1 Introduction
Carpenter ants (genus Camponotus) have established an association with the intracellular endosymbiont Blochmannia (γ-Proteobacteria) found in all of the species studied thus far [1,2]. The function of endosymbionts has not totally been elucidated, but their role in supplying complementary dietary nitrogen was shown through the analysis of the genome sequence of two species of Blochmannia [1,3–5] and in experiments eliminating the bacteria through the administration of antibiotics and the use of chemically–based diets [6,7]. However, the apparent ability of the ants to survive without Blochmannia and the omnivorous behaviour of several Camponotus species suggests that the bacteria perform other functions for the ants. We recently demonstrated that the presence of Blochmannia may be important during the colony-founding phase, and that these bacteria play a role in improving the immune response of the ants [8].
Here, we tested other functions the bacterial endosymbiont carries out for the host ants. It is well known that cuticular surface chemicals, mainly hydrocarbons, protect insects from desiccation [9,10]. These hydrocarbons also play an underestimated role in providing protection from pathogens [11]. We tested the effects of an antibiotic treatment in eliminating or greatly reducing the quantity of endosymbiotic bacteria on the cuticles of the ants and on the melanization processes.
2 Material and methods
2.1 The ants
Incipient colonies of Camponotus fellah were collected by Pr Abraham Hefetz in Tel-Aviv in March 2007. The colonies were placed into plastic containers (20 × 20 × 10 cm) furnished with plaster nests and kept in a climate chamber (constant temperature of 28 °C, 12 h DL), and were fed twice a week with Tenebrio molitor larvae and honey. Each colony contained one queen, and at least one hundred workers and brood. We used 10 control colonies (also fed with Tenebrio larvae and honey) and 10 treatment colonies (fed with Tenebrio larvae and honey for the first week, and then Tenebrio larvae and honey solution containing 1% of the antibiotic rifampicin for the second week and thereafter). The treatment was applied during 3 months. It has previously been shown that the antibiotic treatment has no side effects on medium-sized ants (see Discussion in [6,8]). For the experiments, we used medium-sized foraging workers selected randomly from all of the colonies. We verified that there was no detectable colony effect. In previous studies, we confirmed the efficacy of the antibiotic treatment in eliminating bacteria using real-time quantitative polymerase chain reaction (PCR) and fluorescence in situ hybridization (FISH) tests. It was demonstrated that the antibiotic treatment reduced the amount of bacteria by at least 75% [8].
2.2 Degree of melanization of the cuticle
We dissected the third tergite from 27 naive workers and 29 workers treated with antibiotics. They were placed in Clarion™ medium, and then mounted on glass slides. Each piece was examined under a light microscope and photographed using a digital camera (Olympus DP50). The mean grey value of the cuticle fragment was measured using ImageJ 1.37v software. The background grey value was subtracted to correct the values of the fragments. The mean grey value was obtained by measuring three square plots in each figure. We assumed that the darkest grey corresponded to the cuticle with the highest degree (i.e., totally black) of melanization. Melanization is normally achieved a few days after imaginal eclosion, but changes occur in adults, for example, as they age [12].
2.3 Chemical analyses
Parts (i.e., the head and thorax, including the legs) taken from 27 treated workers and 31 control ants were immersed in 1 mL of pentane for 5 min and then removed, and 5 μl of pentane containing 50 ng of eicosane (C20) was added as an internal standard. For the analyses, the solvent was evaporated until 5 μl remained which were then injected into an FID gas chromatograph (VGM250Q system, Perkin-Elmer) using a DB-5 fused silica capillary column. The temperature was kept at 150 °C during the splitless initial 2 min, raised from 150 °C to 310 °C at 5 °C/min and held at 310 °C for the last 10 min. The cuticular hydrocarbons had been previously identified [13,14]. To also identify the smaller peaks, we analyzed the cuticular profile in greater detail with the same gas chromatograph coupled to a Perkin-Elmer mass spectrometer operating at 70 eV. We used a high temperature column to identify the peaks with more than 30 carbons (DB-5HT, 30 m, 0.251 mm × 0.10 μm). The temperature program was 2 min at 100 °C; 6 °C/min until 350 °C and maintained for 5 min. The areas of the peaks were estimated through peak integration using a TurboChrome Workstation. We then calculated the quantities of substances using the internal standard area (ng per thorax) and their relative proportions. Hydrocarbon classes by percentage and total quantities were compared with the Mann-Whitney U test. Data expressed as a percentage are used with the Arcsine (square root) transformation [15]. The profiles between the two groups were compared with a dendrogram using the single-link Ward method and Euclidian distance.
3 Results
3.1 Degree of melanization
Antibiotic-treated ants showed a significant increase in the degree of melanization when compared to the control group (Student's t-test, t26 = 12.9, P < 0.00001; Fig. 1). Treated ants exhibited a degree of melanization approximately two times superior to that of control ants.
