1 Introduction
In barley and in most Gramineae species, the apex produces leaf and spikelet primordia in chronological order, so that all leaf primordia have been formed when the first spikelet primordium is initiated. Whilst the apex produces spikelet primordia, leaves elongate appearing externally and, therefore, the phasic development of these plants can be described through sequences of changes that occur either internally or externally [1]. Several developmental scales have been elaborated for cereals, but all describe only either apex or plant morphology, and none of them try to find a correspondence between processes that proceed, at least in part, in a coordinated way within the plant [2,3]. Stages of external development are well defined and widely accepted, and the most used scales are that of Zadoks for the entire plant cycle [4] and that of Haun for leaf appearance [5].
Stages of internal morphology are less easy to recognize and descriptions are often not uniform among scales [6,7]. Main internal processes are the initiation of leaf and then spikelet primordia, the differentiation of spikelets and then florets, the end of spikelet initiation and the separation of the spike from the base of the plant (crown). At the time of onset, spikelet primordia can not be distinguished from leaf ones, but authors agree that the beginning of spikelet initiation is associated with the rapid expansion of the growing point [7,8]. The first visible signal that spikelets have been initiated on the apex is the formation of double ridges, but this occurs when around half of the spikelet primordia have already been initiated, with great differences depending on genotype and environmental conditions [9,10]. Differentiation steps of spikelets are lateral expansion, indicated as triple mound in barley, and then the differentiation of glumes [2,7]. The first evidence of floret differentiation is the appearance of stamen initials that is followed by the elongation of awns [6,11]. In barley, also the end of spikelet initiation is difficult to identify. Indeed, the apex initiates an indefinite number of primordia, but the latest-formed do not differentiate into spikelets and die [3,12]. Consequently, the cessation of the activity of the apical meristem does not correspond to the initiation of the uppermost spikelet that is visible at heading.
To link stages of external and internal plant morphology could give deeper knowledge into the coordination of developmental processes in Gramineae species and help plant modelling [3,13,14]. In addition, to estimate apex stage from external signals is determinant for crop management and yield prediction, because it allows identifying sensitive plant stages and the period when potential grain-number is set [7,12,15].
Linear relationships between the number of emerged leaves and the total number of primordia produced on the main culm apex were found for wheat [16–19] and oat [13], but different slopes were reported in dependence on final leaf number and environmental conditions. Hay and Kemp [1] found that the slope changed from the initiation of leaf to that of spikelet primordia, and only the rate of spikelet initiation differed among varieties and in response to sowing date and location. Further attempts to link external and internal plant stages were scarce and, according to Miralles and Slafer [20], any reported relationship is hard to extrapolate due to the different response of leaf appearance and apex stage to major environmental factors. In wheat, Baker and Gallagher [9] and Brooking et al. [17] found that spikelet initiation started during the emission of the fourth leaf (Haun 3–4), whereas Kirby [16] found that it varied between Haun stages 2.4 and 6.5 in dependence on the final number of leaves (FNL). Spikelet initiation ended when leaf-sheaths became erect, according to Baker and Gallagher [9], but in correspondence to the stage of first node detectable, according to Kirby et al. [21]. Rassaa et al. [22] reported that durum wheat plants had five to six leaves at the end of spikelet initiation when well irrigated, but only four to five when water stressed. Finally, George [7] found that, in wheat, culm elongation started at the beginning of spikelet differentiation.
The barley apex differs substantially from that of wheat since it produces an indeterminate inflorescence with determinate lateral branches [11]. Consequently, we expect that also the patterns of primordia initiation and the relationship with leaf appearance may be different in the two species. Miralles and Richards [23] reported that both leaf appearance and primordia initiation proceeded faster in barley than in wheat, but they did not investigate the relationship between the two processes. Moreover, all studies on the coordination between leaf appearance and primordia initiation have overlooked the ontogeny of single leaves before appearance, though it is probable that events occurring at the top and at the base of the growing apex are strongly coordinated [14].
