1 Introduction
Although variously used (e.g., for nest building, plumage cleaning, ectoparasite control, visual communication [1–3]), the bill apparatus is above all a tool for the collection and preliminary process of food items. It is all the more important, since birds have their forearms transformed into wings and thence cannot use them for their nutrition as most Tetrapods do. Therefore, its study appears to be fundamental to understand the evolution of birds and assess which morphofunctional pathways have been followed according to habitat and diet.
Numerous bird species belonging to various orders and families are specialized in the exploitation of plant resources for food. Food items (e.g., leaves, flowers, buds, sprouts, fruits, seeds) vary in size, shape, thickness, hardness, etc. Avian feeding mechanisms have variously been discussed (e.g., [4] and citations therein). We have already described some aspects of the morphofunctional adaptations of the bill and hyoid apparatus of strict leaf-eating species such as the Hoatzin, Opisthocomidae [5], or strictly frugivorous species such as turacos, Musophagidae that feed on the pulp of the fruit they ingest and spit out or defecate the seeds [6]. Biomechanical evolutionary trajectories were thence proposed [2,7].
Pigeons constitute the order Columbiformes represented today by a single cosmopolitan family (Columbidae), the monophyly of which is regarded as well established [8,9] (even the famous Dodo from Mauritius and Solitaire from Rodrigues fit therein [10]). The various species are usually [9,11] grouped into five subfamilies (Columbinae, Treroninae, Gourinae, Otidiphabinae, and Didunculinae) although things might be more complicated (see the phylogenetic relationships depicted by the recently published trees [10,12]). The species live among a wide range of habitats spanning from the most closed to the most open ones, from lowland to upper montane, and from moist tropical forests to arid areas. Animal preys are only accidentally taken. If many are only arboreal (mainly frugivores), many others are both arboreal and terrestrial, and some species are strictly terrestrial, feeding on small seeds or/and on fruits (in fact consuming their seeds) pecked on the ground [9,11].
Sandgrouse (Pteroclididae) are desert or semi-desert terrestrial birds that feed on the ground, picking up mainly small seeds but also green sprouts and buds (e.g., [13,14]). Their classification has been much debated and is still discussed. Some authors put them within the Columbiformes, others group them with the Charadriiformes and others place them in a separate order [15–18] (for reviews of the various classifications proposed, see [8,19]).
Anatomical data on the bill apparatus have been published by various authors (such as [17,18,20–27]; see also references in [8,19]), but the main objective of these studies was systematic, not morphofunctional. Based on lists of characters, these studies aimed at defining how pigeons and sandgrouse could be classified in relation to each other and to other birds, particularly to Charadriiformes. The recent achievements in the domain of functional morphology and the independent elaboration of molecular phylogenies make necessary new analyses and re-examinations of existing data taking into account the fact that phylogenesis is followed up by successive changes of the adaptations in the course of evolution.
The approach chosen for the present study, as descriptive as it would appear, is based on an interpretation of observed character states in terms of functional units. On the one hand, it opens the way to field observations by eco-ethologists and, on the other hand, provides functional anatomists with basic elements (expressed as potentialities) so that, using sophisticated methods (e.g., high-speed video, radiocinematography), they can measure performances of the anatomical structures.
The present paper deals with the question of the evolutionary trajectory (or trajectories), i.e. the succession of anatomical adaptive steps that allowed pigeons and sandgrouse to (1) colonize so diversified habitats where they kept a vegetarian diet and (2) respond to the pressures imposed by these habitats in terms of nature and availability of food items, knowing that the latter can be fixed (i.e., require to be removed from the plant) or free (i.e. are merely picked up on the ground). Does a key adaptation exist that permitted this radiation? Is it an adaptation to remove fixed food items as well as to pick up free ones? Or are these two food-collecting modes indicative of distinct adaptations?
2 Material and methods
2.1 Specimens examined
We examined 15 species of Columbidae representing the five subfamilies usually recognized (e.g., [9,11]; authors we follow here although recent studies [10,12] make one think that the recognition of subfamilies is not yet cleared if indeed justified):
- • eight Columbinae: Caloenas nicobarica*, Chalcophaps indica*, Columba guinea, Columba oenas, Geopelia striata, Geotrygon chrysia, Oena capensis*, Phaps chalcoptera;
- • one Gourinae: Goura cristata*; 1
- • one Didunculinae: Didunculus strigirostris*;
- • four Treroninae: Alectroenas pulcherrima*, Ducula badia*, D. bicolor, Treron australis*;
- • one Otidiphabinae: Otidiphaps nobilis*.
Among Pteroclididae, we examined Syrrhaptes paradoxus*, Pterocles alchata*, and P. orientalis*.
For all species, we examined the skull except asterisked species (*), for which we examined also the cranial muscles and the hyoid skeleton and musculature.
2.2 Working procedure
We made a biomechanical analysis of the skull and dissected the musculature associated with the functioning of the bill apparatus. Syndesmological preparations were handled to simulate all possible movements and allow better analyses. Specimens were dissected under magnifying binocular glasses Leica WILDM3Z and Zeiss SV11; drawings were done with a drawing tube S.
We follow the nomenclature of muscles and aponeuroses [28] that we used elsewhere [5–7,29]. Muscles and aponeuroses originate on the braincase and insert on the mandible and other moving parts.
The general morphofunctional approach to the bill and hyoid apparatus that we adopt here has already been applied to various groups of birds (cf. [2,30–32]). It rests on well broken-in methods that use anatomically based working drawings to conduct Dzerzhinsky's graphical analysis of the static balance of the forces during gripping and manipulation of items [30], a method elaborated in the continuity of [33,34]. Contrasted with available biological, ecological and behavioural data, results bring precision on the mechanical functions of the jaw and hyoid apparatus considered as a single functional unit, functions that can be interpreted in terms of trophic adaptations and generate new hypotheses testable through direct observation of living animals.
Morpho-functional analyses have been interpreted in the light of behavioural and diet data on the species either resulting from our personal experience or borrowed from literature. Numerous data on Columbidae and Pteroclididae exist in the ornithological literature (e.g., [9,11,14,35–42]).
2.3 Precision on some functional definitions used here
In order to lighten the text and make it easier to read and understand, we clarify here some terms that we use frequently and that are conceptually important, as they refer to very particular and fundamental mechanisms.
2.3.1 Ligaments in the mandibular joint
Most birds possess two ligaments in the mandibular joint: the internal and the external jugomandibular ligaments (which for convenience we often call ‘internal or external ligament of the mandibular joint’). Usually, the internal ligament originates at the caudal extremity of the jugal bar, passes around the mandibular joint and caudal to it inserts on the mandible. This ligament constitutes an additional edge to the mandibular joint, an edge that easily fits the shape of the joint during the mutual movements of the quadrate and mandible. The external ligament links the caudal end of the jugal bar to the lateral surface of the mandible, anterior and ventral to the mandibular joint. This ligament is characteristic of birds that use the joint muscular control of the movement of the upper and lower jaws.
2.3.2 The joint muscular control of the movement of the jaws
This mechanism (which is often called ‘joint muscular control’ in the text) is that when upper and lower jaws clamp an item with the sole force produced by the dorsal adductors. In that case, the ventral adductor (m. pterygoideus) cannot lower independently the upper jaw; it just passes onto it the force from the dorsal adductors. When it contracts, this muscle exerts equal but opposed forces: one on the upper jaw, the other on the mandible. The force applied to the upper jaw is passed on to the quadrate by the palatine and the pterygoid, then by the tension of the external ligament of the mandibular joint to the mandible, where its action ‘cancels’ that of the force directly exerted on the mandible. Thus, when the external ligament is present in the mandibular joint, the forces exerted by m. pterygoideus cancel each other, and the muscle cannot play an independent role in the muscular control of the movement of the jaws, which is then of the joint type.
2.3.3 The separate muscular control of the movement of the jaws
This mechanism (which is often called ‘separate muscular control’ in the text) is that when the motions of the mandible are controlled by the dorsal adductors, whereas those of the upper jaw are controlled by m. pterygoideus, which works irrespective of dorsal adductors. The conditions when m. pterygoideus is a retractor of the upper jaw have been discussed by various authors. We follow here the ideas of Marinelli [43,44] and Dzerzhinsky [30,31]. An indicator of such a muscular control is the absence of the external jugo-mandibular ligament in the mandibular joint. In that case, the forces exerted by m. pterygoideus do not cancel each other and this muscle can thence work in an independent manner in the muscular control of the movement of the jaws, which is then of the separate type. Such a control is also indicated either by the fact that the fibres of m. pterygoideus are directly attached to the base of the cranium or by the possibility that this muscle exerts its force on the base of the cranium through the occipito-mandibular ligament.
2.3.4 The coupled kinesis
This mechanism has been described and well studied [30,45] (see also [2,29]). The presence of the post-orbital ligament is required. When m. depressor mandibulae depresses the mandible, the ligament tightens so that the articular end of the mandible pushes the quadrate forward. The upper jaw then protracts and rises, pivoting at the prokinetic hinge. Thus, the bill is open by the symmetrical and synchronous movement of the jaws. However, it is important to stress that this kinesis occurs only when the ligament is stretched by a medially oriented force. This implies that the quadrate does not swing laterally, otherwise, at the instant of the lateral displacement, the tautness of the ligament would be released, and this could not ensure the joint movement of the jaws. The lateral movement of the quadrate can be controlled by a conscious action of the dorsomedial portion of m. pterygoideus [30,46,47]. However, according to a new analysis, such an action would not be the best solution for that task, because this muscle is one of the adductors and therefore might counteract the depressor. Some contraction (moderate as compared with supposed effects) of protractor muscles (of the quadrate as well as pterygoid bone) would be rather more useful in that case. These muscles originate on the brain case more medially comparatively to their insertion, so they inevitably pull the quadrate in a medial direction.
3 Results
3.1 Anatomical characteristics of the bill of the Columbidae
A detailed study on this subject has been done by one of us [48] (for more information, see also [20,21,25–27]). However, for a comparison between pigeons and sandgrouse, a summary of this work (in Russian) is presented here. To define the ‘model’ Columbidae, we base our descriptions on Treron australis.