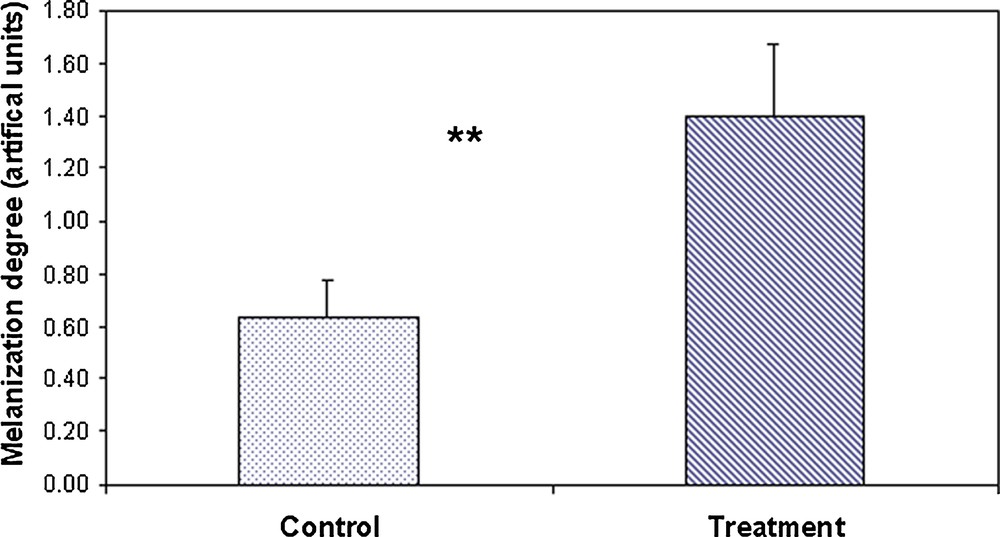
Means (± SE) of the degree of melanisation of the cuticle on control (n = 27) and antibiotic-treated ants (n = 29). Student's t-test, P < 0.0001.
3.2 Chemical analysis
We recorded 62 peaks, of which 58 were identified. Our analysis was very detailed as 43 out of the 58 had not previously been identified using earlier data [13], including all of the components with more than 32 carbons not previously described with a total of 9% (Table S1 and Fig. S1 in the supplementary material). All were hydrocarbons except for a trace of one ester. There were three major peaks in the C27 series: C27 (peak no 11, mean 14.6 ± SD 4.3%), 3-MeC27 (no 16, 25.3 ± 2.9%) and 7-MeC27 (no 13, 7.7 ± 1.2%), and 16 peaks were comprised between 1 and 5%. All of the other peaks were less than 1%, and must be considered as traces and were detectable only in some individual extracts due to the sensitivity of our gas chromatograph (GC).
When comparing the profiles of treated and control ants, we did not find qualitative differences; all of the substances were present in both treated and control groups (all P values > 0.25). The main classes for control ants are n-alkanes (25.30 ± 6.22%), methyl-alkanes (49.56 ± 3.41%), dimethyl-alkanes (18.29 ± 3.29%), trimethyl-alkanes (3.88 ± 1.12%) and a few alkenes (0.27 ± 0.15%) (Fig. S2 and Table S1 in the supplementary material). A dendrogram (Cluster analysis, Ward method, Euclidian distance) did not separate the two groups; the ants were placed indifferently in the control or the treatment group according to their colony (data not shown). Moreover, when we compared the percentages of the different substances, no significant differences were detected, indicating that the chemical profiles of control and treated ants were similar. These data indicate that the cuticular profile of the ants did not change because of the antibiotic treatment. Nevertheless, the antibiotic-treated ants had a total quantity of hydrocarbons significantly higher than the control ants (459.6 ± 260 vs 300.5 ± 159 ng/ant, respectively; P = 0.007, Mann-Whitney U test; Fig. 2).
