1 Introduction
Hydrocarbons (HCs) accumulated on the body surface of insects not only protect against desiccation, but are also used for chemical communication [1]. In particular, social insects use cuticular hydrocarbons to distinguish nestmates [2–5]. During encounters, workers compare their memory of the colony odour, which is referred to as the “template”, with the odours of the others, termed the “cues”. The template is generally thought to be formed through the perception of the hydrocarbon mixture generated by trophallaxis and allogrooming among the colony members (Gestalt model) [6]. When one worker encounters another, it responds according to the difference between its template and the odour spectrum of its counterpart.
Although mature individuals of social insects from different colonies can discriminate each other in confrontations, newly emerged workers, or callows, are accepted by members of other colonies or even by those of other species, with the probability of acceptance declining with age [7,8]. The lack of aggressiveness of older workers against callows has been attributed to the small amount of cuticular hydrocarbons carried by the callows [4,9]. The low quantity may fall below some threshold of hydrocarbon profile difference needed to trigger aggression. The hypothesis that non-aggressiveness is based on the low quantity of hydrocarbons was termed “chemical insignificance” [4], and was later referred to as the “blank hypothesis” in the case of bees [10]. In addition to chemical insignificance, the immobility of newly-emerged callows may contribute to their acceptability [5]. Hence, studies on the ontogenetic differentiation of chemical profiles will help us to understand nestmate recognition.
Cuticular hydrocarbons involved in the colony-specific odours are at least partially derived from hydrocarbons stored in the postpharyngeal gland (PPG) [11]. These substances are originally produced in adipose tissue and transferred to the PPG with hemolymph lipoproteins [1,12]. Lipids in the adipose tissue are also used for the development of eggs in the ovary. Thus, we may expect either: (1) that the quantity of hydrocarbons on the cuticle and/or the PPG is correlated with the degree of ovary development; or (2) that, conversely, lipids in the adipose tissue that are used in ovaries are no longer available for the cuticle or PPG. In this case, one might expect a negative correlation between egg development and cuticular/PPG hydrocarbons.
The hydrocarbons in the PPG are exchanged among nestmates through trophallaxis, allogrooming, and body contact, which leads to the averaging of the chemical profiles of individual colony members, according to the Gestalt model [13–17]. In this model, the non-aggressiveness towards callows due to their low levels of HCs seems to be counterintuitive; if workers frequently perform these behaviours, callows ought to have a normal level of hydrocarbons. One explanation is that the immobility of callows noted by Vander Meer and Morel [5] may reduce the exchange or reception of hydrocarbons, keeping the quantity of hydrocarbons low. Alternatively, callows may have age-specific substances that suppress aggression from older workers, as hypothesised by Jaisson [18]. Age-specific hydrocarbons, if any, should disappear as the callow ages.
Analysing the quantity and composition of cuticular and PPG hydrocarbons with gas chromatography, we examined the ontogenetic differentiation of chemical profiles in workers of the myrmicine ant, Aphaenogaster senilis, aged 0 to 60 days after emergence. We predicted that HC quantities would increase with age. Orphaned workers lay haploid eggs [19], and we predicted HC quantities would be correlated with ovarian development. Although the functions of individual hydrocarbons are not yet clearly known, we examined the HC profiles for hydrocarbons characteristic of callows. It has been shown that individually isolated, mature ants change their HC profile and are not recognised by their nestmates after a few days [20,21]. We examined the effects of the early social environment by rearing workers in isolation. Aphaenogaster workers are known to exchange their hydrocarbons by allogrooming, not by trophallaxis [21,22], and thus require a longer period for gestalt establishment [16]. Therefore, this species may be less sensitive to isolation.
2 Materials and methods
2.1 Preparation of the experiments
We used three laboratory colonies of Aphaenogaster senilis, which were collected in southern Spain in May 2002. These colonies had been maintained at temperatures of 28 °C and humidity of 50% under a photoperiod 12L:12D. The ants were given live calliphoride larvae three times a week and honey solution for bumblebees and oranges once a week.
