1 Introduction
Intercellular pectic protuberances (IPPs) have been observed in many plant taxa and in numerous plant tissues [1–5]. Due to the diverse shapes assumed by these protuberances, they have received many designations in the literature, including: intercellular wall thickenings, pectic strands, pectic filaments, pectic warts, scalae, microprojections, bead-like projections, papilla-like structures, protuberances, intercellular protuberances, and intercellular pectic protuberances (see [6]). The development of these structures is usually associated with the formation of intercellular spaces during cell expansion [4], although the formation of IPPs has been reported even after the development of intercellular spaces – and in these cases they have been observed to form by secretions from the protoplast [7]. There is still much controversy surrounding the origin, chemical composition, and function of these intercellular structures [4,7,8].
According to Potgieter and van Wyk [4], any biological functions attributed to the IPPs are still speculative, and include their involvement in apoplastic transport, hydration of the cell wall, reserve storage, cellular adhesion, and defense against pathogens. Although very few studies have examined this subject in any detail, it is quite possible that their diverse morphological forms do in fact reflect distinct functions – but there is growing evidence that IPPs interconnecting adjacent cells facilitate apoplastic transport [2,9].
The presence of IPPs connecting palisade cells is a common characteristic in the leaves of most angiosperm species [2]. Among the Fabaceae, these structures have also been reported in the seeds (see [1]) and stomata of Vicia faba [10].
We report here the occurrence of IPPs linking parenchyma cells of the mesophyll and secretory cells of the floral nectary of Hymenaea stigonocarpa (a species typical of the cerrado vegetation of central Brazil) and propose hypotheses concerning their function.
2 Materials and methods
The middle third section of the leaflet blade of mature leaves and the secretory tissue of the floral nectary, at anthesis, were collected and fixed in a Karnovsky solution [11] for 24 h. To prepare the specimens for light-microscopy studies, the fixed specimens were processed and embedded in historesin (Leica Embedding Kit) following standard methods, and sectioned transversely and longitudinally at 2 to 4 μm. The sections were mounted on slides and stained with toluidine blue [12]. Ruthenium red was employed to detect pectic compounds [13].
For examination under transmission electron microscopy, samples were fixed in a Karnovsky solution (Karnovsky, 1965) for 24 h, post-fixed in osmium-tetroxide (1%, 0.1 M phosphate buffer, pH 7.2) for 2 h, dehydrated in an acetone series and embedded in araldite resin [14]. Ultra-thin sections were stained with uranyl acetate and lead citrate, and examined using a Philips CM 100 transmission electron microscope at 60 kV.
For observation by scanning electron microscopy, leaf sections were dehydrated in the presence of silica gel at 50 °C until attaining a constant weight, fractured, mounted on aluminum supports, and subsequently gold coated. Hypanthium samples were fixed in a Karnovsky solution [11] as described earlier, dehydrated in an ethanol series, submitted to critical point drying, and subsequently gold coated [15]. The specimens were examined using a Philips SEM-515 scanning electron microscope.
3 Results
3.1 Intercellular pectic protuberances in leaf mesophyll
The mature leaves of H. stigonocarpa have a homogeneous mesophyll formed of various layers of palisade cells that are connected by intercellular pectic protuberances (IPPs) located predominantly on the anticlinal cell walls (Fig. 1A–D), besides usual cell contact by anticlinal walls. These protuberances take the form of filaments with a scalariform pattern (sometimes branching at the extremities), forming a web on the plane parallel to the major axis of the cells they connect (Fig. 1B and C).