3.1.1 Skull
The clinorhynchic skull sensu [49,50] of pigeons shows some peculiarities (Fig. 1). The most striking ones are presented here. The terms clinorhynchy and orthocrany have been introduced by H. Hofer as synonyms of the German words of W. Marinelli [43] – respectively ‘Knickschädel’ and ‘Streckschädel’. In clinorhynchy, the bill is tilted ventrally relatively to the braincase, the horizontal plane of which being indicated by the jugal bars; in orthocrany, the oral cleft is close to that horizontal plane (for illustrations, see [2]).

Particularities of the pigeon skull (Treron australis). A: Lateral view. B: Ventral view of the mandible (shaded: mandibular rami during gaping). C: Ghost position (in black) of the ligament that, if present in the mandibular joint, would limit (1) the movement during the retraction of the upper jaw and (2) the movement of the mandible during gaping. Arrow 1 indicates that during the retraction of the upper jaw the pterygoid pushes laterally the quadrate relatively to the mandible.
The upper jaw is of the schizorhinal type, but the nasal slit goes only through the pliable area of the culmen; it does not extend so deeply backwards as in Charadriiformes; the three pliable areas (culmen and nasal bones) are on the same line. The maxillary process of the nasal and the adjoining area are much inflated. The attachment of the very slender jugal bar to the upper jaw is much more dorsal than that of the palatine. There is no synpalatiny: contralateral palatines are separate. The pterygoid articulates with the base of the cranium by a basipterygoidal joint (except in Goura).
In the quadrato-cranial joint, the medial condyle of the quadrate is much more caudal than the lateral one comparatively to other birds (except in Didunculus, where its position is usual for a bird). The common axis of these condyles is perpendicular to that of the mandibular joint. In the latter, the quadrate has only two condyles instead of three in most birds; the caudal one is missing. The common axis of these condyles continues the longitudinal one of the pterygoid. This latter bone is arched and slides in the basipterygoidal joint, the surface of which is parallel to the axis of the condyles of the quadrate (Fig. 1C). In Didunculus, the medial condyle is relatively high and oriented according to the parasagittal plane, whereas in the quadrato-cranial joint, the common axis of the medial and lateral condyles is in the transverse plane, indicating that the quadrate swings in the parasagittal plane.
The upper jaw is usually hooked (the rhamphotheca is reduced to this hook which is quite expanded in Didunculus). Each ramus of the mandible is made of two parts that angle downward, the caudal one has an oval section and the rostral one is flattened (laterally compressed). One notes that the limit between these two parts is just under the attachment of the jugal bar to the upper jaw.
3.1.2 Cranial musculature
The external mandibular adductor (m. adductor mandibulae externus) has three portions: superficial, medial, and deep (Fig. 2). However, it is important to note that usually in birds, most of the surface of the mandible is covered by the medial portion of the external mandibular adductor, whereas in pigeons (although except in Otidiphaps and Didunculus), the attachment of this medial portion is much more caudal and leaves the place to a very hypertrophied m. pseudotemporalis profundus (Fig. 2B, C). The superficial portion of the external mandibular adductor includes the aponeurosis of insertion as, which is thus pushed caudad by m. pseudotemporalis profundus. The inner edge of as is fused to the aponeurosis of insertion ar of the deep portion of the external mandibular adductor (Fig. 2D). The lateral part of as makes a kind of funnel, inside which insert the fibres of the outer portion originating on the aponeurosis ams. This latter is fused to the aponeurosis of origin am of the medial portion attached to the zygomatic process. In Treron, the deep portion of the external mandibular adductor is particular. Its rostral part is rather typical and originates at the surface of the braincase, where it fills the temporal fossa, quite shallow in pigeons, and insert on the mandible by the aponeurosis ar; the aponeurosis of origin alt overlaps the base of this portion. The part of the deep portion of the external mandibular adductor, which is generally called ‘caudal’, is in reality, at least in Treron, much more medial than usual. The anterior edge of the aponeurosis aci of the insertion of this part is fused to the internal edge of the aponeurosis ar. These aponeuroses have the same orientation, and aci is thus linked to muscular fibres coming from the temporal fossa. Thus, we can say that this ‘caudal’ part repeats the rostral one. This caudal part originates also from the aponeurosis amq, which fuses with am.

Lateral views of the cranial musculature of pigeons (Treron australis). A, B, C: dorsal adductors. D: aponeuroses of the external mandibular adductor.
In pigeons, m. pseudotemporalis superficialis is relatively small but present (contra [18]) with the same orientation, as the rostral part of the deep portion of the external mandibular adductor (Fig. 2A). In many birds, it is usually more vertical.
The hypertrophied m. pseudotemporalis profundus originates at the rostral half of the orbital process of the quadrate and inserts both medially and laterally on the surface of the mandible. It has two well-developed aponeuroses: that of origin app attached at top of the orbital process and that of insertion apm, which covers the inner surface of this muscle and is attached to the inner surface of the mandible.
The posterior mandibular adductor (m. adductor mandibulae posterior) is well developed (Fig. 2C). It originates at the base of the orbital process of the quadrate and extends vertically down to a flat triangular horizontal area on the surface of the mandible, just rostral to the mandibular joint. We failed to find any aponeurosis for this muscle.
M. pterygoideus is rather typical in ventral view (Fig. 3). The aponeurosis alo of the origin of the ventro-lateral portion of this muscle is very thick and extends forward to the visible rostral end of the palatine, i.e., to the point where this bone is attached to the upper jaw. The muscular fibres of this portion insert on the ventral and lateral surface of the posterior part of the mandible; above the ventro-lateral portion are the fibres of the dorso-lateral portion, which originates on the dorsal surface of the palatine and ends on the aponeurosis of insertion ali. The muscular fibres of the ventro-medial portion originate on the dorsal surface of the aponeurosis avo, and also on the surface of the palatine and pterygoid. This portion is inserted at the top of the internal process of the mandible by the aponeurosis avi. One notes that ventral portions are almost parallel to the medial line of cranium. The dorso-medial portion has no aponeurosis. Its muscular fibres link the rostral surface of the base of the internal process of the mandible to the pterygoid. A portion, called caudal, originates on the medial surface of the pterygoid; it is attached near the top of the internal process of the mandible.
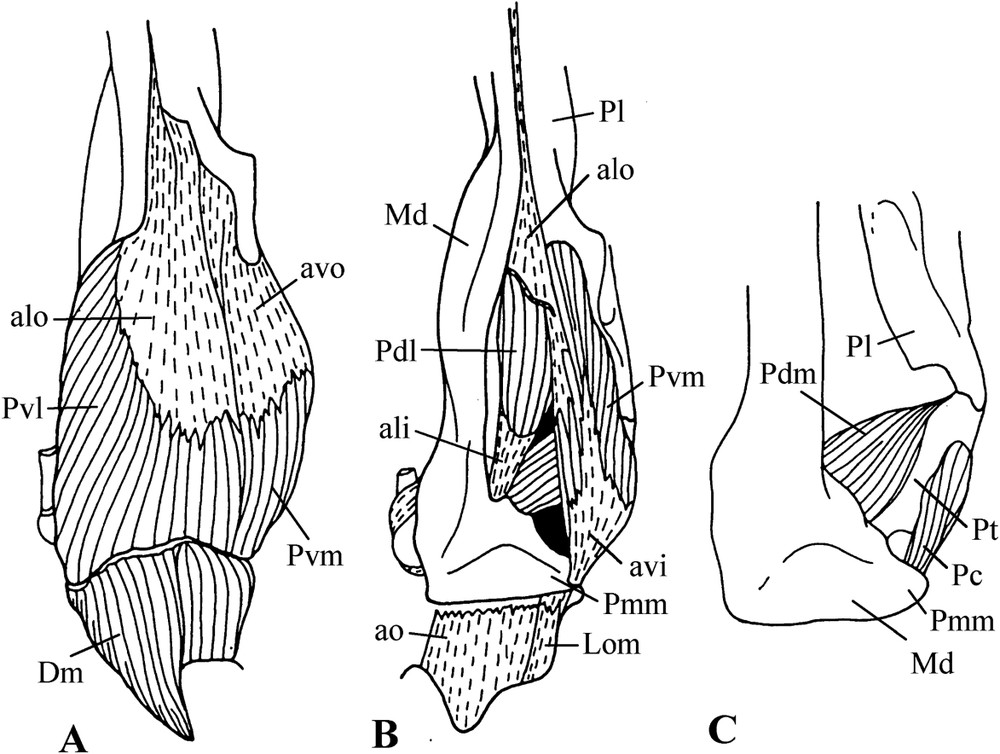
Ventral views of successive planes (A, B, C) of the dissection of pigeon m. pterygoideus (Treron australis).
The protractors of the quadrate and of the pterygoid (mm. protractor pterygoidei et quadrati) are not particularly well developed as for birds in general (Fig. 2A). The depressor of the mandible has two aponeuroses: that of insertion ad is attached to the lower caudal edge of the mandible and that of origin ao, very thick, which extends from the caudal edge of the exoccipital (ala otica) downward almost to the mandible. The inner edge of this latter aponeurosis is fused to the occipito-mandibular ligament.
In Otidiphaps, although well developed, m. pseudotemporalis profundus does not reach the lateral face of the mandible (Fig. 4B). In Didunculus, it is of the usual type, not hypertrophied. On the other hand, in both of these genera, the medial portion of the external adductor muscle is very well developed and bifurcate, passing on both sides of the superficial portion. Moreover, in Didunculus, the origin of this portion extends along the ossified bridge between the post-orbital and zygomatic processes (Fig. 5C). Furthermore, the ventro-lateral portion of the m. pterygoideus is much hypertrophied in Didunculus, inserting on the lateral surface of the mandible as well as on the jugal bar and even on the post-orbital ligament (Fig. 5A). This attachment gives the muscle connection with the postorbital process of the braincase, and, which is important, it allows the muscle to work as an additional adductor. Still in Didunculus, the medial part of m. pterygoideus is well separated from the lateral part and originates from the very thick aponeurosis avo, which rises almost at base of the enormous hook formed by the rostral part of the upper jaw (Fig. 5D). The aponeurosis of insertion of this part of m. pterygoideus is attached to the occipito-mandibular ligament and even immediately to the base of cranium (Fig. 5F).

Cranial musculature of Otidiphaps nobilis. A: Overall superficial view. B: Details (the jugal bar and the post-orbital ligament have been removed).
