1 Introduction
The principle of competitive exclusion [13,17,20,35,47] asserts that two or more competitors cannot coexist indefinitely on a single prey, which was supported by experiments on Paramecium cultures by Gause [15] (see also [28]). It was thought to hold in laboratory settings until Ayala [2] demonstrated experimentally that two species of Drosophila could coexist upon a single prey.
In order to explain Ayala's experiments, various competition models have been proposed. For example, Armstrong and McGehee [1] considered the model:
| (1.1) |
| (1.2) |
Consider an ordinary differential equation model for n interacting biological species:
| (1.3) |
To have the strong version of coexistence, researchers have taken various other factors into account when modeling competition, such as interspecific interference [45,46], spatial heterogeneity [5], stoichiometric principles [34], etc. Another important factor is intraspecific interference within a population of competitors, which includes aggressive displays, posturing, fighting, infanticide, and cannibalism [30]. When the principal effect of the intraspecific interference is a reduced rate of feeding or resource intake, the effects can be modeled via an altered functional response for the consumer [8,10,40]. Recently, Cantrell et al. [6] extended system (1.2) to incorporate conspecific feeding interference for one of the competitors. The functional responses for the competitor w and the prey u are now taken to have the Beddington–DeAngelis form:
On the other hand, when lethal fighting or cannibalism occurs, it is more appropriate to include a nonlinear (density-dependent) mortality term for the consumer into the competition model [30]. In plant population, density-dependent mortality is one of the three principal effects resulting from intraspecific competition [38,49]. To incorporate such intraspecific competition (as well as the interspecific competition) into the model, Ruan and He [39] studied the global stability of a chemostat-type competition model, Kuang et al. [30] investigated the global stability of a Lotka–Volterra competition model. See also [16,32,33] for persistence of n species on a single resource.
Notice that in Refs. [16,30,32,33,39] all competitors are assumed to have density-dependent mortality rates. A very natural and very significant question arises: If one of the competitors does not have a density-dependent mortality rate, does the model still exhibit coexistence and have a stable componentwise positive equilibrium? The numerical simulations of Kuang et al. [30] indicate that, even for a Lotka–Volterra model, a competitor without a density-dependent mortality rate could eliminate another competitor with a density-dependent mortality rate if the first competitor has a lower break-even prey biomass. The problem can be very subtle.
Hixon and Jones [21] found that density-dependent mortality in demersal marine fishes is often caused by the interplay of predation and competition (see also [22]). To study how the nonlinear mortality rate determines the dynamics of such competition models qualitatively, we consider a two-competitor/one-prey model of the form:
| (1.4) |
| (1.5) |
We shall show that the two competitors can coexist upon the single prey. It demonstrates that density-dependent mortality in one of the competitors can prevent competitive exclusion.
As an example, we apply the obtained results to a two-competitor/one-prey model with a Holling II functional response:
| (1.6) |
2 Mathematical analysis
2.1 General functional responses
First of all, we can see that the solutions to the initial value problem (1.4)–(1.5) are nonnegative.
Define and denote . We have:
Next, we consider the existence of a positive equilibrium. System (1.4) has a componentwise positive equilibrium , where:
| (2.1) |
| (2.2) |
| (2.3) |
It is important to note that the key assumption for the existence of the positive equilibrium is . If , then the positive equilibrium simply does not exist. To have , we require , which means that at the steady state the growth rate of the species w must be greater than the linear mortality rate. Notice that from the first equation, we have:
Now we study the local stability of the positive equilibrium . Define:
| (2.4) |
| (2.5) |
| (2.6) |
Since , the characteristic equation (2.6) always has at least one negative real root. Notice that:
- (i) iff
- (ii) iff
| (2.7) |
| (2.8) |
Assume that the positive equilibrium
exists. If
Theorem 2.1
and
(2.9)
then it is locally asymptotically stable.
(2.10)
The conditions (2.9) and (2.10) (corresponding (2.17) and (2.18) in Proposition 2.4) are technical assumptions on parameters. For the specific Holling II functional response, these conditions will be expressed explicitly, see Remark 2.7. Also, since , we can see that implies both (2.9) and (2.10). Thus, conditions (2.9) and (2.10) can be replaced by a stronger conditionRemark 2.2
Assume for certain parameter value, say a critical value of the carrying capacity of the prey population, . Then the characteristic equation (2.6) has a pair of purely imaginary roots given by . If, moreover, the transversality conditionRemark 2.3
holds, then the positive equilibrium becomes unstable and a Hopf bifurcation occurs at when K passes through .
