1 Introduction
In taxonomy, species identification may rely on the morphospecies, or typological, concept which has been used since the beginning of systematics [1]. This concept has been commonly used in palaeontology and zoology for a lot of species described only on the basis of diagnostical morpho-anatomical characters. However, the recent development of various other systematic techniques (DNA molecular sequencing and morphometrics) and new diagnostic characters like karyotypes or chromosome banding patterns have allowed the description of new species [2–4]. It has been reported that karyotyped data can provide good evidence of probable reproductive isolation at specific level, without any sign of morphological discrimination even by more sophisticated methods of morphometrics [5,6]. In other cases, analyses of size and shape by conventional or geometric morphometrics have suggested specific separation [7–10]. In West Africa, the multimammate rat Mastomys is the dominant genus from the rodent communities [11–13]. Using its exceptional ability to adapt and reproduce, this rodent is generally found in all habitats from houses to forests. The taxonomy of the genus has been problematic due to the high morphological convergence between the species which prevents correct assignation to a specific level by traditional systematic analyses [14]. The determination key of Mastomys is based on various morphological features like the mammae number (between 5 to 12 pairs); sperm and penis morphology and dimensions [15,16]; karyotype characters such as the diploid number (2N); and the fundamental number (FN). The use of cytogenetic and molecular tools has recently highlighted presence of many sibling species within the genus, especially in West Africa [14]. There is now a consensus to consider M. natalensis (
2 Material and methods
2.1 Specimens
A total of 133 genetically determined (DNA sequencing for cytochrome b) specimens were used for the morphometric analyses. They were classified into three taxa: M. huberti (
After capture, each individual was measured, weighed and sexed. Age class was determined on the basis of body weight following Leirs [29], Granjon et al. [30] and Lalis et al. [31]. The samples were deposited in the collection of the National Museum of Natural History (France NMNH). As we did not find either significant sexual dimorphism differences between groups (2 way ANOVA,
2.2 Geometric morphometrics analyses of size and shape
Three-dimensional coordinates of 25, 35 and 21 landmarks on the dorsal, ventral and lateral sides of skull, respectively, were acquired using a Reflex Microscope (Reflex Measurements Ltd.) (Fig. 1). Coordinates were superimposed using a generalized Procrustes analysis algorithm (GPA) [32,33]. We investigated size and shape parameters separately and pooled both to analyse the form of skull. Size was eliminated by dividing coordinates by the centroid size, which corresponds to the square root of the sum of the squared distances between the centre of the object and each landmark [34]. Residuals of superpositions (= Procrustes residuals) together with centroid size constitute the shape and size variables respectively used in the univariate and multivariate statistical analyses. Possible effects of sex and age were investigated and possible errors of measurement were detected by a preliminary Principal Component Analysis (PCA) of Procrustes residuals. The analyses were done by pooling the sides.
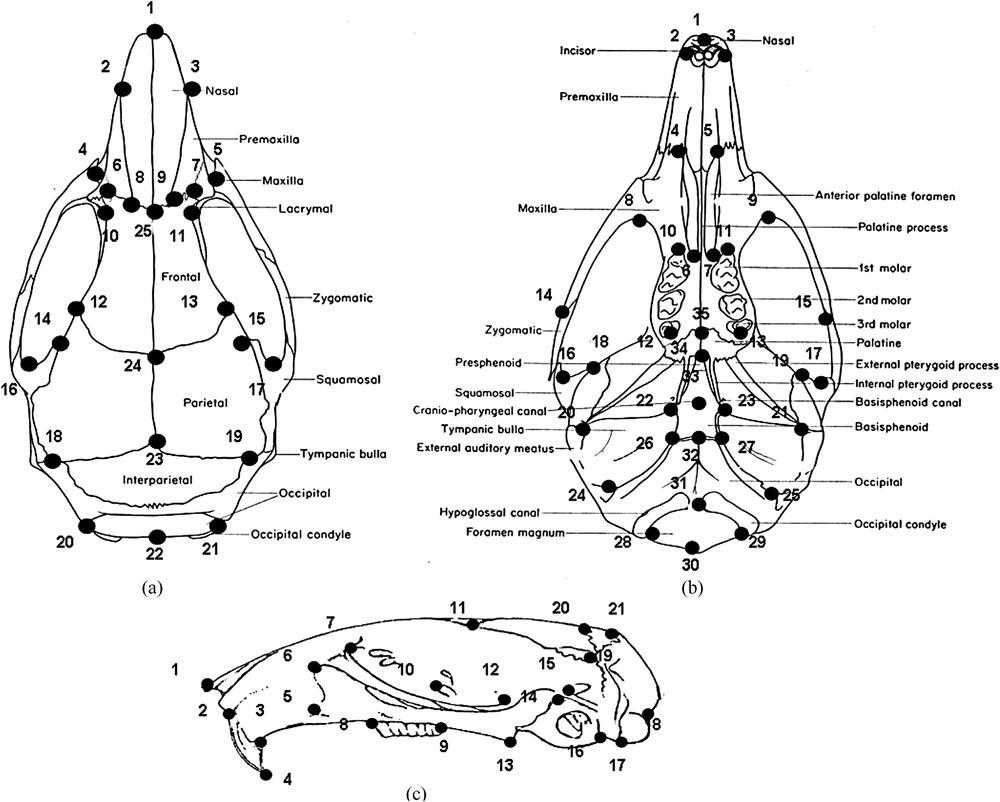