Two nests with 250 workers and 30 or more pupae were made from each of the 3 colonies (6 nests total). When workers emerged from these pupae, they were marked with a felt pen. These workers were either released in the natal nest (grouped workers) or placed individually in a glass tube with water-dampened cotton (isolated ones). Four marked callows from each of the two conditions were killed by freezing at the following ages: 0, 1, 2, 5, 10, 15, 20, 30, 40, 50 or 60 days. Preliminary observations indicated that 60-day workers can be considered mature. For each worker, we dissected the abdomen under a binocular microscope to count the number of eggs in the ovarioles. We also observed the colour of the poison gland as it represents a simple physiological maturation not influenced by social isolation. Most dissected workers had two ovarioles, and there were only two workers with three or four ovarioles. Hence, the ovariole number was not used for any comparison in this study. The colour of the poison gland was assigned to one of three classes (dull white, clear yellow to orange, and dark brown). The frequency of each colour was calculated for each age after emergence. The thorax with legs of dissected workers was individually put in 1 ml pentane to extract the cuticular hydrocarbons and after 5 min the thorax was removed. The extract was left at 25 °C for evaporation of the solvent and kept at −21 °C until chemical analysis. A. senilis is monomorphic but presents some size variations [23]. Therefore, to prevent any bias with size, head width was measured under a binocular microscope, and the head was dissected to obtain the two postpharyngeal glands (PPG). The glands were immersed in 1 ml of pentane and treated in the same way as the cuticular hydrocarbon extracts.
2.2 Chemical analysis
When the extract was analysed, it was dissolved in 50 μl of pentane containing 50 ng of C20 as internal standard, and 5 μl of the dissolved extract was applied to a gas chromatograph (VGM250Q coupled with a TurboChrome Workstation) using a DB-5 fused silica capillary column. Helium was used as the carrier gas at a constant flow rate of 0.1 ml min−1. The injector was at 220 °C and the detector at 330 °C. Temperatures were programmed to be kept at 150 °C in the initial two minutes, increased from 150 °C to 300 °C at 5 °C/min for the following 30 min and kept at 300 °C for the last 12 min. The hydrocarbon pattern was constituted of 32 previously identified peaks [21]. The quantity of individual substances in each worker was estimated by peak integration in comparison to the internal standard, and the proportion of each peak to the total of the 32 peaks was calculated.
2.3 Statistical analysis
Since we picked workers from the same nests throughout this study, to prevent pseudo-replication problems, we used repeated measures ANOVA to examine whether the egg number in ovarioles varied with social conditions (grouped or isolated) and worker age (day after emergence). The numbers were log-transformed and added to 1 to avoid the zero value for workers without eggs. Worker aging was examined by assuming that the colour of the poison gland would become darker with age and then analysing the colour frequencies with repeated measures ANOVA. We also used repeated measures ANOVA to examine the effect of social condition and worker age on the total quantities of cuticular or PPG hydrocarbons, with each datum being log-transformed. Finally, we evaluated the influence of social condition and worker age on the proportions or the quantities of each hydrocarbon on the cuticle or in the PPG by MANOVA. Arcsine transformation was used for the proportions and log transformation for the quantities. We treated the colonies as replicates.
3 Results
Head width was not significantly different between grouped and isolated workers or between days after eclosion (ANOVA,
3.1 Development of ovary and poison gland
In both grouped and isolated workers, the first eggs were found in the 2-day worker ovaries. The egg numbers were significantly different between ages (
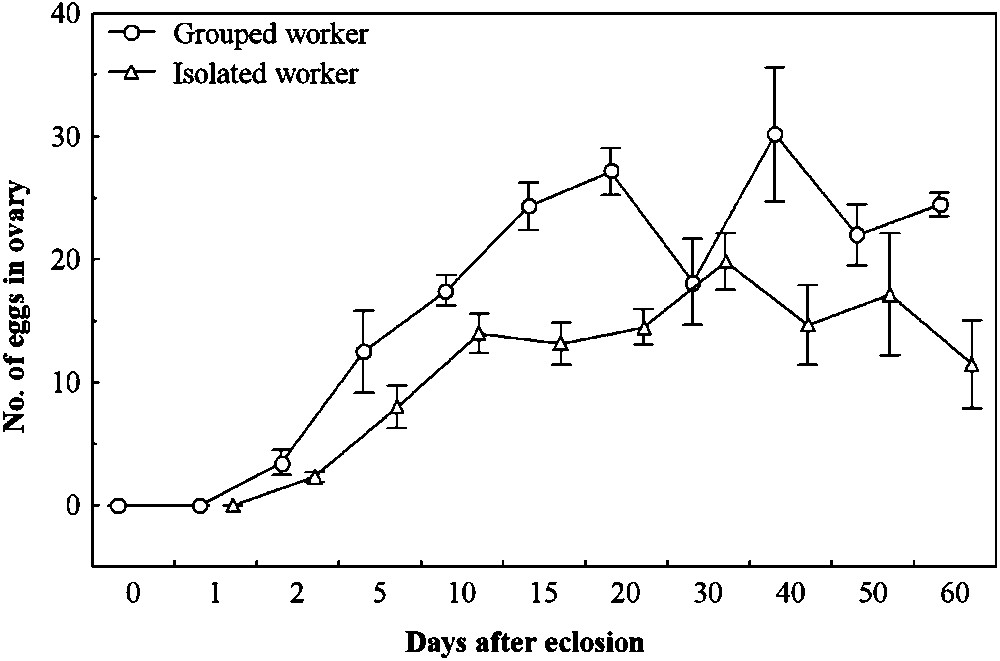
Total number of eggs (mean ± SE) in ovarioles of workers maintained in the natal nest (circles) or isolated (triangles) from eclosion until 60 days of age.