Finally, we discuss the global stability of the positive equilibrium . Choose a Liapunov function as follows:
| (2.11) |
In next subsection, for the model with Holling II functional responses, we will choose proper and γ to derive explicit sufficient conditions for the global stability of the positive equilibrium.
2.2 Holling II functional responses
In this subsection we apply the above results to system (1.4) with Holling II functional responses, namely system (1.6). System (1.6) has a unique componentwise positive equilibrium defined by:
| (2.12) |
| (2.13) |
| (2.14) |
Define
| (2.15) |
| (2.16) |
By Theorem 2.1, we have the following local stability result.
Assume that the positive equilibrium
of system
(1.6)
exists. If
Proposition 2.4
and
(2.17)
then it is locally stable.
(2.18)
Note that , defined by Eq. (2.4), is for general functional responses, while , defined by Eq. (2.15), is for the specific Holling type-II functional response. They both are related to the derivative of the right-hand side function of the prey equation, that is, the growth of the prey population at the positive equilibrium.Remark 2.5
Finally, we give a sufficient condition for the global stability of the positive equilibrium for system (1.6).
Assume that the positive equilibrium
of system
(1.6)
is locally stable. If
Proposition 2.6
then it is globally stable.
(2.19)
Let be the Liapunov function defined by (2.11), where α, β, and γ are positive constants to be determined. Along any trajectory of system (1.6), we have:Proof
Choose
Then we have
The coefficient for is always negative. The coefficient for is:
Thus, if (2.19) is satisfied, then and if and only if , , . This completes the proof. □
Using (2.12), (2.15) and (2.19), one of the local stability conditions (2.17) becomes:Remark 2.7
and the global stability condition (2.19) reduces to(2.20)
We can see that G plays a role in the local stability condition (2.20). Once the positive equilibrium is locally stable, K, the carrying capacity of the prey, plays a role in the global stability. If the value of K is increased so that condition (2.21) is not satisfied, the positive equilibrium becomes unstable and a Hopf bifurcation can occur (see Figs. 4 and 5).(2.21)
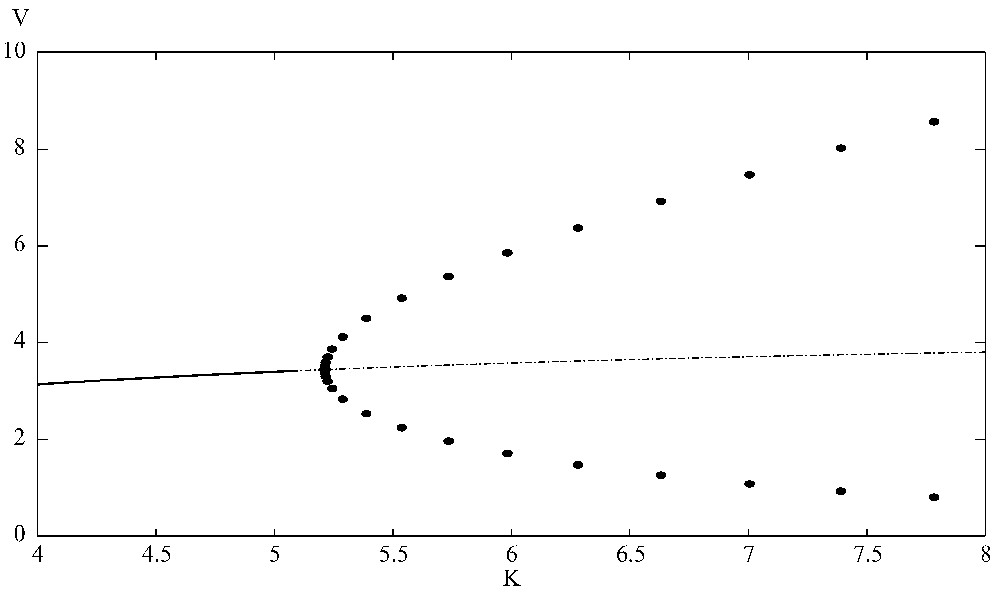
The bifurcation diagram shows that the positive equilibrium is stable when K⩽5.21. At K=5.21, it loses its stability and a supercritical Hopf bifurcation occurs. Here r=1.5, a=0.45, b=0.35, d=0.45, e=0.55, A=0.55, B=0.35, D=0.45, E=0.65, G=0.1. XPPAUT was used for the simulations.