The present research aimed at giving insight into the coordination of the processes of leaf appearance, spike development and stem elongation in barley. In order to do that, between emergence and heading, we determined plant phasic development and the time courses of leaf appearance, primordia initiation and apex differentiation. To test variations caused by genotype and environment, we investigated the performances of three contrasting (winter, alternative and spring) two-rowed barley varieties sown in autumn and in spring.
2 Materials and methods
2.1 Growth conditions
The research was carried out between autumn 2005 and spring 2006 at the Department of Agronomy and Agroecosystem Management of the University of Pisa (43° 41′ N, 10° 23′ E, Tuscany, Italy). The experimental site is located at approximately 15 km from the sea and 1 m above sea level, where climate is a hot, humid Mediterranean, with a cold winter, and an arid summer (Csa, Köppen), and photoperiod ranges from 8 h 46′ to 15 h 14′. For the entire period of the research, the minimum and maximum daily temperatures and daily rainfall were obtained from a weather station located at the experimental site. For the period November 2005–May 2006 the average mean temperature was 10.6 °C and the total rainfall 473 mm. Mean temperature was in line with long-term averages, whereas rainfall was by 22% lower.
The research was carried out in growth containers of 100 L volume (0.25 m2 surface area and 0.4 m depth) placed outdoors and spaced 10 cm in two adjacent rows. Spaces were filled with expanded clay to smooth daily fluctuations in soil temperature. Containers were filled with a field-collected loam soil tamped to about original soil bulk density, and were equipped with a drainage system. Fertilizer application was that conventionally used for barley crops in central Italy. Weed control was performed manually. When necessary to avoid water stress, irrigation was performed by means of sprinkler irrigation.
2.2 Treatments
Treatments were two sowing dates, 4 November 2005 (autumn) and 14 February 2006 (spring), and the two-rowed barley (Hordeum vulgare ssp. distichum L.) varieties Baraka, Ninfa and Tunika, classified as winter, alternative and spring types, respectively. In order to distinguish single plants, the soil surface was covered with a net of 2 cm × 2 cm meshes before seeding. Seeds were manually placed, one per mesh, in three rows spaced 16 cm apart. Final seed rate was 250 seeds m−2.
2.3 Measurements and calculations
The timings of emergence, tillering, first node detectable, booting and heading were recorded following the Zadoks’ scale [4]. These stages were considered uniformly achieved when they were observed on more than 50% of the plants of each container. Between emergence (stage 8–10) and booting (stage 40), plants were harvested at weekly intervals for the autumn sowing, and at 3–4 day intervals for the spring one. At each harvest, four plants per combination “variety × sowing date” were collected, taking two plants from each container. All measurements were carried out on the main culm that was distinguished from tillers by means of a red wool-thread tied around the sheath of the fourth leaf.
On harvested plants, we determined the number of tillers and the leaf stage, the latter as the number of completely emerged leaves plus the fraction of the last emerging leaf-sheath relative to the next older one [5]. After dissection under a stereomicroscope (Leica Z16 APO) with magnification up to 92×, we determined: (i) the number of enclosed leaves; (ii) the number of primordia, identified as leaf or spikelet ones, when possible; (iii) the apex stage, following the B&W code [6]; (iv) the length of the spike; and (v) the distance of the spike from the base of the plant. The total number of leaves was calculated, for each plant, as the sum of emerged leaves, enclosed ones and leaf primordia. We considered as “enclosed”, leaves that were completely hidden within the sheats of emerged leaves and were longer than 1 mm, and as “leaf primordia”, structures with a clear leaf shape that were shorter or equal to 1 mm. The total number of primordia produced on the main culm apex was obtained as the sum of the total number of leaves plus the number of spikelet- and undifferentiated primordia.
At heading, ten plants were collected from each container and the FNL and the number of complete spikelets were recorded. Since the total number of primordia exceeded the sum of final leaves and spikelets recorded at heading, we introduced the concept of “productive primordia” to indicate primordia that effectively developed into mature structures, either leaves or spikelets. Consequently, we indicated as last spikelet primordium the last productive one, and not the uppermost primordium initiated on the apex (maximum primordium). The first and the last spikelet primordium were identified in retrospect by subtracting from the number of total primordia, respectively, FNL or the number of productive primordia.