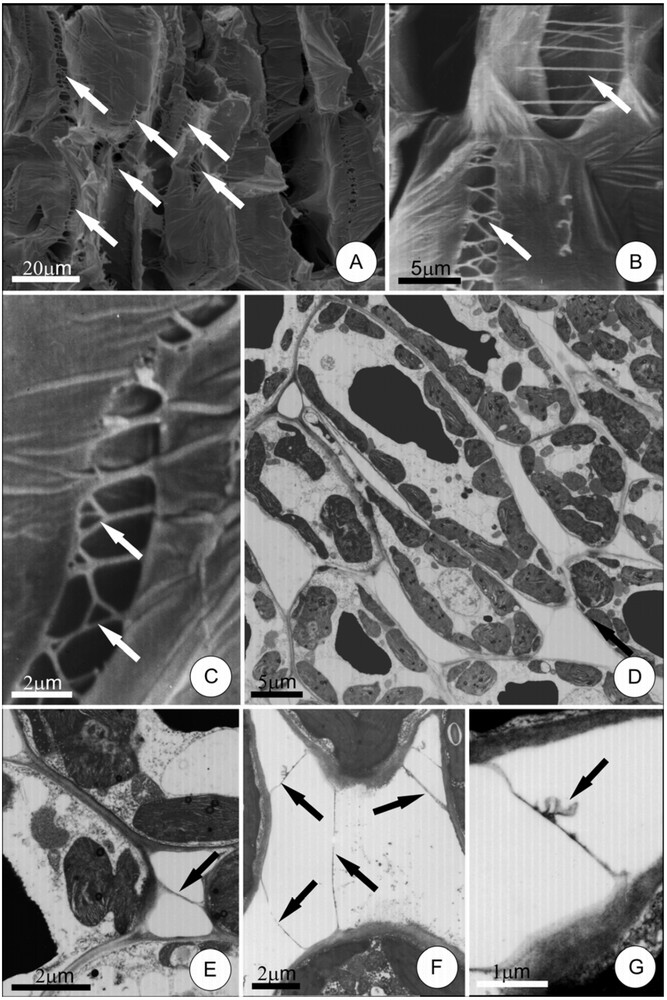
Pectic protuberances between cells in the leaflet mesophyll of Hymenaea stigonocarpa. A–C, protuberances (arrows) connecting parenchyma cells, as seen by scanning electron microscopy. Note their filamentous form and their ramifications. D, general view of palisade cells. E–G, details of intercellular pectic protuberances under transmission electron microscopy (arrows). Note, in G, dilated portion (arrow) at protuberance.
The IPPs in the leaf mesophyll have frequently small, uniform diameters (0.05 to 0.4 μm) along their entire length – except at their extremities where discrete dilations increase their contact with the cell wall (Fig. 1E). These IPPs have a very homogeneous structure and no channels through their interiors were observed. Some IPPs demonstrated dilated sections and ramifications (Fig. 1F and G).
IPPs are difficult to observe under light microscopy as their diameters are very close to the resolution limit of this type of equipment. However, these structures stained deeply with ruthenium red and could be observed in some situations.
3.2 Intercellular pectic protuberances in floral nectaries
The flowers of H. stigonocarpa have well-developed floral nectaries vascularized with phloem, and the secretory parenchyma occupies a large portion of the hypanthium. Secretion is abundant, with the nectar being secreted into the space between the hypanthium and the stipe.
Sections through the parenchyma secretory region demonstrated numerous IPPs linking adjacent cells (Fig. 2A–D). These protuberances took the form of rarely branched strands and were distributed apparently randomly, without forming any discernable network. As in the leaf parenchyma mesophyll, these IPPs had a uniform thickness (0.05 to 0.2 μm) and were dilated only at their extremities, where they connected to the plant cell walls (Fig. 2D and E). The dilations observed at the extremities of the IPPs in the hypanthium were larger than those seen in the mesophyll.
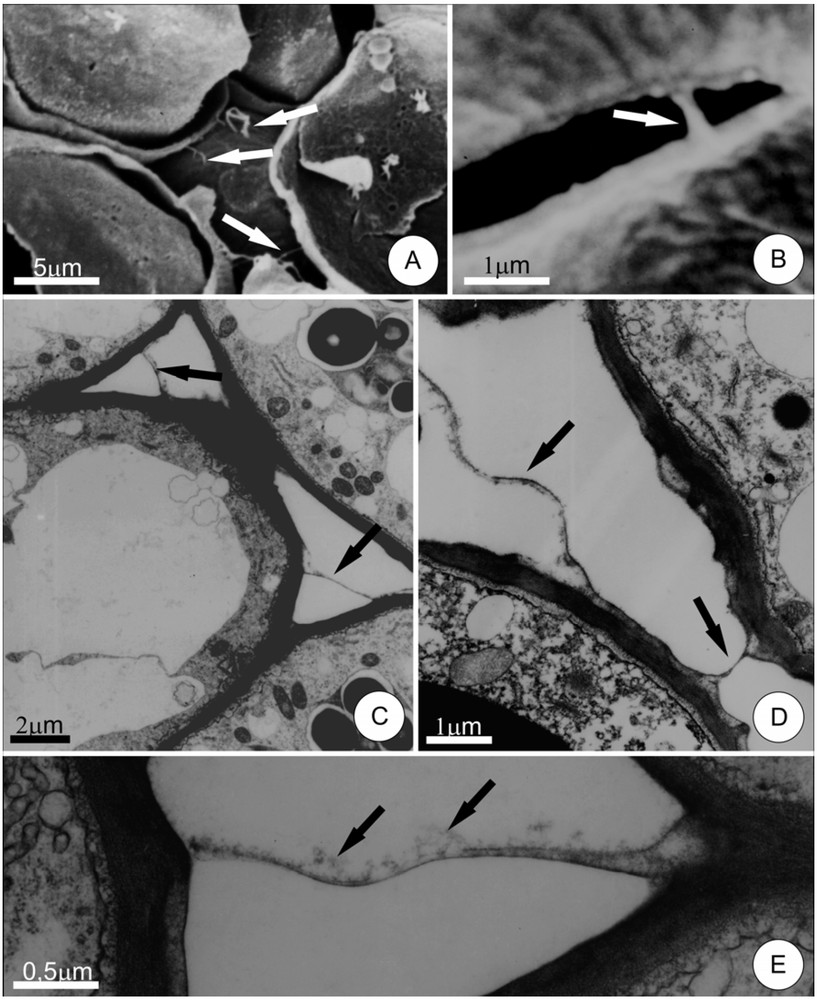
Pectic protuberances between cells in the secretory tissue of floral nectary of Hymenaea stigonocarpa. A, general view of the secretory tissue, as seen by scanning electron microscopy; arrows indicate the location of a pectic protuberance. B, detail of a filamentous intercellular pectic protuberance (arrow) linking adjacent cells. C and D, intercellular pectic protuberances (arrows) viewed under transmission electron microscopy. Note dilation at the extremities of the protuberances. In E, note the presence of flocculated material, probably secretion residues, along the protuberance (arrows). Masquer
Pectic protuberances between cells in the secretory tissue of floral nectary of Hymenaea stigonocarpa. A, general view of the secretory tissue, as seen by scanning electron microscopy; arrows indicate the location of a pectic protuberance. B, detail of a ... Lire la suite
Neither the cells of the mesophyll nor the secretory parenchyma of the floral nectaries demonstrated plasmodesmata directed towards the IPPs, nor was any other cell wall specialization observed that would indicate its involvement in the liberation of components to construct the protuberances. Likewise, there was no evidence of abnormalities or preferential disposition of cytoplasmic organelles near the IPPs.
There was a negative correlation between the length and the diameter of the IPPs in both the leaf mesophyll and secretory parenchyma of the floral nectary – and the shortest IPPs tended to be the thickest.