Landmarks location for (a) the dorsal, (b) the ventral and (c) the lateral views of the skull. Dorsal view: tip of the nasals (1), most anterior points at nasal–premaxillary suture (2, 3), anterior projection of zygomatic (4, 5), intersection of premaxilla and frontal (6, 7), nasal–frontal suture (8, 9, 25), suture of premaxilla and maxilla over lachrymal capsule (10, 11), frontal–parietal suture (12, 13, 24), most medial point at interorbital constriction (14, 15), back of zygomatic notch (16, 17), intersection of parietal–interparietal and supraoccipital sutures (18, 19), back of the lateroccipital protuberances (20, 21, 22), midpoint of parietal interparietal suture (23). Ventral view: tip of the nasals (1), antero-lateral extremity of left or right incisive alveolus (2, 3), anterior palatine foramen (4, 5), posterior palatine foramen (6, 7), back of zygomatic plate (8, 9), intersection between left or right anterior end of molar and mandible (10, 11), intersection between left or right posterior end of molar and mandible (12, 13), suture between jugal and squamosal in the left or right zygomatic arch (14, 15), front of glenoid fossa on squamosal root of zygomatic arch (16, 17), suture between left or right parietal and squamosal (18, 19), back of external opening of auditory bullae (20, 21), lateral points of sphenoccipital suture (22, 23), styloinastoid foramina at the posterior border of external auditory meatus (24, 25), anterior extremity of occipital (26, 27), posterior intersection between foramen magnum and occipital condyle (28, 29), posterior extremity of foramen magnum (30), anterior extremity of foramen magnum (31), anterior extremity of occipital (32), cranio-pharyngeal canal (33), contact point between palatine and presphenoid (34), contact point between maxilla and palatine (35). Lateral view: tip of nasal (1), upper point of incisors (2), margin of the alveolus at the back of incisors (3), tip of incisors (4), inferior margin of the infraorbital foramen (5), front of the zygomatic plate (6), anterior point at maxillary root of zygomatic (7), front of first molar (8), back of toothrow (9), dorsal intersection of alysphenoid and maxillary (10), lateral point at parietal–frontal suture (11), front of the squamosal root of the zygomatic (12), tip of pterygoid process (13), upper and inferior point at the posterior margin of the hamular process squamosal (14, 15), anterior and posterior extremity of occipital (16, 17), point above occipital condyle (18), interparietal–occipital–squamosal interaction (19), front of interparietal (20), back of interparietal (21). Masquer
Landmarks location for (a) the dorsal, (b) the ventral and (c) the lateral views of the skull. Dorsal view: tip of the nasals (1), most anterior points at nasal–premaxillary suture (2, 3), anterior projection of zygomatic (4, 5), intersection ... Lire la suite
2.3 Statistical analyses
Differences in the log of centroid size of taxa were depicted by boxplots and their significance tested with analyses of variance (student t-tests and ANOVAs) and pairwise comparisons using Bonferroni tests. Canonical Variate Analyses (CVA) has been used to characterize and analyse the shape variability among taxa; Multivariate Analyses of Variance (MANOVAs) to test the significance of differences between taxa. To visualize the shape differences, deformations along factorial axes were calculated by multivariate regressions [35]. In order to facilitate the visualization of shape differences, deformations were amplified by a factor of three. Classification percentages were estimated by multiple discriminant functions using shape parameters and leave-one-out cross validations [36] to test the validity of the a priori taxonomic assignments. Due to the relatively small sample sizes and the large number of variables (81 three-dimension landmarks), statistical analyses of shape were done using the dimension reduction approach advocated in Baylac and Friess [37]. We tested and removed allometries in shapes by a regression between the CVA projections and the log centroid size. The overall phenotypic similarities between taxa ware depicted using a neighbour-joining tree (NJ) computed from the matrix of Mahalanobis's
3 Results
3.1 Size
Log centroid-size of the three taxa differed significantly for all views (dorsal side:

Skull size variability among taxa. Boxplots of the sum of dorsal, ventral and lateral log centroid sizes. The analyses were done by pooling the sides. Boxplots present medians and 25 and 75 percentiles; limits are the 95 confidence intervals. ERY1: Mankountan; ERY2: Bantou.