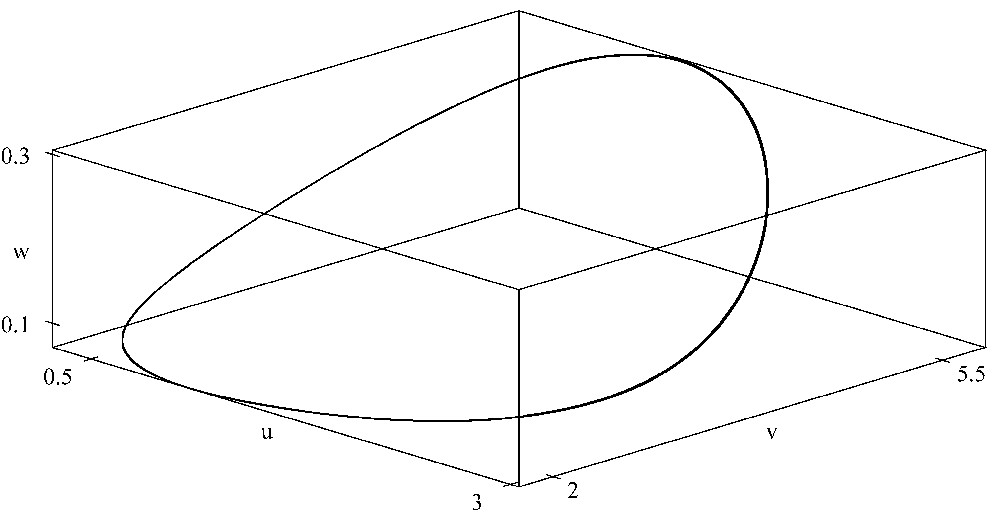
When K=6, there is a periodic orbit bifurcated from the interior equilibrium in the three-dimensional space which is asymptotically stable. Here r=1.5, a=0.45, b=0.35, d=0.45, e=0.55, A=0.55, B=0.35, D=0.45, E=0.65, G=0.1. XPPAUT was used for the simulations.
The plot of indicates that for some parameter values (see Fig. 1). So the assumption in Theorem 2.6 makes sense.
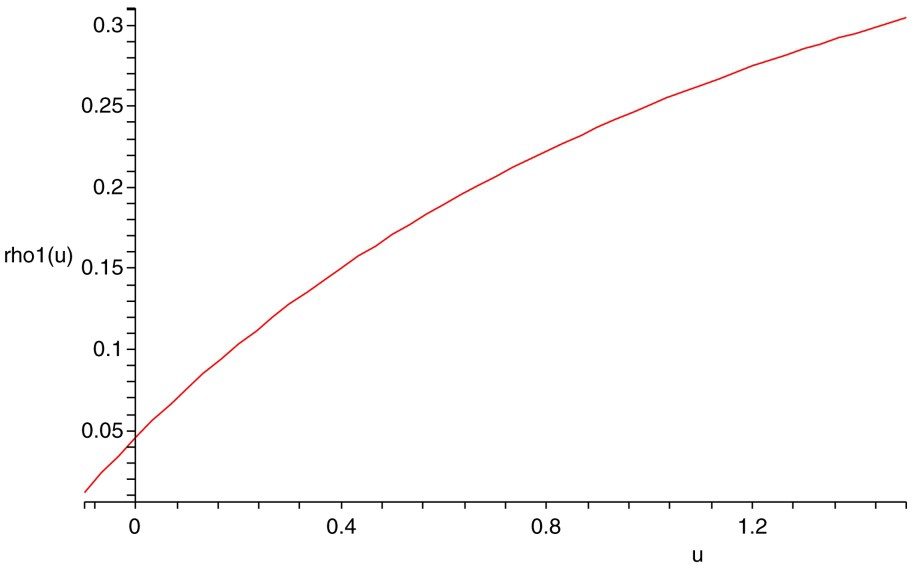
The plot of the function . Here r=1.5, K=3, a=0.45, b=0.35, d=0.45, e=0.55, A=0.55, B=0.35, D=0.45, E=0.65, and G=0.05.
3 Simulations and discussion
It is well known that density-dependent mortality terms (closure terms) can greatly affect the outcome of plankton models [42]: not only limit cycles [11] but also chaos [7] can occur in such models.