2.4 Experimental design and statistical analysis
The experiment was arranged in a split-plot design. Sowing date was the main treatment, variety the secondary one, and two containers were used for each combination of variety and sowing date. Replicates were single plants collected from both containers at each harvest. Results were statistically treated by ANOVA, in order to test the main effects of sowing date and variety and their interactions. Means were compared by Duncan's multiple range test (P < 0.05). The stage of Haun was plotted against the number of either total or only productive primordia (leaf + spikelet primordia). For each couple of data series, we calculated the best fitted interpolation curve and the correlation coefficient.
3 Results
3.1 Final number of leaves and spikelets
The FNL recorded on the main culm at heading was approximately 10 in the variety Tunika, and 11 in the varieties Baraka and Ninfa, both for the autumn and the spring sowing (Table 1). Moreover, in no case, we found plants with less than nine or more than 12 leaves. At the same stage, the number of completely differentiated spikelets ranged from 23.3 to 24.9, without significant differences among varieties and sowing dates. Starting from these data, the number of productive primordia (leaves + spikelets) was always close to 35, primordium n. 11–12 developed into the first spikelet and primordium n. 35 into the last one, whereas all primordia initiated after n. 35 aborted during spike differentiation.
Final number of leaves (FNL), of completely developed spikelets and of productive primordia on the main culm of three two-rowed barley varieties sown in autumn and spring. Productive primordia are the sum of final leaves and fully developed spikelets. Data are means ± SE, n = 20.
| Variety | Sowing | Final Leaf Number | Spikelet Number | Productive Primordia |
| Baraka | Autumn | 11.1 ± 0.1 a | 23.6 ± 1.0 a | 34.7 ± 0.4 a |
| Spring | 11.1 ± 0.2 a | 24.2 ± 0.5 a | 35.3 ± 0.3 a | |
| Ninfa | Autumn | 10.7 ± 0.2 a | 23.3 ± 1.0 a | 34.0 ± 0.7 a |
| Spring | 10.5 ± 0.3 a | 24.0 ± 0.8 a | 34.5 ± 0.4 a | |
| Tunika | Autumn | 9.9 ± 0.1 b | 24.9 ± 0.4 a | 34.8 ± 0.5 a |
| Spring | 9.7 ± 0.2 b | 24.5 ± 0.5 a | 34.2 ± 0.3 a |
3.2 Coordination between leaf appearance and primordia initiation
The total number of primordia produced on the main culm apex was strongly correlated (r = 0.97) to the stage of Haun and, for all varieties and sowing dates, the relationship was described with the logistic curve y = 51/(1 + 21 × exp[−0.68 x]) that had an horizontal asymptote at y = 51(x) (Fig. 1). According to this equation, approximately three primordia were already present on the main culm apex at plant emergence, and the maximum number of primordia was reached with the emission of the 10th leaf. The curve also indicated that the initiation of primordia markedly slowed down after the complete appearance of the sixth leaf.

Relationship between Haun stage and total number of primordia formed on the main culm apex in the two-rowed barley varieties Baraka, Ninfa and Tunika, sown in autumn and in spring (n = 250). Data were fitted by non-linear regression (asymptotic) excluding those referring to primordia abortion (n = 231).
By plotting only the number of productive primordia versus the stage of Haun, we found strong linear relations that differed among varieties, but not between sowing dates (Fig. 2). According to the equations of fitted lines, the first spikelet primordium (n. 11–12) was initiated at Haun stage 2.4–2.5 in the varieties Baraka and Ninfa, whereas it started slightly earlier, at Haun stage 2.1, in the variety Tunika. The initiation of the last spikelet primordium (n. 35), occurred at Haun 6.6 and 7.0 in the varieties Baraka and Ninfa, respectively, and at Haun 5.5 in Tunika. The equations also evidenced that the initiation rates of productive primordia, relative to leaf appearance, were similar for the varieties Baraka and Ninfa, respectively 5.5 and 5.1 primordia per emerged leaf, and higher for Tunika, 7.1.

