments such as mutagenesis and to the study of the effect of various heavy metals on various plant species.
Acknowledgements
We thank the Tunisian National Institute of Agronomic research (INRAT) in Tunis (Tunisia) for kindly providing seeds of Brassica napus var. Drakkar. We are grateful to Dr. Nathalie Poupart (LEBHAM–IUEM–UBO) in Brest (France) for valuable advices.