In the case of two competitors competing for a common prey, our results indicate that density-dependent mortality of one competitor not only ensures the long-term survival of itself, but also guarantees the existence of the other competitor, which would otherwise be out-competed.
To illustrate the results numerically, consider system (1.6) with Holling II functional responses. Choose , , , , , , , , , , and let G (the density-dependent mortality parameter) vary. When , that is, when there is only linear mortality for the competitor with density w, numerical simulations show that the competitor with density w out-competes the competitor with density v (see Fig. 2). Note that system (1.6) does not have any positive equilibrium when .
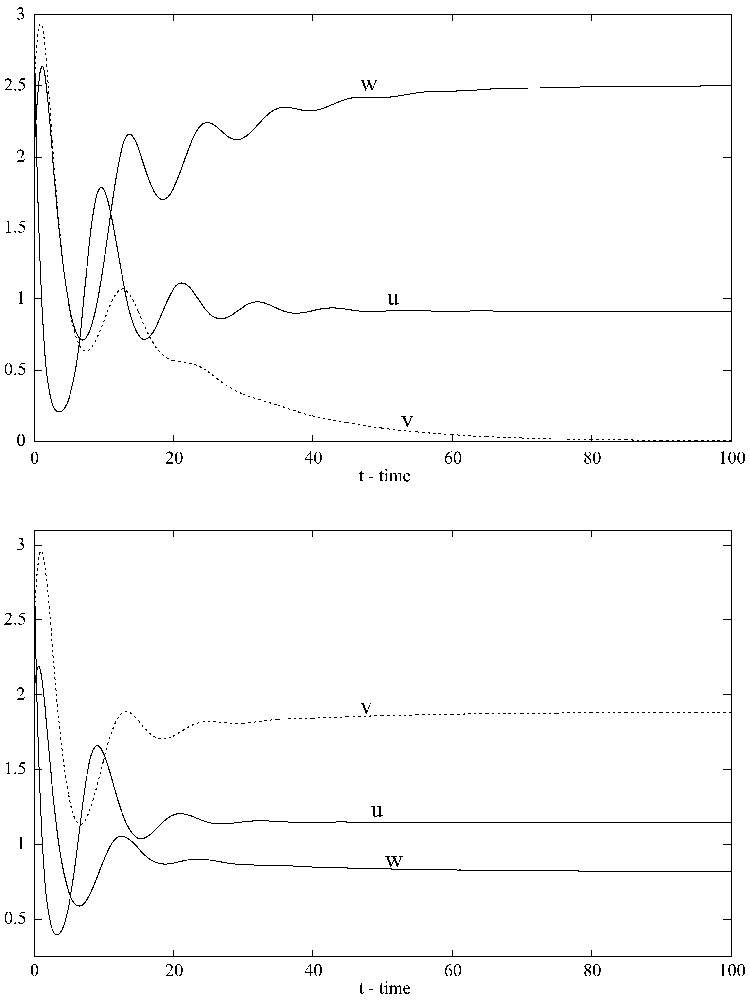
When G=0, the strong competitor with density w wins the competition and the weak competitor with density v tends toward extinction (top). When G=0.1, both competitors coexist and the solution converges to the positive equilibrium (1.1465,1.8837,0.8197) (bottom). XPPAUT was used for the simulations.
Introducing density-dependent mortality only for the competitor with density w (the stronger competitor) makes the system coexistent not only in the sense of uniform persistence, but also in the sense of existence of a globally stable positive equilibrium (Fig. 2). With the above parameter values and , all conditions in Proposition 2.6 are satisfied. Thus, system (1.6) has a positive equilibrium , which is globally stable. A possible explanation for this phenomenon is that density-dependent mortality in the stronger competitor prevents it from reducing the density of the prey below the threshold value (the value – see [44]) needed for the weaker competitor to be able to maintain itself. In fact, the steady-state values of v and w are functions of G (see Fig. 3).