Relationship between Haun stage and number of productive primordia formed on the main culm apex in the two-rowed barley varieties Baraka (A), Ninfa (B) and Tunika (C) sown in autumn and in spring. Data were fitted by linear regression. n = 57 (Baraka), 54 (Ninfa), 45 (Tunika).
3.3 Coordination between internal and external plant development
In order to find a correspondence between crucial stages in primordia initiation and stages of apex and plant development, we described the morphology of the apex and of the entire plant at the beginning of spikelet initiation, at the stage the last spikelet primordium was initiated and when the maximum number of primordia was achieved.
In all varieties and sowing dates, at the time the first spikelet primordium was initiated, the apex of barley had a slightly elongated shape and two to four single ridges were visible (Table 2, Fig. 3A). The cowl-shaped leaf primordium hulling the apex corresponded to Leaf 6 in the varieties Baraka and Ninfa and to Leaf 5 in Tunika, indicating that, in all varieties, six leaves were still at the stage of primordium when spikelet initiation started (Fig. 3B). Tillering was proceeding and all dissected plants had two to three tillers (data not reported).
Characteristics of the barley apex at the stage the first (n. 11–12) and the last (n. 35) spikelet primordium were initiated, and at the stage of maximum primordia. Corresponding codes according to the developmental scales of Banerjee and Wienhues [6], Haun [5] and Zadoks et al. [4] are reported. Data refer to three barley varieties sown in autumn (A) and in spring (S). Data are means ± SE with n = 5.
| Baraka | Ninfa | Tunika | ||||
| A | S | A | S | A | S | |
| Stage of 1st spikelet primordium | ||||||
| Number of ridges on the apex | 3.7 ± 0.3b | 3.5 ± 0.5b | 2.7 ± 0.3a | 3.7 ± 0.3b | 3.0 ± 0.0a | 2.7 ± 0.3a |
| Number of most apical Leaf Primordium | 5.7 ± 0.3b | 5.5 ± 0.2b | 5.5 ± 0.3b | 5.0 ± 0.0a | 4.8 ± 0.2a | 5.0 ± 0.0a |
| Distance from crown (cm) | 0 | 0 | 0 | 0 | 0 | 0 |
| Codes in developmental scales | ||||||
| B&W | 2–3 | 2 | 2 | 2–3 | 2 | 2 |
| Haun | 2.5 | 2.6 | 2.5 | 2.5 | 2.2 | 2.4 |
| Zadoks | 22–23 | 23 | 23 | 23 | 22 | 22 |
| Stage of last spikelet primordium | ||||||
| Spike length (mm) | 1.8 ± 0.3a | 2.3 ± 0.3b | 1.8 ± 0.3a | 1.8 ± 0.3a | 1.8 ± 0.3a | 1.8 ± 0.3a |
| Number of basal Leaf Primordium | 11.5 ± 0.5c | 11.5 ± 0.5c | 11.5 ± 0.5c | 11.0 ± 0.0b | 9.5 ± 0.4a | 9.5 ± 0.5a |
| Distance from crown (cm) | jpa | 0–jp | 0–jp | jp | 0–jp | 0–jp |
| Codes in developmental scales | ||||||
| B&W | 6a | 6a | 6a–7 | 6b | 6a–6b | 8 |
| Haun | 6.3 | 6.1 | 6.6 | 6.4 | 5.2 | 5.4 |
| Zadoks | 30 | 30 | 30 | 30 | 30 | 30 |
| Stage of maximum primordia | ||||||
| Spike length (mm) | 6.5 ± 0.9bc | 5.7 ± 1.1b | 4.1 ± 0.5a | 5.7 ± 1.0b | 6.8 ± 1.2c | 4.5 ± 1.0a |
| Number of total primordia | 52.8 ± 1.3d | 53.3 ± 0.3d | 48.2 ± 0.7b | 45.8 ± 0.5a | 51.8 ± 0.7c | 46.7 ± 0.7a |
| Distance from crown (cm) | 1.4 ± 0.4a | 2.5 ± 1.8bc | 0.9 ± 0.4a | 1.8 ± 0.7ab | 3.4 ± 1.3c | 1.5 ± 0.9a |
| Codes in developmental scales | ||||||
| B&W | 11a–13 | 12–13 | 10–11a | 10–11 | 12–13b | 11a–13 |
| Haun | 9.1 | 8.6 | 8.7 | 7.4 | 8.5 | 7.5 |
| Zadoks | 30–31 | 31 | 30 | 30–31 | 31–32 | 30–31 |
a Culm elongation just perceivable.