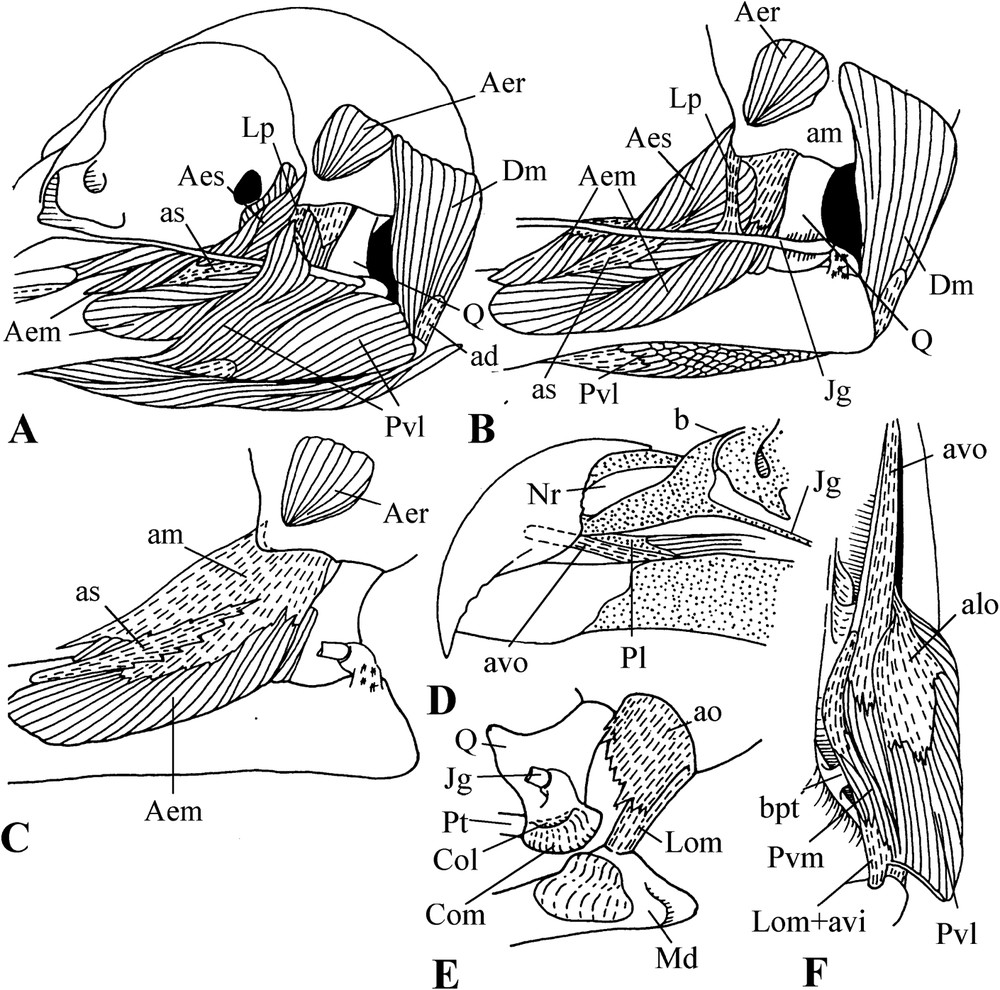
Cranial musculature and details of the skull of Didunculus strigirostris. A: Overall lateral view. B: Exposed lateral view of m. pterygoideus. C: Details of the internal structure of the external mandibular adductor. D: Details of the rostral part of the skull. E: Mandibular joint. F: Ventral view of m. pterygoideus.
3.1.3 Ligaments
All pigeons lack the internal ligament of the mandibular joint; they possess only the external ligament (Fig. 2A). However, the external ligament is also missing in Didunculus and Otidiphaps (apparently, Burton [24] failed to note this) which thus have no ligament in their mandibular joint (Fig. 4A and B). The occipito-mandibular ligament is very thick (Fig. 3B). In the parasagittal plane, it is very slanting and, in the frontal plane, it almost matches the axis of the m. pterygoideus. The post-orbital ligament is present and of a classical type; however, it varies in thickness and orientation (Fig. 2A). Many pigeons show a link between the base of the post-orbital ligament and the zygomatic process (so-called zygomatic ligament). Didunculus differs from the other pigeons by its post-orbital ligament attached to the jugal bar, not to the mandible (Fig. 5B).
3.2 Anatomical characteristics of the bill of the Pteroclididae
In this part, we describe in details the cranial osteo-muscular morphology with a particular focus on the characters important for comparisons among sandgrouse and between them and pigeons. The species taken as a reference is Syrrhaptes paradoxus.
3.2.1 Characteristics of the skull
The skull is clinorhynchic [sensu 49,50]; however, the clinorhynchy is slight: the angle between the palate and the base of the skull is very obtuse (Fig. 6). The bony nostrils extend over more than half the length of the upper jaw. The rhamphotheca is reduced to the distal part, less than half of the bill. Tomia are relatively sharp. When the bill is closed, the edges of the rhamphotheca abut almost without overlap (Fig. 6A(a) and B(b)). This is however not the case with Pterocles alchata: the edges of its rhamphotheca are much more chamfered, giving a wider contact area (Fig. 6C(c)). The bill tip is not hooked; there is just a short maxillary overhang.
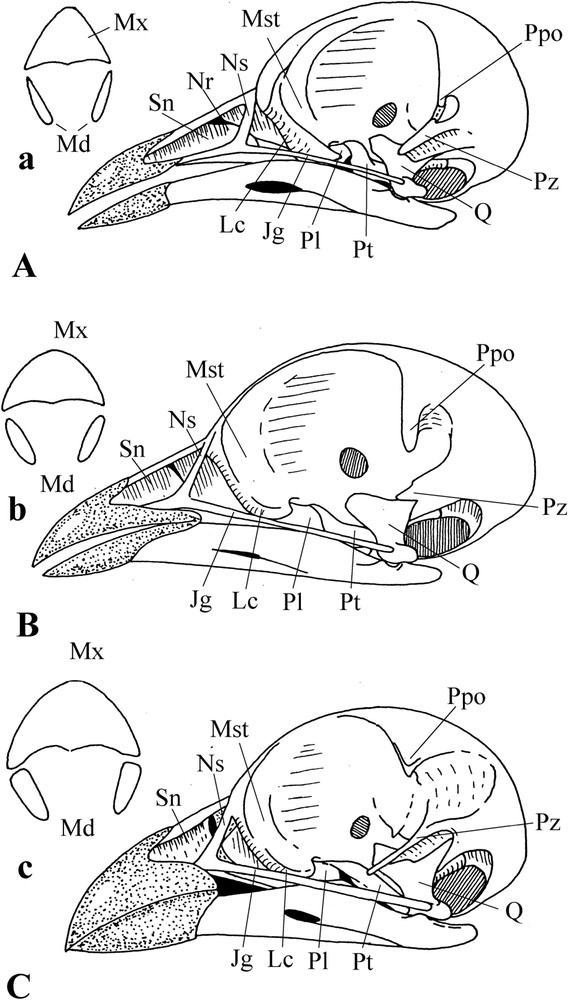
Particularities of the sandgrouse skull. A: Syrrhaptes paradoxus. B: Pterocles orientalis. C: Pterocles alchata. a, b, c: Transversal section of the bill of the corresponding species.
The posterior edge of the naris is rounded. The apparent holorhiny (pliable area of the culmen frontal to the posterior edge of the nostrils, well frontal in Syrrhaptes and P. orientalis, not much in P. alchata) is due to a slit between the nasal septum and the anterior crest of the mesethmoid (Fig. 6). In typical holorhiny, the pliable area is caudal to the posterior edge of the nares. Thus, we deal in Syrrhaptes and P. orientalis with an atypical holorhiny.
Syrrhaptes and P. orientalis show a second pliable area, much more caudal to the first, at the base of the maxillary processes of the nasal bones. In spite of an apparent holorhiny, the upper jaw does not keep monolithic but gets out of shape during movements: in fact, the upper jaw is rhynchokinetic and atypically holorhinal. On the other hand, in P. alchata, the situation is different. The nasal capsule is ossified at the level of the caudal part of the naris and the positions of all the three pliable zones of the culmen and maxillary process of the nasals coincide; thus, although the pliable area is in three parts, the upper jaw works as a monolith. Furthermore, there is a ligament extending from along the maxillary process of the nasal to the upper edge of the lacrimal; this ligament limits the twisting movement of the maxillary process of the nasal when the upper jaw protracts, strengthening this zone (against the passive contraction), and making it, because of its elasticity, out of danger of breaking.
The palate is of the schizognathous type (the contralateral parts of the upper jaw are widely separated). The extremities of the very thin palatine processes of the maxillaries do not join on the medial line of the palate, being well separate. The relatively slender jugal bar is attached to the upper jaw almost at the same level as the palatines (Fig. 6). The latter are relatively long, narrow, and straight. The straight part is at first elongated and almost parallel to the jugal bar and ramus of the mandible, then it bends toward the base of the inter-orbital septum to which it applies by an enlarged extremity (Fig. 8E and F). In Syrrhaptes, no medial wing is present on the palatines. In P. orientalis, there is a kind of medial membranous flange, almost transparent, but m. pterygoideus is not attached to it. On the other hand, in P. alchata, this wing is not only present, but is also ossified and on its dorsal surface are attached the muscular fibres of the dorso-lateral part of m. pterygoideus (Fig. 8D–F).

M. pterygoideus and details of the skull of sandgrouse. A, B, C, E: Syrrhaptes paradoxus. D: Pterocles orientalis. F: Pterocles alchata. In E, 1 = axis of the mandibular joint; 2 = axis of the quadrato-cranial joint; 3 = axis of the pterygoidal joint.
The contralateral palatines are well separated, do not joining above the parasphenoidal rostrum. There is no synpalatiny. Extremities of the pterygoid bones apply on each side of the basisphenoid rostrum (on the ventral border of the interorbital septum). Pterygoids have a particular shape in Syrrhaptes and P. orientalis: their anterior part is laterally swollen and their medial part articulates with cranium base by a relatively elongated basipterygoidal joint (Fig. 8E). In P. alchata, pterygoids are straighter, without enlarged anterior lateral swelling, but with a small medial wing (Fig. 8F).