In both grouped and isolated ants, the poison glands were white until the 10th day. Thereafter, the proportions of yellow glands increased. The frequencies of yellow poison glands increased rapidly until day 15, and all workers had yellow poison glands after 50 days (Fig. 2, top). A brownish poison gland is characteristic of older workers, particularly foragers (unpublished data), and therefore we did not find any in the 60-day workers. As predicted (see Fig. 2, bottom), the maturation of the poison gland is not influenced by the social condition, indicating that the physiological maturation of the isolated ants was not modified.
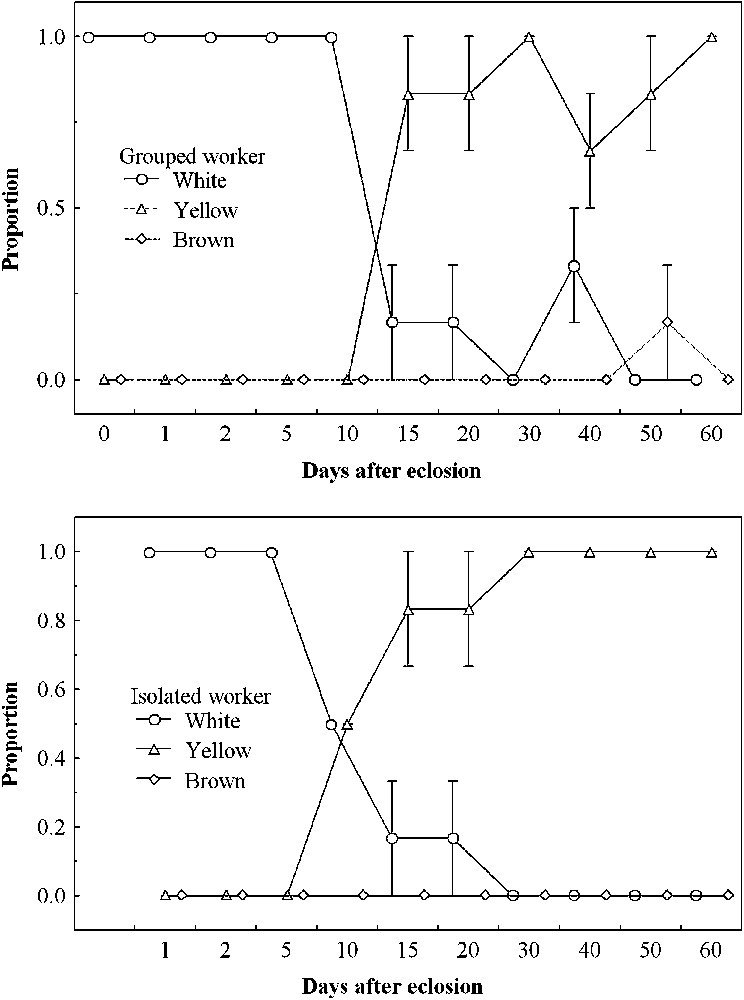
Proportions (mean ± SE) of three colour classes of the poison gland in workers from eclosion until 60 days old under the grouped (top) and isolated conditions (bottom).