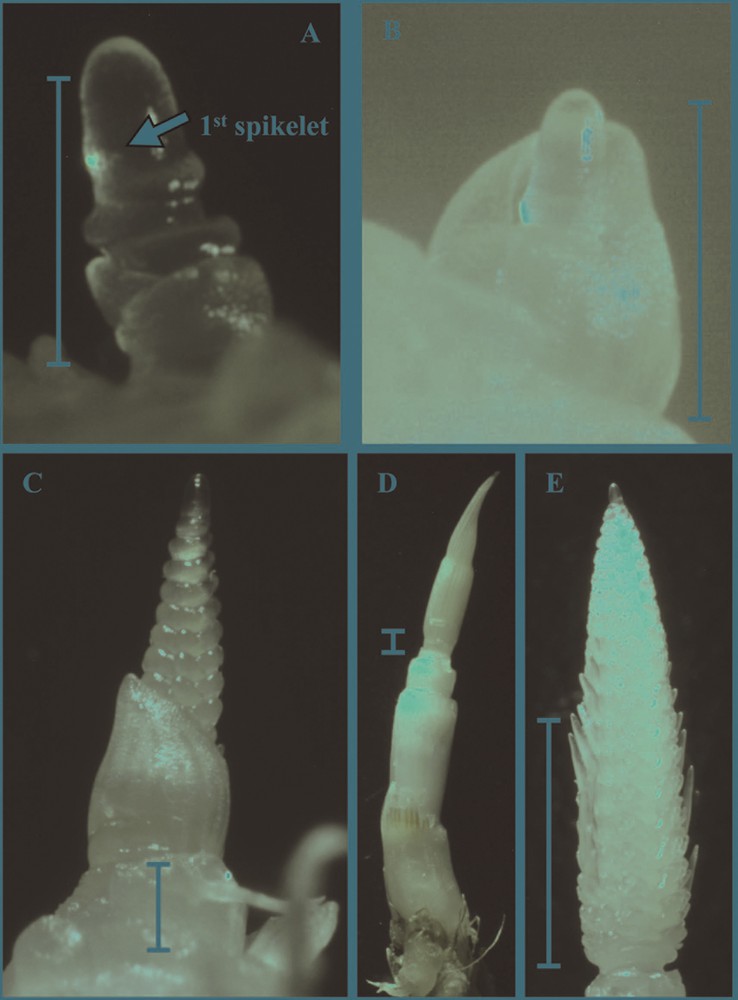
Main culm apex of two-rowed barley (variety Baraka). A. Stage of first spikelet initiation. The basal leaf primordium is L7. On the elongated apex the primordium of L8, three complete ridges and the primordium of the first spikelet, are visible. B. Stage of first spikelet initiation. The apical leaf primordium hulling the apex is L6. C. Stage of last spikelet initiation. The basal leaf primordium is the flag leaf (L11), spikelet primordia protrude laterally, the first glume initials are visible and the spike is already separated from the crown. D. Stage of maximum number of primordia. The flag leaf (L11) hulls the spike and internode distension is evident. E. Stage of maximum number of primordia. Awns are elongating in the central part of the spike. In all figures the bar corresponds to 1 mm.