The contact between the quadrate and the mandible is relatively wide in sandgrouse. The quadrate is quite particular. It has two condyles in the mandibular joint: one lateral and one medial. The latter is relatively high and thrusts into the mandibular groove. Its lateral sliding surface is oriented along the longitudinal axis of the pterygoid and is parallel to the flat surface of the basipterygoidal joint (Fig. 8E). The lateral condyle is flattened and oval; it slides along the flat though slightly concave surface of the mandible. Caudal to the direct contact of this condyle with the mandible, the quadrate makes a kind of specific swollen scroll overhanging the mandible when the bill is closed. In P. orientalis, the mandibular joint is organized as in Syrrhaptes. On the other hand, in P. alchata, although the principle remains the same, the medial condyle is relatively higher and the mandibular groove is deeper, and the surface of the mandible facing the lateral condyle is more clearly concave.
In the quadrato-cranial joint, the quadrate has two condyles: one lateral and one medial, the latter being much more caudal than the former. The common axis of these condyles is almost perpendicular to the groove in which the medial condyle slides, and to the axis of the basipterygoidal joint (Fig. 8E).
The base of the inter-orbital septum is specifically bulky. The wings of the mesethmoid are very thick and laterally extend beyond the jugal bar. The lacrimal bones and the lateral wings of the mesethmoid fuse without clear-cut dividing line (a limit is however visible in P. orientalis). The post-orbital process is curved inward. The zygomatic process is very large and flattened in the parasagittal plane in Syrrhaptes and P. alchata, but is much shorter in P. orientalis (Fig. 6).
The mandibular symphysis takes up all the part covered by the hypotheca. The mandibular rami are slender with flattened anterior part, and progressively increase in height up to a maximum at the level of the mesethmoid. The thickness of their caudal part, where the adductors attach, increases backward. This is the case for all the sandgrouse; however, P. alchata is particular in that this caudal part shows in this species a more important backward decrease in height than in the other ones.
On the caudal end of the mandible, there is a retro-articular process where the depressor of the mandible attaches, and a widening against which rests the lateral condyle of the quadrate. The internal process of the mandible lies against the bony plate of the cranium base that fringes the inferior part of the exoccipital otic wing.
3.2.2 Characteristics of the cranial musculature
When the skin is removed, a large superficial aponeurosis is visible which passes round the eye and covers a great part of the cranial musculature. Its lower part is very thick and links the inferior part of the lacrimal to the zygomatic process. The external adductor (m. adductor mandibulae externus) shows the three classical superficial, medial, and deep portions (Fig. 7).

Dorsal (A–E) and external (F) adductors of sandgrouse. A: Pterocles orientalis. B, C, D: Syrrhaptes paradoxus. E, F: Pterocles alchata (E′: aponeuroses of m. pseudotemporalis superficialis).
It is the insertion of the medial portion which covers most of the lateral surface of the mandible where inserts also the posterior adductor. The muscular fibres of the superficial portion originate at the aponeurosis am of origin of the medial portion. They insert on the aponeurosis as which passes rostrad under the mesethmoid and attaches along the upper edge of the mandible, and caudally fuses with the aponeurosis ar of insertion of the rostral part of the deep portion (in Pterocles, the superficial portion originates also with the aponeurosis ams).
The medial portion of the external mandibular adductor is very well developed. Its aponeurosis of origin am is fastened on by a very wide base along the ventral border of the enormous zygomatic process. This process is as much developed in P. alchata as in Syrrhaptes, whereas in P. orientalis it is much smaller and more in accordance with that usually found in birds. The hypertrophy of the zygomatic process is obviously due to a basal ossification of the aponeuroses am and amq (Figs. 6 and 7).
The anterior edge of am folds and constitutes amq, which belongs to the caudal part of the deep portion of the external mandibular adductor; furthermore, the muscular fibres of this part are also inserted on the aponeurosis ace, between am and amq, as well as on the aponeurosis aci, deeper than amq. The aponeurosis aci is the caudal extension of the aponeurosis ar, which belongs to the rostral part of the deep portion. This rostral part originates on the braincase and extends toward the mandible through the window delineated by the post-orbital process, the residual post-orbital ligament, the zygomatic process, and the surface of the braincase. In P. alchata, this rostral part is well developed and consequently the post-orbital ligament has vanished. The basement of origin of this rostral part of the deep portion of the external mandibular adductor is covered by the aponeurosis alt, usually small but well developed in P. alchata, attesting still here to the great development of this rostral part in that species. In this same part, there is also the aponeurosis of origin amr, which is fastened on along the lower edge of the post-orbital process and shows an anterior part that folds when entering the temporal fossa. In P. alchata, it is also attached to the very short post-orbital process as well as to the bottom of the temporal fossa, whereas in P. orientalis it shows four distinct attachments, the separate fasces reuniting into a single aponeurosis, and moreover there is a link between the most ventral one and its closest dorsal neighbour (alt).
It is important to point out here that in P. alchata the aponeuroses are globally more developed and thicker than in Syrrhaptes and P. orientalis, which testifies that the external mandibular adductor is much greatly enlarged in this species (Fig. 7F).
The origin of m. pseudotemporalis superficialis on the posterior wall of the orbit contains the aponeurosis ai and extends to the mandible, where it inserts by the aponeurosis aps, very close to but more medial than the aponeurosis ar. This muscle is almost the same in Syrrhaptes and P. orientalis, but is much more developed in P. alchata (Fig. 7E).
M. pseudotemporalis profundus is well developed (Fig. 7C and E). It originates by the aponeurosis app on the rostral half of the orbital process of the quadrate and extends far down and forward to insert below the lateral wing of the mesethmoid on the inferior border of the medial surface of the mandible, where is the aponeurosis amp.
The posterior adductor (m. adductor mandibulae posterior) is quite well developed; it originates on the proximal half of the orbital process of the quadrate and attaches to the lateral surface of the mandible, caudal to the medial portion of the external mandibular adductor. The aponeurosis of origin apq is very well developed (Fig. 7D–F).
The protractor of the quadrate (m. protractor quadrati) and that of the pterygoid (m. protractor pterygoidei) are difficult to separate at their origin on the inter-orbital septum, but insert differently. The protractor of the pterygoid inserts on the caudal extremity of the pterygoid, whereas that of the quadrate does it a little more caudally, towards the quadrate. These protractors are united in P. alchata, whereas they are more developed and distinct at their origin in P. orientalis.
The ventral part of m. pterygoideus has two portions: one ventro-lateral and one ventro-medial (Fig. 8). The ventro-lateral portion originates along the straight part of the palatine by the aponeurosis alo and inserts at the base of the internal process and on the lateral surface of the mandible. The ventro-medial portion attaches by the aponeurosis avo to the bent part of the palatine and inserts by aponeurosis avi on the distal part of the internal process of the mandible. In Syrrhaptes there is also an aponeurosis of insertion asi.
The muscular fibres of the dorso-lateral portion of m. pterygoideus originate on the dorsal surface (very narrow in sandgrouse) of the palatine and insert at base of the internal process of the mandible by the aponeurosis ali. The dorso-medial portion is well separated from the dorso-lateral one; its muscular fibres originate by the aponeurosis ado at the level of the swollen part of the pterygoid. This dorso-medial portion inserts on the mandible by the aponeurosis adi.
Sandgrouse have a retractor of the palatine (retractor palatini) parallel to the median axis of the skull, with muscular fibres originating on the widened part of the palatine and inserting at the base of cranium, which makes a pocket there in Syrrhaptes, but not in Pterocles (Fig. 8B and D). In P. alchata, the muscular fibres of the dorso-lateral portion extend forward up to the base of the palatine process of the maxillary, and they attach also to the aponeurosis at the junction of jugal bar and nasal process; furthermore, these fibres cover the dorsal surface of the medial wing of the palatine.
The depressor of the mandible (m. depressor mandibulae) extends from the occipital part of the braincase to the aponeurosis ad at top of the retro-articular process of the mandible (Fig. 7D). The aponeurosis of origin ao is very well developed, attaching along the lateral border of the exoccipital otic wing and fusing with the occipito-mandibular ligament.
3.2.3 Characteristics of the cranial ligaments
We did not find full post-orbital ligament in sandgrouse: in these birds, there is just a reduced ligamentous part, the rest being ossified and attached to both post-orbital and zygomatic processes. There is no trace of this ligament between the zygomatic process and the mandible. In P. orientalis, it is short with a reduced ossified part at the extremity of the post-orbital process, and a ventral part fused with the superficial great aponeurosis (aponeurosis magna). In P. alchata, this ligament is completely missing (Fig. 7A and D).
It is important to mention here that in sandgrouse there is no ligament, either external or internal, in the mandibular joint, a fact apparently unnoticed until now (Fig. 7).
The occipito-mandibular ligament is rather slanting and oriented along the direction of the mandible. Its insertion is more laterally located than its origin in P. orientalis, comparatively to Syrrhaptes and P. alchata (Figs. 7 and 8).
In P. alchata there are two additional ligaments (i.e., that we failed to find in Syrrhaptes and P. orientalis). First, there is a ligament laterally oriented along the maxillary process of the nasal: it attaches on one side to the swollen part of the lacrimo-mesethmoidal area, and on the other side to the maxillary process of the nasal. The second ligament, triangular, fills the corner at the junction jugal bar–nasal (Fig. 6C).
3.3 The particularities of the hyoid apparatus in pigeons and sandgrouse
In this part, we do not describe in details the hyoid apparatus, as Fig. 9 contains the necessary and sufficient information for the purpose of this paper (for pigeons, see [24,26,51]). We highlight the main particularities of this apparatus in pigeons and sandgrouse, and focus on the main differences between or within these two groups.