The IPPs linking secretory cells of the floral nectaries demonstrated amorphous and flocculated material (Fig. 2E). This material may be secretion residues, once its presence is coincident with the secretory stage of nectary.
4 Discussion
The pectic protuberances of H. stigonocarpa are found in cells that are characterized by intense metabolic activities that require exchanges with the apoplast. The presence of elevated potassium levels within the IPPs of the palisade cells (see Carr et al. [2]) indicates that these structures act as functional links in apoplastic transport, as this ion is known to be highly mobile within the xylem.
The formation of scalae between palisade cells of H. stigonocarpa leaf appears to result from the distancing of the cells from each other during leaf expansion and formation of intercellular spaces. According to Carr and Carr [8], the cylindrical palisade cells establish contact with each other along tangential lines, which results in the formation of planar networks (scalae-like, as per Potgieter and van Wyk [4]) during expansion and their concomitant separation.
Cell walls are modified during their expansion phase (see [16]) and, in some cases (as in fruit maturation), the intercellular spaces become filled with a pectin solution [17], apparently due to the dissolution of the middle lamella through enzymatic reactions. As such, it is possible that the physicochemical alterations of the middle lamella accompanying cell expansion and separation are linked to IPPs formation.
The degree of connection between mesophyll cell walls affects lateral water transport. According to Romberger et al. [18], reduced contact area between palisade cells reduces lateral transport. In this way, IPPs can make this transport more efficient, especially in terms of solutions originating in the xylem. Our results indicate that the IPPs in a scalae pattern in the mesophyll of H. stigonocarpa facilitate lateral transport by increasing contact between cells. It is important to emphasize that mesophyll cells absorb water and salts from the apoplast, and the efficient translocation of this solution depends on the continuity of the cell wall matrix. Potgieter and van Wyk [4] and Heide-Jorgensen [9] indicated that the IPPs can aid in lateral water conduction by increasing the cell wall surface area. Thus, the presence of ramifications in the IPPs, as observed in the mesophyll cells of stigonocarpa, amplifies the cell surface area and may facilitate apoplastic transport by amplifying the contact surface and the volume of cell wall active in this transport.
During the period of active secretion in the floral nectary of H. stigonocarpa, nectar is secreted into the space between the hypanthium and the stipe by way of stomata on hypanthium epidermis [19]. Ultrastructural examination of the secretory cells performed by Paiva and Machado [19] indicated that the nectar is initially secreted into the intercellular spaces and flows through these spaces until they are liberated through the stomata. It is possible that the IPPs connecting the secretory cells serve as a matrix for nectar movement through the apoplast, as Romberger et al. [18] proposed for liquid transport through the apoplast. The presence of IPPs in the hypanthium parenchyma was also observed by Carr and Carr [8] in species of Eucalyptus.