When the primordium of the last spikelet was initiated, the apex was at the triple mound stage, showing three rows of round blisters expanded laterally, and measured approximately 2 mm (Table 2, Fig. 3C). In the varieties, Baraka and Ninfa spikelets were completely undifferentiated, stage 6a and 6b of the B&W scale, whereas in Tunika glume initials were already visible in plants from the spring sowing, stage 8 B&W. In all varieties, the most basal leaf primordium corresponded to the flag leaf, indicating that FNL was achieved at this stage. Finally, a distance between the base of the spike and the crown was just perceivable, evidencing that culm elongation had started (Fig. 3C). Independently of sowing date, the stage of Haun was approximately 6.2 in Baraka, 6.5 in Ninfa and 5.3 in Tunika, and plants were at the growth stage of pseudo stem erection that is when leaf-sheaths elongate becoming erect (30 Zadoks’ scale) (Table 2). Above results indicate that the initiation of productive spikelets ends when the flag leaf primordium starts to elongate and the last completely emitted leaf corresponds to the leaf primordium that hulled the apex at the beginning of spikelet initiation. The number of this leaf differed among varieties (L6 in Baraka and Ninfa and L5 in Tunika), but in all five leaves had still to appear.
At the stage the maximum number of primordia was achieved, culm elongation was evident and all plants were around the stage of first node detectable (Table 2, Fig. 3D). Floret differentiation was in progress and awns were growing in all varieties and sowing dates (Fig. 3E). Both spike and awn length greatly differed among plants, but awns exceeded the top of the spike only in some plants of Tunika from the autumn sowing. In all varieties, the maximum number of primordia was achieved at an earlier stage of Haun in the spring sowing and, correspondingly, maximum values were slightly lower than in the autumn one (Table 2).
4 Discussion
Results obtained from barley varieties differing in thermophotoperiodic requirements (winter, alternative and spring types) indicated that leaf appearance and primordia initiation were highly coordinated processes in barley, and the relationship could be represented with an asymptotic curve. A coordination between the processes of leaf appearance and primordia initiation was reported also for wheat [16,24] and oat [13], but relationships differed according to FNL which, in turn, was strongly influenced by environmental conditions [18,19,21,25]. In the present research, FNL varied among genotypes but was not affected by sowing date, and the number of spikelets was fairly stable across both. These results confirm the hypothesis that FNL is under strong genetic control [27] and support the findings of Baker and Gallagher [9] and Juskiw et al. [26] that sowing date has a minimal influence on the FNL of winter wheat and spring barley. In addition, García del Moral et al. [15] reported that also the patterns of primordia initiation and abortion were not affected by environment in barley. Thus, we suggest that the equation of the asymptotic curve describes fairy well the time course of total primordia initiation, relative to leaf appearance, of two-rowed barley varieties that produce 10 or 11 leaves on the main culm (FNL). The rate of primordium initiation decreased progressively after the initiation of the last spikelet, whereas the initiation of productive primordia (n. 1–35) was linearly, and highly significantly, related to leaf appearance. Moreover, the rate of initiation did not change appreciably form the initiation of leaf- to that of spikelet primordia, it was higher with lower FNL and it was independent of sowing date. Consequently, we can state that in barley, the initiation of leaf and spikelet primordia proceeds at a constant rate, relative to leaf appearance, and this rate is determined only by genotype. Kirby [16] and González et al. [19] reported an inverse relationship between primordia initiation rate and FNL also in wheat, but these authors found that spikelet initiation proceeded faster than leaf initiation.
Present results indicate that, in barley, crucial steps in primordia initiation can be related to developmental stages of the apex and of the plant. In particular, the beginning of spikelet initiation occurs when the third leaf starts to appear externally, the apex turns from a rounded shape to an elongated one, and six leaves are at the primordium stage (shorter than 1 mm). This correspondence is not affected by sowing date, and it changes slightly depending on the FNL characteristic of each variety. Tillering started widely before the initiation of the first spikelet and, therefore, it is not coordinated with the switch of the apex from vegetative to reproductive. The initiation of spikelets occurred earlier than what generally reported in wheat [9,16] and it indicates that, in barley, the actual reproductive phase starts at a very early stage of development.