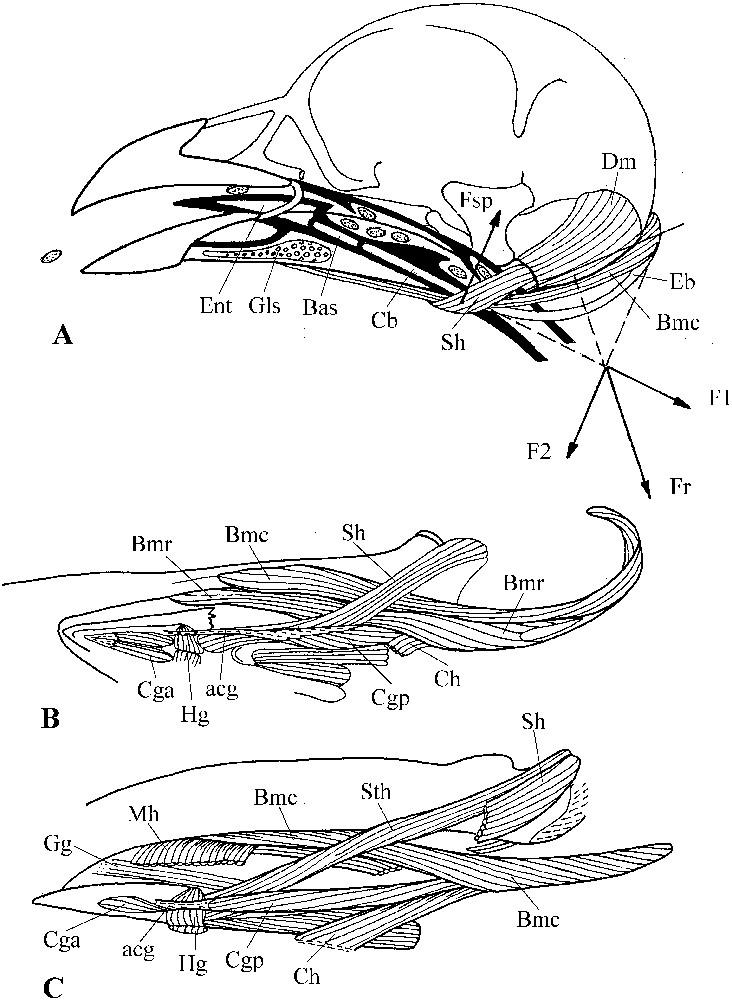
A: The tongue-raising mechanism in sandgrouse (Syrrhaptes paradoxus); bold line = epithelium of the buccal cavity and of the entrance of the oesophagus; small seeds are dotted; Fsp = component of the force exerted by m. serpihyoideus; Fr = reaction force; F1 and F2 = components of Fr; F2 and Fsp constitutes a couple of forces which rotates the hyoid apparatus and lifts the tongue towards the palate. B, C: musculature of the hyoid apparatus in sandgrouse (Pterocles orientalis, B) and pigeons (Oena capensis, C). Masquer
A: The tongue-raising mechanism in sandgrouse (Syrrhaptes paradoxus); bold line = epithelium of the buccal cavity and of the entrance of the oesophagus; small seeds are dotted; Fsp = component of the force exerted by m. serpihyoideus; Fr = reaction force; F1 and F2 = components ... Lire la suite
The tongue is well developed in pigeons and sandgrouse. Its caudal edge is armed with relatively hard notches. Likewise, the area caudal to the glottic slot and the palate are crenellated just above the notches of the tongue; however, Treroninae, Didunculinae and Otidiphabinae have a smooth palate. In sandgrouse, pliable valves at the entrance of the œsophagus are similarly crenellated. In Otidiphaps these valves are present but without fringes; in Treron at that place are found two particular porous, crescentic swellings.
Movements of the tongue are produced by m. ceratoglossus anterior and m. hypoglossus (Fig. 9B). The former can modify the shape of the tongue; its presence shows that this latter has potentialities for the manipulation of items in the buccal cavity.
In pigeons, m. serpihyoideus shows a thin muscular band originating at the base of cranium whereas the main part of the muscle originates on the lateral surface of the caudal extremity of the mandible (Fig. 9C). We did not find this band in sandgrouse.
The most interesting for comparison is m. branchiomandibularis (Fig. 9B and C). In pigeons (except Otidiphaps) it is not divided, whereas in Otidiphaps and sandgrouse there are two portions: one rostral and one caudal. The fibres of the rostral portion originate on the ceratobranchial and epibranchial and form a kind of muscular sheath around each hyoid horn. During the protraction of the hyoid apparatus, the hyoid horns are most probably pushed out of their sheaths. The fibres of the caudal portion originate on the distal part of the epibranchial and extend dorsally along the sheath that surrounds each horn. They attach to the lateral surface of the mandible caudal to the rostral portion, which inserts on the medial surface of the mandible. The main difference between Otidiphaps and sandgrouse is that in the former the hyoid horns are shorter than in the latter and do not apply against the depressor of the mandible.
The other muscles of the hyoid apparatus in pigeons and sandgrouse do not provide any information useful for our purpose.
4 Discussion
4.1 Which evolutionary morphofunctional pathways can be proposed for Columbidae?
4.1.1 The key adaptation of pigeons: removal of attached items or picking up free ones?
Three broad categories of pigeons are admitted [11]: arboreal, terrestrial, and arboreal-terrestrial (which would be the more numerous). As said above, pigeons can be considered as strictly vegetarian and, according to their location in the plant or on the ground, they will have either to remove fixed items (leaves, buds, fruits, etc.) or to pick up items fallen on the ground. The various authors who discussed the adaptation of the bill for removing objects [e.g., 5,7,31,52–54] have shown that the removal of attached items requires particular devices (to ensure the clamping of the item in the jaws; to oppose the protraction of the upper jaw induced by the resistance of the item; etc.), whereas the picking up of free items (for a very detailed study of the pecking behaviour of Columba livia see [26]) can be done by the mere utilization of the general functional properties of the bill apparatus that works under the joint muscular control [30]. In this case, do all pigeons show a single key adaptation, and if so, is it for the removal of attached food items or for the picking up of free ones?
It is noteworthy that pigeons are among the best flying birds. Their adaptation to flight gives them access to a wide range of habitats and thence food sources, and above all allows them to respond easily to the variations in both space and time of these resources. They can have well-separate breeding and feeding sites. This capability for far and rapid movements in order to track food availability allowed them to remain strict vegetarians.
The cere on the bill of all pigeons can be explained as a protection of the olfactory area (i.e., nasal capsule projecting out of the naris), but can also be seen as facilitating the formation of the hook at the tip of the bill during embryogenesis: it would save the matricial layer from a change in orientation [55].
The hook is important to remove items. When this hook is present, the joint muscular control requires a very slanting orientation of the resulting force from the adductors [30]. This condition is also necessary to maintain the upper jaw in a fixed position when the bird removes the item (see [2]), because at removal, the attached item exerts a resisting force that tends to protract the upper jaw and weakens the clamping forces so that the item could slide out of the bill. The adductor giving the more slanting orientation to this force passing by the quadrato-cranial joint is m. pseudotemporalis profundus. This muscle is particularly hypertrophied in pigeons, except in Otidiphaps and Didunculus, which obtain the necessary slanting orientation through the development of the medial portion of the external mandibular adductor. Furthermore, because of the caudal position of the medial condyle of the quadrate, the centre of the quadrato-cranial joint is displaced backwards, which, comparatively to the general case with the medial condyle more medial comparatively to the lateral one, makes still more slanting the orientation of the forces from m. pseudotemporalis profundus. Thus, the bill apparatus of pigeons shows arrangements that allow the most intense force that gives the mechanical advantage to the upper jaw for removing the item. Indeed, even in the species without well-marked hook, m. pseudotemporalis profundus and the quadrato-cranial joint are of the type described above. This means that the key adaptation of pigeons is really the capability to remove attached food items.
We can go farther. First, note that all pigeons lack the internal jugo-mandibular ligament in the mandibular joint. Usually this ligament originates under the external ligament at the caudal extremity of the jugal bar then goes round the quadrate and inserts on the mandible. In Treron, the longitudinal axis of the lateral and medial condyles of the quadrate in the mandibular joint and the basipterygoidal joint are on the same arch and in the same plane (Fig. 1): when the upper jaw retracts, the mandibular and basipterygoidal joints slide on the same arch and the quadrate is laterally pushed by the pterygoid, apart from the mandible. If there were an internal ligament, its tension would limit the retraction of the upper jaw. The absence (probably secondary) of this retraction-limiting element attests to how important are the effects resulting from the retraction of the upper jaw.
Pushing an item between the mandibular rami is among the most obvious of these effects. Small items (less than the distance between mandibular rami) pass without difficulty, moved by inertia by way of jerky head movements. These movements are inadequate for large items (diameter larger than the intermandibular distance). An additional force is supplied by the active retraction of the upper jaw [47,56]. Usually, in strict frugivores, the upper limit of the size of the fruits that can be swallowed as a whole is dependent on the intermandibular distance (gape size, see e.g., [57–59]). Thus, for these birds, the adaptation to the deglutition of whole fruits (the size of which is dependent on that of the bird) appears as the principal factor at the origin of the main aspects of the bill apparatus. This is why usually frugivores have a wide skull, thence a wide gape. Pigeons do not follow the rule, as they are microcephalic. Swallowing a big fruit requires a force coming from the retracting upper jaw and pushing the fruit between the mandibular rami. We noticed above that the jugal bars are attached to the upper jaw more dorsally than the palatines. In Treron and other frugivorous pigeons, there is a swollen area at the base of the upper jaw, at the junction of the maxilla and maxillary process of the nasal. This area constitutes a ‘piston’ that pushes the item between the mandibular rami. Moreover, when the bill is closed, the palatines are more ventral than the jugal bars, which are at the level of the upper border of the mandible. This very low position allows the palatines to force the item down between the mandibular rami, which may move aside if necessary. This passive gaping of the mandibular rami is easily reproduced on syndesmological preparations. Manipulations show that the flatten part of each ramus gets out of shape just below the ‘piston’, and that at this moment the caudal part of the ramus makes a rotation relative to the quadrate (Fig. 1B and C). The structure of the mandibular joint is thus adapted to two motions: retraction of the upper jaw and rotation of the mandible during the gaping.
There is also an active gaping in Ducula, produced by a couple of forces acting upon the caudal extremity of the mandible. One force, oriented forward, comes from m. pterygoideus, which retracts the upper jaw; the other with a backward slanting orientation comes from the post-orbital ligament. The slanting orientation of this latter force is due to the fact that the ligament is bifurcate and, in addition, has a more caudal attachment to the zygomatic process. As in the passive gaping, the location of the condyles in the mandibular joint does not limit the rotation of the mandible. Being in line, thus linking the mandible to the inter-orbital septum, the protractors of the quadrate and pterygoid and m. pseudotemporalis profundus can act so as the mandibular rami actively come near again, and have a part in the preparation of the deglutition of the item.