The link between size and shape is not significant (
3.2 Shape
Shape analyses were realized using the 15, 17 and 12 first principal components (PCs) extracted from Procrustes residuals which explained 78.4, 74.2 and 68.4% of the total variance for the dorsal, ventral and lateral sides.
MANOVA results revealed highly significant differences between taxa for the three pooled sides (
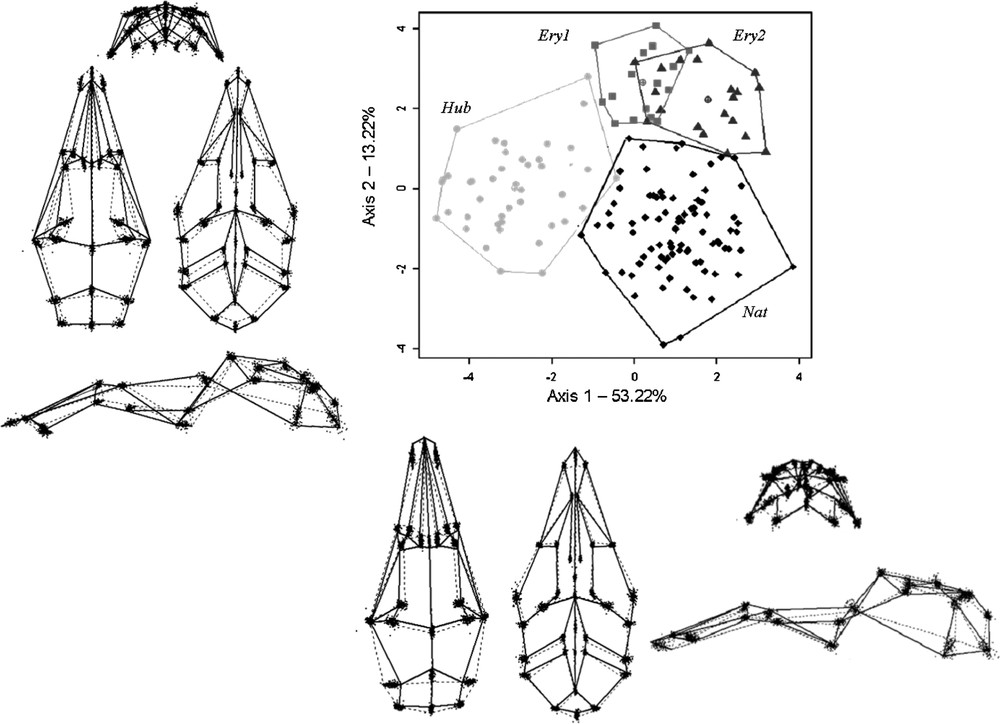
Shape variability among taxa: first two axes of the CVA, computed for the three pooled sides of skull (for 21 PCs included). Light grey: M. huberti; dark grey: M. erythroleucus; black: M. natalensis. Shape differences along the CVA axes one and two. Solid lines depict shapes on the positive side of the CVA axis, dotted lines on the negative one.
Classification results of the discriminant analysis by species.
| Actual group | N | Predicted group membership | ||
| HUB | ERY | NAT | ||
| HUB | 43 | 36 (83.7%) | 3 (6.9%) | 4 (9.3%) |
| ERY | 60 | 1 (1.7%) | 57 (95%) | 2 (3.3%) |
| NAT | 30 | 2 (6.7%) | 1 (3.3%) | 27 (90%) |
According to the NJ tree based on Mahalanobis distances, M. natalensis and M. huberti seemed to be morphologically more similar to each other than they are to M. erythroleucus (Fig. 4).