3.2 Temporal changes in cuticular and PPG hydrocarbons
The total quantity of cuticular hydrocarbons increased from 1 to 3–4 microgrammes both in the grouped and isolated workers until 15 to 20 days after emergence and subsequently remained stable (Fig. 3, top). It was not significantly different between the two groups (
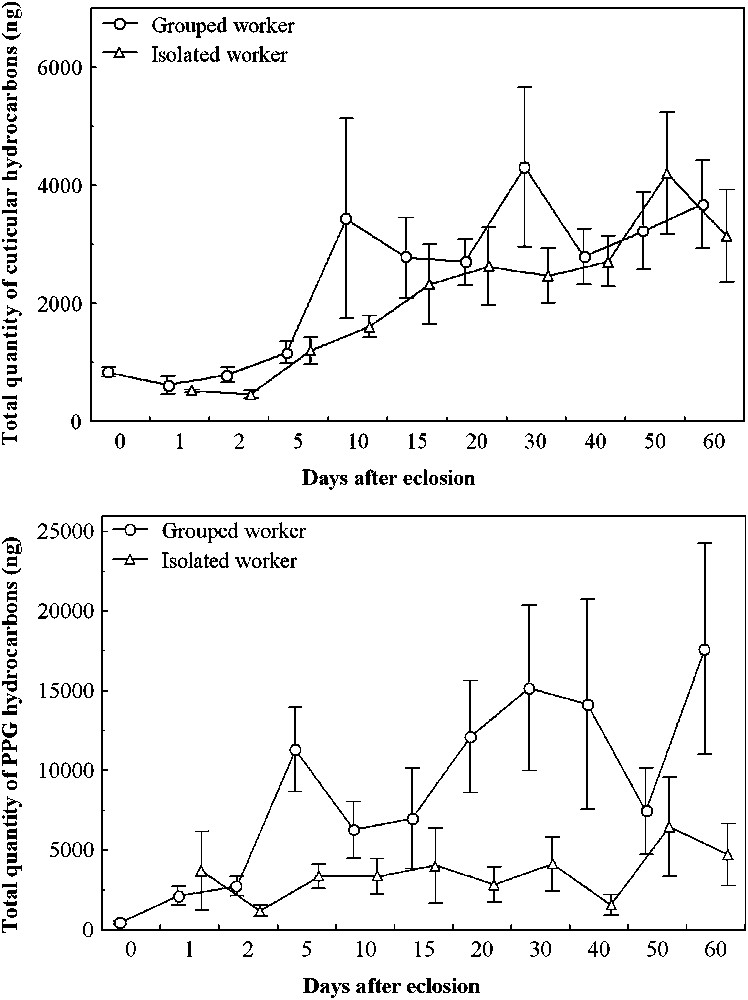
Total quantity of the cuticular (top) and postpharyngeal gland (bottom) hydrocarbons (mean ± SE) of workers grouped (circles) or isolated (triangles) from eclosion until 60 days old.
The PPG hydrocarbon quantities also increased from less than 1 to 15 microgrammes in grouped workers, but remained low (4–5 microgrammes) in isolated workers (Fig. 3, bottom). The total quantity of PPG hydrocarbons was significantly different between grouped and isolated workers (
3.3 HC quantities and ovarian development
A significant, positive correlation was obtained between the total egg number and the quantities of both the cuticular (
3.4 Rearing and age effects on individual hydrocarbons
MANOVA performed on all cuticular hydrocarbons showed that both the proportions and quantities of hydrocarbons on the cuticle differed significantly with worker age, but were not influenced by social condition (Table 1). In contrast, the quantities of hydrocarbons on the PPG were significantly influenced by the social condition in addition to worker age, while the proportions were not significantly influenced by either social condition or age. The interaction of condition and age was not significant in any comparison. We were not able to differentiate any hydrocarbon that was characteristic of callow workers.
MANOVA performed on the individual HCs proportions and quantities on the cuticle and in the PPG related to the condition of workers (grouped or isolated) and age. In bold significant values.