In Goura, manipulations of syndesmological preparations show that when the mandible is depressed, the post-orbital ligaments are stretched and each mandibular ramus swings around its longitudinal axis, and because of the slanting orientation and pliability of the delineation area of its constitutive parts, gets out of shape and increases the distance to the other ramus. A similar mechanism is known for Caprimulgus [30–60].
In pigeons, the quadrate does not swing around its vertical axis because of the placement of the condyles in the quadrato-cranial joint. When the mandible rotates, the quadrate remains still. If an internal ligament were present in the mandibular joint, it would limit the rotation of the caudal part of the mandible, and thence any passive or active gaping.
It is important to underline here that we did not observe any tongue-raising mechanism similar to the one found in sandgrouse and which indicates a particular adaptation to the collecting of very small seeds (see below). This absence shows that in pigeons, although the tongue can be used in what Zweers [26] called the ‘slide and glue’ mechanism, the pecking of small seeds on the ground by various Columbinae does not depend on a particular adaptation.
Thus all the main features of the pigeons' bill apparatus design can find a coherent and integrated interpretation in terms of a key adaptation to a diet based on the exploitation of attached items of varying although not very small size.
4.1.2 Some distinctive features of Didunculus: biting fruits to take out their seeds?
The head anatomy of Didunculus strigirostris has been described [21,24]. Burton [24] found some resemblance with parrots and concluded that this species would be a relict survivor of a specialized group of pigeons that diverged early from the main stock of Columbiformes and had retained anatomical features from the stock ancestral to parrots. Field studies of this pigeon have been conducted in the Samoa archipelago [61]; they provide very interesting and useful data on its ecology and feeding behaviour. The author says that he observed it high up in trees usually between 15 and 20 m, never below 5 m, contrary to other accounts (e.g., [62]) that mention it on the ground. It is therefore interesting to note that it has been considered [63] that, because of the arrival of Man and the accompanying set of predators, the species would have become rapidly more and more arboreal, and less and less terrestrial.
In Didunculus, to the hypertrophied state (absolute relatively to other pigeons) of m. pterygoideus corresponds the absence of the external ligament of the mandibular joint (a character not mentioned by Burton), which means that, like parrots, Didunculus possesses the separate muscular control (see Section 2). The absence of this ligament and the fact that m. pterygoideus is also attached to the base of cranium allow this muscle to retract the upper jaw in a completely independent way, and to resist efficiently the passive protraction of the upper jaw when an attached item is removed.
Aside this adaptation to remove fixed items, Didunculus appears apt to process these items at the extremity of its bill (see also [24,61]). Beichle [61] describes how Didunculus destroys the pericarp of attached fruits to take out kernels: he says the bird plunges the hook of its upper jaw in the pericarp and moves longitudinally its mandible. However, we failed to find any structure allowing such motions of the mandible, so that they are probably more apparent than real, due to an optical illusion because of the pronounced protraction and retraction movements of the upper jaw. Really both jaws may swing simultaneously up and down, with inevitable mutual longitudinal displacement.
Because of the structure of the quadrato-cranial and mandibular joints (Fig. 5E), the quadrate can swing in the parasagittal plane, not transversal as in other pigeons. The independence of the movements of the upper and lower jaws during the access to the kernels is indicated by the arrangement of the post-orbital ligament, which is attached to the jugal bar in Didunculus and thus does not limit the independence of the movements, being merely suspensory of the jugal bar. This suspension is important as m. pterygoideus is also attached to the jugal bar. Because of this suspension, m. pterygoideus can apply very intense forces to the relatively slender jugal bar, and, because of its very dorsal attachment, can play the very important role of an additional adductor of the mandible.
Various characters show a strengthening of the upper jaw in Didunculus: the rostral part of the bill is monolithic and hooked; the caudal part is quite reduced. The basal part gets a little out of shape during protraction and retraction movements, because the prokinetic hinge and the pliable area of the swollen process of the nasal are on the same line and ensure a holorhinal prokinesis (cf. [64]). The palatine (as in parrots) and the aponeurosis of origin for the dorso-lateral portion of m. pterygoideus are attached to the base of the monolithic part of the bill; so the force is directly exerted on the hook, working part of the bill during the digging of the fruit (Fig. 5D).
A twofold trend is visible in the dorsal adductors: an enhancement of the power and a more vertical orientation of the external adductor. The former is expressed by the very well developed medial and superficial portions; the latter is due to the fact that the attachment of the aponeurosis of origin for the medial portion extends from the zygomatic to the post-orbital processes.
All this shows that Didunculus tends to enter the feeding niche of parrots (see [24,65,66]). It is particular among pigeons in that it is not only able to collect fruits but also to bite, crush or dig them and tear off the pulp either to eat it or to extract seeds, with the capability to process food items at the extremity of its bill.
4.1.3 Otidiphaps: a peculiar terrestrial pigeon?
It has the characters of a generalist bird that collects items on the ground but nevertheless shows specific specialized characters.
With its moderately developed m. pseudotemporalis profundus and absence of external ligament in the mandibular joint, which indicates a separate muscular control of jaw movements, Otidiphaps is on the same evolutionary morphofunctional pathway as Didunculus. Both species share the characteristic trend of a developed and bifurcate medial portion of the external mandibular adductor, passing on both sides of the superficial portion (Figs. 4A and B, and 5A and B). In Didunculus, the medial portion is more vertical, whereas it has a more slanting orientation in Otidiphaps. If this is natural for Didunculus, it is rather surprising for Otidiphaps because the muscular control, here of the separate type, requires a more vertical force. In Otidiphaps, the eye is relatively enlarged. Were the factors responsible for this development linked to life in dark forest understory? With the increase in size of the orbital cavity, the origin of the medial portion of the external mandibular adductor would have been pushed backwards, which would have prohibited a vertical orientation of this portion (cf. [2,29]).
So Otidiphaps uses the mechanical advantages of the separate muscular control only within the limits imposed by the conditions of its habitat, whereas in Didunculus the apparatus allows a quite specialized use of this type of muscular control.
4.1.4 Which hypotheses can be formulated about the trophic adaptation of pigeons?
The most obvious character is the ability of pigeons to make rapid, powerful, steady and long-distance flights [9,11, and pers. obs.]. This primary adaptation allows them to cover wide areas in search of their food, which is often patchily distributed (patches varying in space and time). This has permitted the ancestral group to spread over the world and settle in islands. This flying ability allows also exploiting feeding grounds away of breeding sites, which has probably been a pre-adaptation to the colonization of very open habitats.
The key adaptation is the ability to remove attached food items, particularly fruits. This adaptation developed in the frame of the joint muscular control. In pigeons, this mechanism permitted obtaining an additional force during the retraction of the schizorhinal upper jaw. Until now, the force retracting the upper jaw was attributed to the joint action of the external mandibular adductor and m. pseudotemporalis profundus, but in pigeons (except in Didunculus and Otidiphaps), this latter has become the main source of this slanting force. This allowed pigeons to remove rather large items and swallow them by utilization of the movement of pushing them between the mandibular rami. This movement has been important for pigeons because of their microcephaly. The antagonism between the characteristic microcephaly of pigeons and the need of a wide intermandibular space in frugivores eating large fruits has been resolved by the acquisition of the capability to widen the intermandibular distance as much actively as passively.
By conserving along their history all the advantages in bill functioning provided by the joint muscular control and associated specialized characters, pigeons could be arboreal as well as terrestrial in their search for food in closed or open habitats. Thus, Zenaida galapagoensis with the well-developed force of retraction of its upper jaw can scratch volcanic soils [9] and feed on seeds like a pheasant [52]. Likewise, Phaps chalcoptera is a strict granivore [9], though it still possesses characters of a frugivore (in spite of the loss of the hook): strengthened maxillary, insertion of the jugal bar, hypertrophy of m. pseudotemporalis profundus. Oena capensis, which takes small seeds on the ground, could be another example with the structure of its m. pseudotemporalis profundus and its lack of internal ligament in the mandibular joint, a legacy of the key adaptation of all pigeons.
We can point out that along their evolution based on the strict exploitation of plant resources, pigeons in the whole adapted only to just remove and swallow an attached item, whereas other vegetarian birds, e.g., hoatzin, turacos, mousebirds, parrots (cf. [2,6,7,65–67]), adapted also to process items in the bill; only Didunculus is able to do so.
Isolation in islands left two extant representatives (Otidiphaps in New Guinea, Didunculus in Samoa) of pigeons that succeeded to shift from the joint to the separate muscular control, i.e., became still more specifically adapted to feeding on attached items. In Otidiphaps, niche widening by ecological release must have entailed mainly modifications of its locomotor system in relation to terrestriality. More secondarily, its bill was not much modified. The overall structure of its bill apparatus is thus not much different from that of other pigeons; only the external ligament of the mandibular joint has been lost. On the other hand, Didunculus went farther, however less than parrots, by keeping rhynchokinetic.
4.2 Which evolutionary pathway did Pteroclididae follow relative to Columbidae?
4.2.1 Key adaptation of sandgrouse bill as shown by morphofunctional analysis
The most remarkable particularity of the sandgrouse bill is the absence of ligaments in the mandibular joint. Moreover, some parts of m. pterygoideus (i.e., m. retractor palatini) attach to base of the cranium and, in the frontal plane, the occipito-mandibular ligament is oriented almost along the longitudinal axis of m. pterygoideus and thus passes the force from this muscle onto the base of the cranium. Thus, all the conditions required for the separate muscular control (see methods) are present.
Among all sandgrouse, m. pterygoideus is specialized to transmit its force directly to the upper jaw, without any significant additional effect. This is due to the fact that the force from this muscle tends to be directed along the palatine, a trend indicated by the absence of medial wing on this bone, which acts also as the axis of symmetry for most of m. pterygoideus. If this is the case in P. orientalis as well as in Syrrhaptes, on the other hand P. alchata shows in addition a strong increase in power of m. pterygoideus: the palatine has a medial wing where muscular fibres attach and increase the volume and power of m. pterygoideus, which extends to the base of the upper jaw.
In the separate muscular control, to be efficient in terms of clamping force required to remove an attached item, the adductors of the mandible need their resulting force be more vertically oriented than in the joint muscular control. This trend towards a more vertical orientation is quite obvious in sandgrouse. It is very clear in Syrrhaptes and P. alchata, which have ossified basal parts of the aponeuroses am and amq, which constitute a long process making very rostral the origin of the muscular fibres that thus reach the mandible more vertically than in P. orientalis. In addition, P. alchata differs from the others by the great expansion of its m. pseudotemporalis superficialis, which, among the adductors, shows the more vertical orientation (Fig. 7E). This hypertrophy and the presence of this process suggest that P. alchata uses much more fully than the other ones the biomechanical advantages of the separate muscular control.