Although the floral nectaries of H. stigonocarpa are generally well vascularized, they contain large quantities of secretory parenchyma that is practically devoid of xylem elements [19]. According to Lüttge [20], the movement of water to the nectaries by way of the xylem dilutes the nectar solution and avoids plasmolysis of the secretory cells. The water transported by the xylem must therefore move from the cortical region of the hypanthium to more internal regions where the secretory parenchyma is located, and in order that solutes transported by the xylem reach areas relatively distant from the tracheal elements, there must be contact between the cells – in this case amplified by the IPPs (and reinforcing their role in nectar transport).
The IPPs observed in H. stigonocarpa apparently originate in the middle lamella, and there is no evidence that they are later modified through the actions of the cells that they interconnect – and their formation thus represents a purely mechanical event resulting directly from cellular expansion. The placement of the IPPs, occupying the same positions as did the middle lamella before cell expansion, suggests that these structures are formed from this region of the cell wall during cell expansion, as was proposed by Carr et al. [2] and by Potgieter and van Wyk [4]. The longest IPPs are the thinnest, which reinforces the previous hypothesis, as their thickness appears to depend directly on the degree of expansion to which they were subjected. The junction of short protuberances forming filaments that cross the intercellular space was observed by Davies and Lewis [3], reinforcing that evidence that cellular expansion constitutes the driving force necessary for the formation of IPPs. Evidently IPPs only develop if cell expansion leads to formation of the intercellular spaces, as we observed in the present study.
During cell expansion, the pectin matrix of the middle lamellae is physically and chemically modified. Enzymatic degradation of pectin is precisely targeted [21] and the stresses that tend to separate cells are not distributed evenly over the cell surface, but are concentrated at the cell corners (tricellular junctions) and at the corners of intercellular spaces [22]. Precisely at these points, there are reinforcing zones that can be distinguished under the electron microscope and differ in polymer composition from both the primary cell walls and the middle lamella [23]. These changes in middle lamellae must be involved in IPPs formation, but this process is still few studied.
Leroux et al. [6] reported that the intercellular pectic protuberances observed in Asplenium spp. were not derived exclusively from the middle lamella, because (among other reasons) these structures are not joined to the cell walls of adjacent cells. On the other hand, the IPPs of H. stigonocarpa are seen to be continuous with the adjacent cell walls, which is compatible with the hypothesis that they are derived from the middle lamella, and suggests that they are derived from the separation of these cells by cell expansion, drawing the pectic compounds from the middle lamella to form bridges between the cells.
Machado and Sajo [5] presented evidence for the participation of protoplasts in the synthesis of IPPs in Paepalanthus superbus (Eriocaulaceae), as did Butterfield et al. [24] for Cocos nucifera, and Veys et al. [25] for some species of Azolla. However, in these cases the protuberances were voluminous, not forming strands or scalae. Importantly, in these cases, there was no evidence that these protuberances were derived from the middle lamella as a consequence of cellular expansion – but rather they were formed after the development of the intercellular spaces and required the active participation of protoplasts (see [22]). Miller and Barnett [26], in their study of sitka spruce (Picea sitchensis), verified the presence of projections on the surface of callus cells where these were not in contact with other cells. In this way, pectic protuberances appear on the outer surface of the cell walls of tomato callus cells in a graft union, according to Jeffree and Yeoman [27] in about 48 h after grafting. Barnett and Weatherhead [28] relate an increase of protuberance numbers on the surfaces of Picea sitchensis callus cells with the age of these cells, reinforcing the idea that, in this case, the protuberances are formed independently of cell expansion.
In light of these apparently contradictory observations concerning the origins of the different types of IPPs, it appears reasonable to assume that there is no single ontogenetic process linked to their formation, and Leroux et al. [6] observed that “pectic protuberances with a similar morphology are not necessarily ontogenetically, chemically or anatomically identical”. Short projections that do not seem to connect adjacent cells appear to result from de novo synthesis by protoplasts see [5,6,21,23,24]; filamentous projections (as observed in H. stigonocarpa) are probably derived from the middle lamella, as proposed by Carr et al. [2], Carr and Carr [8], Carr et al. [10], and Parameswaran [29], and supported by ontogenetic evidence [4].
Our results (like those of Carr et al. [2]) suggest that the principal functions of the IPPs of H. stigonocarpa are related to amplification of intercellular contacts and the promotion of lateral transport – both in the leaf mesophyll and the hypanthium.
Acknowledgements
The authors thank FAPESP (Brazil) for its financial support of this work (Programa Biota 00/12469-3), and the technical team of the Centro de Microscopia eletrônica, Instituto de Biociências, UNESP Botucatu, for their help in preparing the samples. S.R. Machado received a research grant from CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil).