Overall shape differences among taxa: Neighbour-joining tree of the Mahalanobis
4 Discussion
Geometric morphometrics analyses clearly demonstrated that the three species largely overlapped in centroid size but exhibited large differences in skull shape. The results of skull shape analyses showed also that M. natalensis and M. huberti fit the classic definition of sibling species because they seem to be morphologically more similar to each other than they are to M. erythroleucus. According to Duplantier [20] has a significantly smaller mandible than the two other species while the dental rows of M. erythroleucus were higher than either M. natalensis or M. huberti. Three other works have also been more or less successful in attempting to discriminate between sympatric species of Mastomys. First, Dippenaar et al. [39] in South Africa found similar arithmetic means of skull measurements between M. natalensis and M. coucha. In the Ethiopian rift valley, Lavrenchenko et al. [40] studied three sympatric species of Mastomys using classical PCA methods on skull distances. The authors showed that M. natalensis was separated from a group made by M. erythroleucus and M. awashensis representatives. However their study was limited by the number of specimens used (only 4 M. natalensis). Finally, Bronner et al. [41] showed that the inclusion of subadult specimens reduced a posteriori classification accuracy below 95% confidence levels between M. natalensis and M. coucha in South Africa, implying that the age-related variation is sufficiently pronounced to obscure interspecific craniometric differences.
Our results suggest that the three species can be differentiated owing to their skull shapes with a high probability (90%). However, we did not analyze the overall intra-specific variability, as only one site of trapping was used for M. huberti and M. natalensis. Indeed, our results within M. erythroleucus samples suggest potential high intra-species variability.
The main shape changes between species concerned the height of braincase, the length of the rostrum and the larger size of the zygomatic bar, possibly implying a modification in the attachment of masseter muscles. These anatomical elements are important for the biomechanics of mastication [42,43]. It has been shown that diet is an important selective factor acting upon the evolution of skull morphology [35,44,45]. Hence, a source of variability could result from competition for food. This competition could act as a prime selective pressure among sympatric species and could possibly allow for character displacement between and within Mastomys species. Very few data concerning Mastomys feeding behaviour are available. In general, the three species are characterised by their opportunism from their omnivorous-granivorous feeding habits [46]. The feeding habits of the three species are very similar but they distinguish themselves in the details, each species consuming more particularly the food available in its micro-habitat [47]. In Mankountan, M. huberti is primarily observed in swamps (95.1%); M. natalensis occupies houses in Bantou (64.1%); M. erythroleucus is primarily found in houses at Mankountan (84.3%) and in fields at Bantou (89.6%). Smithers [47] observed in the field that M. natalensis primarily eats grass and grains. Kingdon [48] showed that this species can feed itself with animals (insects) or vegetation. Jackson and Van Aarde [49] showed evidence in which M. erythroleucus was able to adapt itself to the less demanding feeding habits of the two other cogeneric species. M. erythroleucus would then present an adaptive capacity higher than the two other species. M. erythroleucus shows a broader and higher skull shape with a length and strength greater than that of M. huberti or M. natalensis. It is possible that this more resistant and adaptive feeding habit would require more from the chewing muscles, leading to a more developed insertion zone. Furthermore, the competition among available resources could explain the high variability in skull size and shape for M. erythroleucus in Bantou. Similar observations have already been made for populations of the European wood mice Apodemus sylvaticus and flavicollis [50], in Murinae [51], and in Tanzanian M. natalensis populations [52]. Furthermore, it is interesting to note that a study has compared several samples of M. natalensis issued from all Africa and has showed that in Tanzania, when not in sympatry, the M. natalensis are larger (Denys, pers. com.). These results clearly confirm that there is a high intraspecific variability at a local scale in Mastomys.
In conclusion, the present geometric morphometrics study based on skull shape allowed us to partially discriminate three morphological groups that are congruent with the three species suggested by molecular identification. In Mastomys, the evolution of cranial length and shape could be interpreted as a link to the competitive pressure between close species by ecological segregation. We confirm that selection may play a crucial role in the evolution of skull shape. Future studies on Mastomys species should be focused on ecological partitioning of the habitats and diets in the three sibling species of Guinean Mastomys. The cranial characteristics using the statistical formalism of geometric morphometrics are sufficient to be a valid identification criterion in such a case of sibling species, a condition previously reached by Dobigny et al. [19] and Cordeiro et al. [21]. It is only a combined set of characters (external, skull, dental morphology) that allows determination of the species with 100% confidence. The integration of all these techniques (molecular, cladistic, phylogenetic and cytogenetic) together provides a powerful tool in allowing identification of sibling species. In the future, the Barcode of life initiative may also represent a good alternative to solving the problems of sibling species identification [53,54].
Acknowledgements
We would like to thank the field staff: Project PFHG Guinea. We are grateful to C. Houssin, A. Delapré, F. Kourouma and P. Guilavogui for the preparation of specimens. We thank M. Baylac for the use of morphometric platform. This work was supported by the European Community (INCO-DEV grant ICA4-CT2002-10050).