| Proportion | Quantity | ||||||
| df | MS | F | P | MS | F | P | |
| Cuticular hydrocarbons | |||||||
| Condition | 1 | 0.000 | 0.097 | 0.756 | 3.521 | 1.806 | 0.182 |
| Age | 9 | 0.006 | 2.729 | 0.007 | 24.532 | 12.581 | 0.001 |
| Condition × age | 9 | 0.002 | 0.700 | 0.707 | 0.445 | 0.228 | 0.990 |
| Total | 100 | 0.002 | 1.950 | ||||
| PPG hydrocarbons | |||||||
| Condition | 1 | 0.004 | 1.970 | 0.164 | 165.509 | 36.350 | <0.001 |
| Age | 9 | 0.003 | 1.245 | 0.277 | 10.717 | 2.354 | 0.019 |
| Condition × age | 9 | 0.003 | 1.299 | 0.247 | 4.204 | 0.923 | 0.508 |
| Total | 100 | 0.002 | 4.553 |
4 Discussion
Queenless workers of Aphaenogaster senilis lay haploid eggs which develop into males [19]. Individually isolated workers had poison glands similar to workers that were reared in their natal nest with nestmates and developed their eggs in the ovary (Figs. 1, 2), although the egg numbers were smaller compared to those of grouped workers (Fig. 1). One possible explanation is that grouping allows for better feeding due to cooperation or some other nutritional factors. Another explanation could be that isolated workers lay their eggs more rapidly than grouped ones, and thus had fewer in the ovary when dissected. We did not measure egg laying rates precisely, but initial observations did not confirm this hypothesis. Since ovary development is highly correlated with the production of lipids that are precursors of cuticular hydrocarbons [1], the differences in the ovary development should be associated with a corresponding difference in the hydrocarbons between grouped and isolated workers. We found a correlation between the cuticular and PPG hydrocarbon quantities and egg number in the ovary, an index of fecundity. It is well known that in Diptera, an increase in JH is correlated to vitellogenesis (see for example [24]). In the wasps Polistes dominulus, the size of the corpora allata (the producer of JH) is positively correlated with ovarian development in polygynous colonies [25]. In a recent paper on the ant Myrmicaria eumenoides, it was observed that cuticular HC modifications are related to the JH III concentration changing with foraging tasks [26], but it is probably not associated to fecundity, as these workers are sterile (Martin Kenne, pers. com.). Laying individuals in ant colonies (queens, gamergates) are generally characterised by a specific hydrocarbon profile (see [27,28]), which is considered to be a fertility signal. In the ant Streblognathus peetersi, the vitellogenin level (used as fertility index) in the haemolymph is linked to the HC profile, but no measures of fecundity were given [29]. Our data show for the first time a link between fecundity and hydrocarbon quantities in ants. It will be interesting to measure the HC quantities of fertile individuals in these species with gamergates.
Interestingly, our data showed a difference in the maturation of the cuticular and the PPG HCs, as the latter were modified by individual social isolation. Workers secrete their own self-produced hydrocarbons directly through their cuticle, and this appeared to enable isolated workers to have normal cuticular HC levels. In many species like Camponotus or Cataglyphis, workers exchange PPG hydrocarbons with nestmates through trophallaxis and cuticular HCs through allogrooming [11,13]. A. senilis however do not practice trophallaxis [16], and as allogrooming is absent in individually isolated workers, they cannot fill their PPG normally. Isolation may lead a worker to feed less unless food is distributed ad libitum, and a change in nutrition could be proposed as an explanation for the low quantities of PPG hydrocarbons. It has been shown that isolated workers produce less octopamine, a behavioural stimulant that may induce a nutritional deficit [30]. However, this explanation is unlikely because (1) decreased feeding should also induce a deficit in cuticular HCs and (2) it has been observed in other cases that individual isolation does not modify feeding behaviour [20]. Rather, the effects of social isolation confirm the role of PPG in the gestalt model of nestmate recognition: the PPG serves to store the exchanged HCs. In isolated workers, the gland is not fulfilled.
Our study confirmed that callows have lower quantities of hydrocarbons than mature ants. Callows aged up to 5 days had less than 1 microgram of cuticular hydrocarbons, a quantity increasing in older workers (Fig. 3). Future studies should use more precise measures immediately at emergence, for ex. 6 hours. The low quantities (chemical insignificance) may explain the tolerance by non-nestmates. This seems to be a general characteristic of callow insects [4,9,31–33]. Differences in HC composition between nurses and foragers are well-known [26,34,35], but few studies report the ontogenetic differences in the composition of cuticular and PPG hydrocarbons. We were not able to find a callow-specific substance. In Cataglyphis iberica there are both low hydrocarbon quantities and substances specific to callows [36], but it is the only known example. In various species, the HCs quantities increase with age and stabilise at one month [37,38]. Workers of A. senilis increased the quantity of hydrocarbons both on the cuticle and in the PPG until 15 to 20 days and then remained stable. This closely corresponds to the results obtained in Cataglyphis niger [39]. These results suggest that Aphaenogaster ants need to spend at least two weeks accumulating sufficient hydrocarbons in their PPG for the acquisition of their colony gestalt odour. As this species does not exchange HCs by trophallaxis, the gestalt homogenisation takes longer than in other ants [16]. It would be interesting to observe the behavioural ontogenesis of callow workers through the first 20 days to find when they cease to be immobile. It would be also useful to carry out the same experiments on a species which exchanges hydrocarbons by trophallaxis.
Acknowledgements
This study was performed during Dr. Ichinose's stay in France with a fellowship from the French Government. We thank J.-Ph. Christidès for gas chromatography analyses, Raphaël Boulay and two anonymous reviewers for helpful comments. Gloria Maria Luque and Hannah Reynolds revised the English.