The specific particularity of sandgrouse is thus a strongly developed adaptation to use the separate muscular control. The apparatus allowing such a control is adapted to a very efficient removal of attached items. Such a mechanism is characteristic of Galliformes and Anseriformes (Gallo-anserae), whose key adaptation is linked to a vegetarian diet [31]. For this adaptation, which appeared in other groups of birds, e.g., trogons [68], the mechanical problem to solve was how to overcome the resisting force opposed by the attached item, which tends to protract the upper jaw, thus weakens the clamping, and increases the risk that the item slides out of the bill before removal. The efficiency of the removal depends on the ability to maintain firmly the upper jaw, so that it resists the passive protraction. In the joint muscular control, when the item is removed, the upper jaw is maintained still by the adductors, which change the balance of forces to its advantage. However, in some birds with joint muscular control, the upper jaw is maintained still by m. pterygoideus, which, in these birds, attaches directly to the base of the braincase (e.g., in trogons, [68]). One of the advantages of the separate muscular control is the possibility for the adductors to take an orientation much more vertical than the slanting one characteristic of the joint muscular control, and to apply a more efficient force to adduct the mandible. At the same time, independent m. pterygoideus can use the most favourable arms of lever determined by the height of the upper jaw at the level of the prokinetic hinge and by the fact that the upper jaw is much shorter than the mandible. The birds able to use the separate muscular control can thus produce more intense clamping forces at bill extremity and maintain the upper jaw still, particularly when the item is removed with a rapid jerky shake of the head. This movement is very important to remove a distinct and often particularly nutritive part of the plant. The particularities of the sandgrouse show that the key adaptation of the bill apparatus of these birds has been primarily directed towards the removal of attached plant fragments.
We can still add that most probably there exists in these birds a strict control of jaw motions, which avoids for instance that the mandibular joint jams during jerky headshakes. As noted above, the medial condyle of the quadrate slides at the articular surface of the mandible in a groove, the axis of this groove being parallel to the sliding surface in the basipterygoidal joint (Fig. 8E). This attests to the fact that the pterygoid moves to and fro in a well-definite manner along its axis. These well-definite movements are due to the orientation of the axis of the medial condyle of the quadrate and to the fact that the axis of the quadrato-cranial joint, along which the quadrate swings, is almost perpendicular to the axis of the medial condyle of the quadrate and to the sliding surface in the basipterygoidal joint. To these well-definite movements corresponds the disposition of the muscular fibres that determines and controls the trajectory of the pterygoid, on which bone these fibres attach symmetrically relatively to its longitudinal axis (Fig. 8C). The pterygoid being clearly bent towards the basipterygoidal joint, the symmetry of the arrangement of the muscular fibres has been maintained thanks to the laterally swollen pterygoid, a bulge that offsets the medial curvature. These well-definite and directed movements, facilitated by the symmetrical arrangement of the muscular fibres, help to avoid unexpected jamming of the mandibular joint.
At the instant of the removal of a food item with jerky head movements, in spite of the absence of ligaments in the mandibular joint, the quadrate can gain stability from the very wide articular surface because of the very lateral position of its lateral condyle. Furthermore, in sandgrouse, the posterior adductor is very well developed. It is attached to the base of the orbital process of the quadrate, very close to the mandibular joint. It cannot produce rotation (or a very little one) of the quadrate, but can efficiently clamp surfaces in the mandibular joint, thus increasing the friction force and immobilizing the quadrate, stopping its movement relative to the mandible, which is fixed by the strong occipito-mandibular ligament, and resisting the passive protraction of the upper jaw when the item is removed. This phenomenon has been described in Galliformes [31,53].
In the case of a separate muscular control corresponding to an adaptation to take motionless items, mechanisms such as the coupled kinesis (see Section 2), which plays an important role in the capture of mobile small items, are not necessary or at least unimportant. Thus, the absence in sandgrouse of the post-orbital ligament is not a surprise and does not deserve further analyses.
An important part of the diet of sandgrouse is made up of seeds, particularly very small seeds [14,36,37,40–42]. Picking up these seeds does not require particular mechanical features for birds with a pointed bill tip. Nevertheless, we can foresee the problems linked to the transfer of minute items from the extremity of the bill to the oesophagus in relatively large birds.
Without doubt, the tongue plays the main role in the transfer of the items in a way recalling what has been described in detail by Zweers for Columba livia, picking up and ingesting small seeds [26]. The tongue is well developed in sandgrouse and, which is very important, there is in the hyoid apparatus a particular tongue-raising mechanism constituted by m. branchiomandibularis and the arched hyoid horns that apply against the depressor of the mandible. M. branchiomandibularis is well differentiated in sandgrouse, with two portions: a rostral one and a caudal one (Fig. 9B). The rostral portion ensures the protraction of the hyoid apparatus, whereas the caudal one, acting as the string of a bow, maintains the internal stress in the decurved horn, thanks to which it presses the surface of the depressor. The component of the reaction force of the depressor to that push and the force produced by m. serpihyoideus (which does not allow the horns to slope down) make a torque that rotates the hyoid apparatus so that its rostral extremity is lifted towards the palate (Fig. 9A) and raises the tongue.
The advantage of this tongue-raising relative to that due to m. mylohyoideus is that the tongue is thus lifted well above the upper edge of the mandible where this muscle attaches. Thus, the tongue plays the role of an additional mandible as it can press small items against the palate and maintain them here when the bird opens its bill to take another item. This mechanism increases pecking efficiency, as the bird does not loose time in swallowing items separately; the bird can accumulate them in its buccal cavity and transfer them episodically to the oesophagus. The transfer is facilitated by the crenellated and backward-oriented ridges of the palate (Fig. 9A). Minute seeds pose a particular problem, as the bird cannot project them deep in its buccal cavity because being very light, their inertia is too weak. In sandgrouse, the salivary glands are placed near the mandibular symphysis and thus play an important role (Fig. 9A). Minute seeds can be rapidly glued with the salivary secretions and, by the movements of the tongue, be aggregated in the buccal cavity and then transferred to the specific fold (valve) visible on the palate at the entrance of the oesophagus. This mechanism is much reminiscent of the ‘slide and glue’ mechanism used by Columbia livia when feeding on small (2 mm) seeds and precisely described by Zweers [26].
Thus, we see that in sandgrouse the bill and hyoid apparatus constitutes a functional unit strongly and specifically adapted to remove pieces of attached plant items as well as to pick up efficiently minute seeds, despite these birds are of relatively large size.
4.2.2 Is bill apparatus in sandgrouse an important source of information on the ecological specializations of these species?
In spite of great similarities, the three species we have examined show specific particularities; this is notably the case of P. alchata. Some of the peculiarities have already been mentioned above; however, we will insist on some that appear important by the questions they raise.
So P. orientalis differs from the others by his aponeuroses am and amq not ossified and thus not constituting the process which in Syrrhaptes and P. alchata allows the muscular fibres of the external mandibular adductor to be more vertically oriented. This implies that, in these two latter species, the bill apparatus would be more adapted to produce clamping forces necessary to remove more firmly attached items.
In P. alchata, the trend toward an increase of the forces of the cranial musculature is quite obvious. It is at first indicated by the fact that the aponeuroses of the external mandibular adductor are very well developed and thicker in this species than in the others (Fig. 7F). This shows an increase in the number of muscular fibres, thence of the intensity of the forces produced by this muscle, which, in addition, has a rostral part of its deep portion well developed and covering a large surface of the braincase. The increase of this rostral part of the external mandibular adductor resulted in the complete disappearance of the post-orbital ligament (which is vestigial in Syrrhaptes and P. orientalis). To the trend of the adductors to exert their forces more vertically, corresponds also the hypertrophy of m. pseudotemporalis superficialis. Furthermore, in this species, the prokinetic area of the culmen is located at the base of the upper jaw, i.e. in the highest part of this jaw. M. pterygoideus can thus resist the passive protraction during the removal of the item with the most advantageous lever arm. In addition, the bones of the skull of P. alchata are thicker than those of the others are. The nasal capsule, ossified in its caudal part, makes the upper jaw more monolithic, which indicates the importance of the forces acting in the bill apparatus of this species. The contact area of the tomia, along all the rhamphotheca, is particularly wide and flat, and the extremity of the bill is more rounded. This can be interpreted as a potentiality, characteristic of this species, to crush items with a great force.
It is thus possible to propose that P. orientalis and Syrrhaptes share the same specializations, whereas P. alchata is somewhat different, appearing clearly able to remove and even crush before ingestion plant parts more hard than those taken by the others are. How these specializations are expressed in terms of habitat selection and ecological segregation in relation to sympatric or even syntopic congeners requires field eco-ethological studies. It would be interesting to see how much P. alchata and P. orientalis diverge in their foraging behaviour, food quality (physical and nutritional characteristics), mechanical food processing, etc., and this as a function of the environmental conditions. Do not forget that sandgrouse are above all birds of sub-desert steppes, even of sandy or stony deserts and that for them water is an important resource they get of course at watering places (often after quite long flights), but also from plants (in particular succulent plants such as Salsolaceae and Chenopodiaceae), which they crush to extract water, but also mineral salts and other substances. It would likewise be interesting to see whether other sympatric species-pairs (e.g., P. senegallus/P. coronatus, P. exustus/P. gutturalis, P. exustus/P. indicus or P. decoratus/P. quadricinctus) show differences like those found between P. alchata and P. orientalis. It would also be important to examine more species to verify that the recorded differences are really the expression of an ecological radiation, not a phylogenetic imprint. It is thus symptomatic that in his evolutionary hypothesis based on coloration patterns; Maclean [69] gives a particular place to P. orientalis, but close to P. gutturalis, thus joining Wolters [70], who admits the sub-genus Eremialector, which does not include alchata.
4.2.3 Is the evolutionary pathway of sandgrouse the same as that of pigeons?
Our material leads us to think that sandgrouse followed an evolutionary pathway similar or at least partly similar to that of pigeons, but unlike them, they are strongly specialized both in picking up very small even minute seeds and to remove attached items.