To identify the end of spikelet initiation is of high concern in crop management since it allows making early predictions of yield [12]. In barley we found two distinct events, the initiation of the last productive primordium (last spikelet) and the achievement of maximum primordium number, the latter, corresponding to the definite end of initiation activity. At the time the last productive spikelet is initiated, the flag leaf is evidently elongating and no more leaf primordia are present at the base of the spike. The apex stage is comprised between triple mound and glume initials, which indicates that spikelets have started to differentiate but florets not yet [6]. At the same time, the entire barley plant assumes an erect position and internode distension is visible under the microscope. Thus, we can state that only spikelet primordia initiated before the start of floret differentiation and culm elongation, will complete differentiation. Though it is not clear which is the driving process, the beginning of floret differentiation and internode distension coincide with the extension of the last leaf (flag leaf). Starting from the functional model of Fournier et al. [14], we can hypothesize that the end of cell division at the base of the spike triggers floret differentiation and activates intercalary meristemes. At the same time, the initiation of new primordia at the top of the spike is markedly slowed down. Thus, we suggest that meristematic activity ceases in parallel at the top and the base of the spike and, therefore, the first steps of flag leaf elongation can be used to signal the end of productive spikelet initiation in barley. The stage of Haun corresponding to the initiation of the last spikelet differed among varieties in dependence of FNL, but the number of leaves still to emerge was approximately five, irrespective of variety and sowing date. A correspondence of apex stages with leaves to emerge is in line with the hypothesis that the appearance of a given leaf is synchronized with the ontogeny of subsequent leaf primordia, which, in turn, is coordinated with their initiation [1,14].
In barley, the definite end of apex activity is not signalled by the formation of a terminal spikelet like in wheat, but Nicholls [11] found that it corresponded to the spike stage of stamen initials under continuous light, and to the appearance of awns at short daylength. In our research, when the maximum number of primodia was achieved, awns were actively growing in all varieties and sowing dates, and plants were around the stage of first node detectable. A correspondence between the end of activity at the top of the apex and the phase of rapid culm elongation was observed in barley, oat, rye and wheat [12,16,21] and it seems, therefore, to be a common feature in cereal development. Conversely, we did not find a constant relation between maximum number of primordia and leaf stage, because both the number of emerged leaves and those to emerge differed among varieties and sowing dates. Since in the spring sowing one leaf more had to emerge compared to the autumn one, we suggest that, in the former, culm elongation and spike differentiation proceeded more rapidly than leaf appearance. These findings are consistent with the hypothesis that both culm elongation and spike differentiation are sensitive to photoperiod and are probably the driving factor, while leaf appearance proceeds at a constant rate that is genetically determined [23,27]. An influence of both variety and environment on the Haun stage at terminal spikelet was reported for other cereals, but in wheat the number of leaves to emerge was constant [16,22] whereas in oat it varied [13].
5 Conclusions
In two-rowed barley, primordia initiation and leaf appearance are coordinated processes that proceed at a constant rate without appreciable differences between the initiation of leaf and spikelet primordia. The Haun stages corresponding to the initiation of the first and the last spikelet, and the rate of initiation depend on genotype, probably due to the different FNL, but not on environmental conditions. The first spikelet is initiated very early, when the third leaf tip becomes visible. The last productive spikelet is initiated when the flag leaf primordium starts elongating, five leaves have still to appear and both floret differentiation and internode distension are beginning. The definite end of primordia initiation is, probably, determined only by competition with rapid culm elongation, and its correspondence to Haun stage and primordium number differs among varieties and sowing dates. Finally, we observed that a given primordium stage corresponded to a rather wide range of apex stages. This suggests that the initiation of primordia proceeds in parallel to leaf appearance and depends only on genotype, while spikelet differentiation and culm elongation are also affected by environmental factors and are accelerated at later sowings.