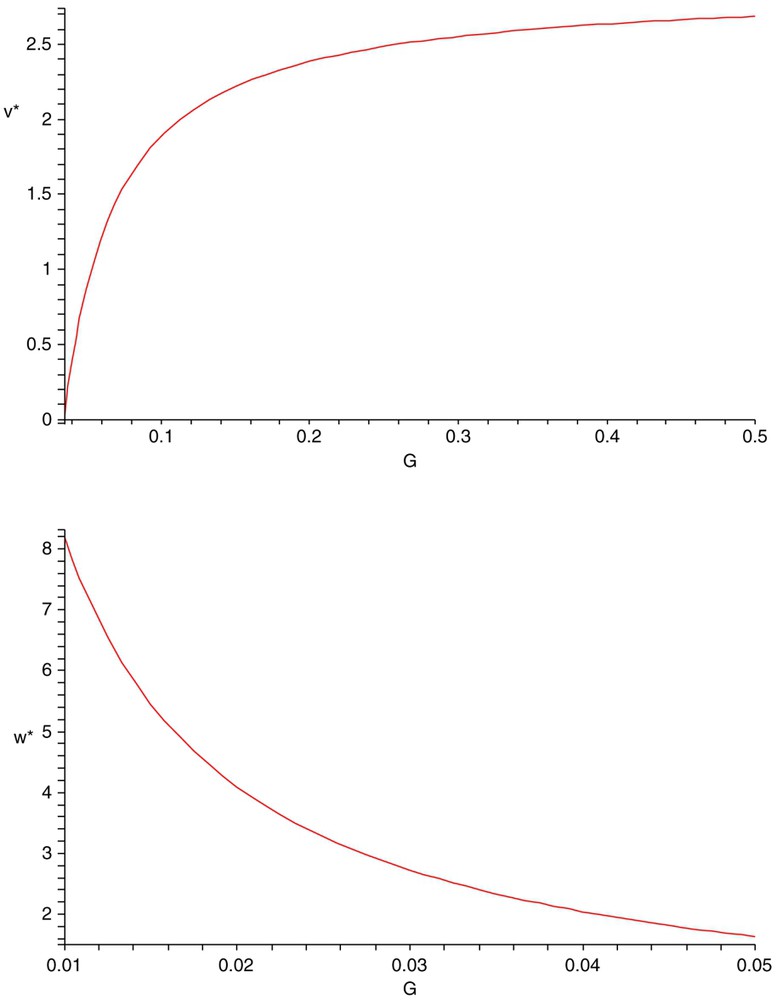
The steady-state value of the weak competitor with density v is an increasing function of G (top) and the steady-state value of the strong competitor with density w is a decreasing function of G (bottom). Here r=1.5, K=3, a=0.45, b=0.35, d=0.45, e=0.55, A=0.55, B=0.35, D=0.45, E=0.65.
Is the situation represented by model (1.4) a feasible one ecologically? It is a variation on the model of Tilman [44] for two consumers competing exploitatively for a single prey. Tilman's model predicts that the consumer that reduces the prey to the lower steady-state value will displace the other competitor. Model (1.4) includes the mechanism of density-dependent mortality, or biomass loss, , on the superior competitor. We believe that this represents one way in which a fitness ‘tradeoff’ might occur between two species. It is reasonable to suppose that one species, w in this case, has superior fitness in its ability to exploit prey, but this is compensated for by a vulnerability to mortality. Increased mortality could reasonably occur under the circumstance that the superior competitor is also the one that is more of a risk taker; for example, that it seeks prey in areas where there is also a greater risk of mortality, say through predation. However, the non-linear form of the mortality, , still requires justification. A nonlinear response of this form might occur if the foraging area contains a diversity of subareas having different relative risk factors. In that case, individuals of species w can tend to use areas that are relatively low in predation or other mortality risk (although higher than members of species v) when the population of species w is low, but can be forced into areas of higher risk as the population size increases. This situation could easily lead the better competitor to have also a density-dependent mortality rate. This might also occur if the better competitor is more prone to dispersal; the probability of dispersal of individuals increases with density, and dispersal increases the chances of mortality. Therefore, we believe that model (1.4) may represent a fairly common situation among consumers competing for a common prey.
It is interesting to observe that, when the carrying capacity K of the prey is increased, the positive equilibrium loses its stability and a Hopf bifurcation occurs when K passes a critical value. With parameters given above, a supercritical Hopf bifurcation occurs when (see Fig. 4). A three-dimensional positive periodic solution bifurcates from the positive equilibrium via a Hopf bifurcation with (see Fig. 5). This is an example of the well-known paradox of enrichment [25].
It is also interesting to notice that, for the subsystem without the competitor v, that is, the predator-prey system with density-dependent mortality:
| (3.1) |
Acknowledgements
The authors are very grateful to the anonymous referee for his careful reading, detailed comments, and helpful suggestions. The first author would like to thank Prof. G. Bard Ermentrout and Dr. Rongsong Liu for their help in using XPPAUT for the numerical simulations.