The absence of internal jugo-mandibular ligament in the mandibular joint in sandgrouse has not yet been functionally interpreted. We suggest a rather ancient legacy of an ancestral evolutionary stage, common to sandgrouse and pigeons, linked to the adaptation to exploit large plant items, most probably fruits.
Sandgrouse would have evolved along the same pathway as Didunculus and Otidiphaps, the morphofunctional novelty of these species being the appearance of the separate muscular control with disappearance of the external jugo-mandibular ligament. Sandgrouse would have then diverged, keeping the tongue-raising mechanism with a well-differentiated m. branchiomandibularis. The latter shows still some indications of differentiation in Otidiphaps (mechanism still present but incomplete, thus not functional), but none in Didunculus (disappearance of tongue-raising mechanism).
The characteristic bill and hyoid apparatus of sandgrouse allowed these birds to live in very open, herbaceous and much harsher habitats than pigeons. They could realize or fill very specific trophic niches based on the removal of selected attached items and, in spite of their relatively large size, the exploitation of very small seeds.
5 Conclusion
The synthesis of the elements we have presented on the most probable adaptive stages in the light of morphofunctional analyses of the bill and hyoid apparatus leads us to propose the hypothesis of an evolutionary scenario common to pigeons and sandgrouse (Fig. 10). This scenario is not to be considered as a genealogical tree of pigeons and sandgrouse (i.e. not a phylogenetic tree), but as a testable hypothesis about how in the course of their evolution pigeons and sandgrouse developed the particular adaptations of their bill apparatus, which can be detected by morphofunctional analyses conducted on present-day species.
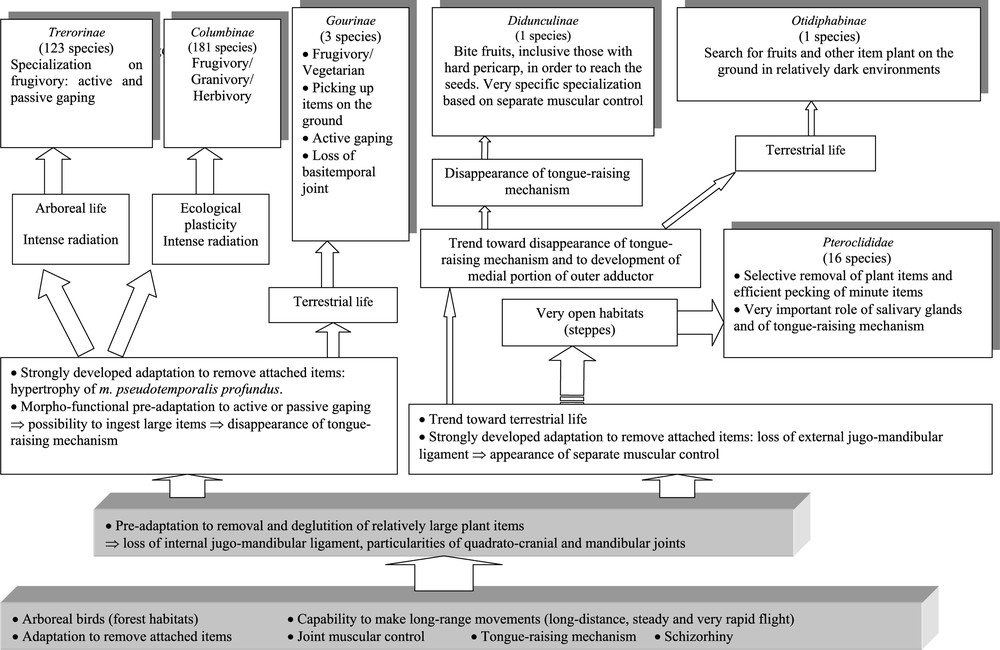
Hypothetical scenario of the successive adaptive steps that paved the way followed by pigeons and sandgrouse during the evolution of their bill and hyoid apparatus. This diagram does not imply that pigeons and sandgrouse are necessarily closely related, because it does not exclude parallel or convergent evolutions. It means that the ancestors of modern pigeons and those of present-day sandgrouse passed through the same adaptive stages in the course of the evolution of their bill and hyoid apparatus. By strongly developed adaptation, we want to stress that the birds went further in their adaptation by developing additional morphofunctional characters. Masquer
Hypothetical scenario of the successive adaptive steps that paved the way followed by pigeons and sandgrouse during the evolution of their bill and hyoid apparatus. This diagram does not imply that pigeons and sandgrouse are necessarily closely related, because it ... Lire la suite
From a key adaptation to the removal and deglutition of attached food plant items, two pathways would have been followed: one based on the joint muscular control (followed by many species: Treroninae, Columbinae, and Gourinae), the other based on the separate muscular control (followed by only a few forest and insular pigeons represented today by Didunculus and Otidiphaps). It is from this latter pathway that would have diverged that followed by the sandgrouse for their radiation in sub-desert or even plainly desert habitats. The morphofunctional study shows clearly that the key adaptation for them was the ability to remove attached plant items in a very selective fashion as well as to pick up particularly small seeds.
Along the pathway with conservation of the joint muscular control, Treroninae would have remained very arboreal and strongly specialized on frugivory, whereas Columbinae would have been more ecologically plastic in both habitat selection and diet. Because of their insularity, Gourinae would have increased their size and turned to a terrestrial life.
Along the pathway with acquisition of the separate muscular control, only Didunculus would have acquired particular morphofunctional characteristics allowing to process food items in the bill, though, in sandgrouse, the question of such a capability could be asked for P. alchata.
An unexpected result of the present study is that it brings to the fore strong differences between the sympatric (or even syntopic) P. orientalis and P. alchata. This raises a series of ecological (niche differentiation) and behavioural (modalities of foraging and food processing) questions that call for new field studies. It is not unimportant to note also that if we did likewise and examined several species in the various pigeon genera, we should most probably have found differences of the same kind. This reveals how important it is to perform such studies on a diversified material, allowing a wide evaluation of the variations within the group under study. This demonstrates once more the usefulness and interest to make studies of species including both actual knowledge of their biology and hypotheses derived from morphofunctional analyses of their various apparatuses based on an intimate knowledge of their anatomy.
Acknowledgements
We are most grateful to the curators in the ‘Muséum national d'histoire naturelle’, Paris, and the British Natural History Museum, Tring, who gave us access to material in their care. We acknowledge particularly the very friendly help of Douglas G.D. Russell in Tring, who facilitated the loan of a spirit specimen of Otidiphaps from the collection of the Natural History Museum. We thank Prof. V. Bels and Prof. A. Casinos as well as an anonymous referee, and particularly Prof. Walter Bock, for their help and most useful comments and suggestions on this paper. Specimens were obtained during field trips organized by the direction of the Vietnamese-Russian Tropical Center (Hanoi) with the participation of M. Kalyakin and V. Trunov. This study benefited from grants RFBR 03-04-48958 and Russian University NUR 07-03-003.
Appendix A
Abbreviations
| Muscle aponeuroses | |
| ace | aponeurosis caudalis externa |
| aci | ap. caudalis interna |
| acg | ap. ceratoglossis |
| ad | ap. depressoris |
| adi | ap. dorsalis insertionis |
| ado | ap. dorsalis originalis |
| ai | ap. interna |
| ali | ap. lateralis insertionis |
| alm | ap. lateralis mandibularis |
| alo | ap. lateralis originalis |
| alt | ap. lateralis temporalis |
| am | ap. medialis |
| amq | ap. medioquadrata |
| amr | ap. mediorostralis |
| ams | ap. mediosuperficialis |
| ao | ap. occipitalis |
| apm | ap. pseudotemporalis mandibularis |
| app | ap. pseudotemporalis profunda |
| apq | ap. posterior quadrata |
| aps | ap. pseudotemporalis superficialis |
| ar | ap. rostralis |
| as | ap. superficialis |
| asi | ap. superficialis insertionis |
| avi | ap. ventralis insertionis |
| avo | ap. ventralis originalis |
| Other abbreviations | |
| Aem | m. add. md. ext. medialis |
| Aer | m. add. md. ext. profundus rostralis |
| Aes | m. add. md. ext. superficialis |
| Ap | m. add. md. posterior |
| b | prokinetic hinge |
| Bas | basihyale |
| Bm | m. branchiomandibularis |
| Bmc | m. branchiomandibularis caudalis |
| Bmr | m. branchiomandibularis rostralis |
| Bpt | basipterygoidal joint |
| Cb | ceratobranchiale |
| Cga | m. ceratoglossus anterior |
| Cgp | m. ceratoglossus posterior |
| Ch | m. ceratohyoideus |
| cl | lateral condyle of the quadrate in the quadrato-cranial joint |
| cm | medial condyle of the quadrate in the quadrato-cranial joint |
| Col | lateral condyle of the quadrate in the mandibular joint |
| Com | medial condyle of the quadrate in the mandibular joint |
| Dm | m. depressor mandibulae |
| Eb | epibranchiale |
| Ent | entoglossum |
| Gg | m. genioglossus |
| Gls | salivary gland |
| Hg | m. hypoglossus |
| HypLji | hypothetical ligamentum jugomandibulare internum |
| Jg | jugale (arcus jugalis) |
| Lc | lacrimale |
| Lje | ligamentum jugomandibulare externum |
| Lom | lig. occipitomandibulare |
| Lp | lig. postorbitale |
| Md | mandibula |
| Mst | mesethmoideum |
| Mh | m. mylohyoideus |
| Mx | maxilla |
| Nr | nostril |
| Ns | nasale |
| Pc | caudal portion |
| Pdl | m. pt. dorsalis lateralis |
| Pdm | m. pt. dorsalis medialis |
| Pl | palatinum |
| Pim | proc. internus mandibulae |
| Ppm | proc. palatinus (ossis) maxillaris |
| Ppo | proc. postorbitalis |
| Pr | m. protractor pterygoidei et quadrati |
| Prq | m. protractor quadrati |
| Psp | m. pseudotemporalis profundus |
| Pss | m. pseudotemporalis superficialis |
| Pt | (os) pterygoideum |
| Pvl | m. pt. ventralis lateralis |
| Pvm | m. pt. ventralis medialis |
| Pz | proc. zygomaticus |
| Rpl | retractor palatini |
| Q | quadratum |
| Sh | m. serpihyoideus |
| Sn | septum nasale |
| Sth | m. stylohyoideus |