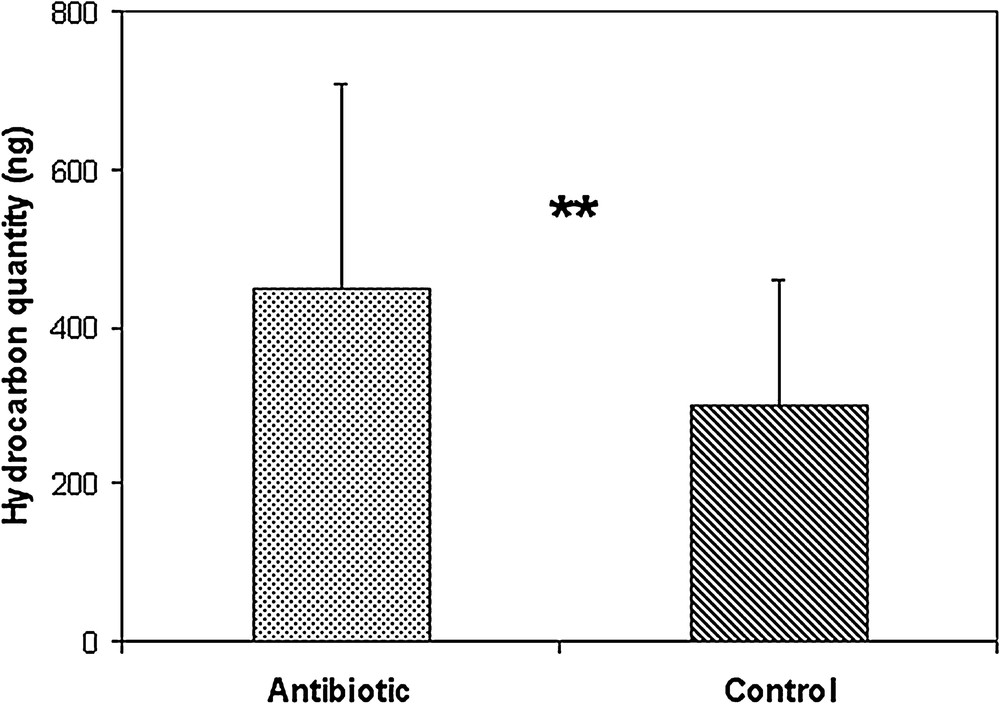
Mean quantities of cuticular hydrocarbons on the thorax (ng/ant ± SD), Mann-Whitney U test P = 0.008.
4 Discussion
The treatment of the ants with antibiotics was correlated to an increase in the degree of cuticular melanization. This response can be linked to other responses observed in a previous study conducted on C. fellah: the increase in the encapsulation response after antibiotic treatment [8]. It is well known that a highly-melanized cuticle can block microbial invasion [16]. It is also known that melanization is a part of the encapsulation process of parasites [17], and, therefore, that it increases the ant's immunological defense. We suggest that the ants compensate for a deficiency in Blochmannia through physiological responses that increase their cuticular melanization so as to be better protected from potential invasions by pathogens and parasites.
Our results also show that the ants’ cuticular hydrocarbon profile did not change after the antibiotic treatment. Although it was not quantified, observations of the ants suggest that nestmate recognition was not modified. As we previously observed [13], non-nestmates are normally rejected while nestmates are easily reintroduced into their colony. Only one research study (on the termite Reticulitermes speratus [18]) has indicated that there is a link between bacteria and nestmate recognition, but this is a unusual case as nestmate recognition in these insects is directly dependant on nutrition and bacteria are intestinal guests. In C. fellah, the cuticular profile is very stable; the profile remains similar for colonies collected during different years from the same site in Israel [13]. The antibiotic treatment suggests that nestmate recognition in these ants is not related to the bacterial endosymbionts. Since it has been shown that the quantity of the bacteria decreases with the age of the ant [19], it seems logical that recognition occurs regardless of the presence of symbionts.
If the cuticular hydrocarbon profile was not modified by the antibiotic treatment, we did observe an increase in the quantities of hydrocarbons. This is the first time that a change in the quantity of the cuticular hydrocarbons has been correlated to an antibiotic treatment. Data on the quantities of hydrocarbons in ants are scarce. It is known, however, that the tropical ant Ectatomma ruidum has very few (125–200 ng/worker) [20] and that Aphaenogaster senilis has 2 to 4 μg per ant [21]; whereas Cataglyphis niger, an ant that is well adapted to hot and dry climates, has 15 to 50 μg per ant (i.e., 1/1000 of its body weight) [22]. The composition and quantity of epicuticular lipids act to waterproof the cuticle, and, therefore, play a role in preventing desiccation [23,24]. Long chain compounds are generally thought to enhance desiccation resistance, although this is not always the case (reviewed by [9] and see [25]). Arthropods like desert Drosophila that dwell in warm, dry environments tend to have hydrocarbons with a longer chain length than their counterparts in more mesic environments [26]. The environmental conditions experienced by ants in different task groups may induce changes in the cuticle. Workers that perform outside tasks are more exposed to higher temperatures, lower humidity and ultraviolet light. In the ant Pogonomyrmex barbatus, the relative abundance of n-alkanes was 20% higher for foragers and patrollers than for nest maintenance workers. This may enhance the desiccation resistance of workers exposed to a desert environment. However, in the study on Pogonomyrmex, the foragers did not have longer chains alkanes [27]. In the same species, the composition (i.e., percentage) for founding queens changed at the founding stage, but the total quantity of hydrocarbons did not [28]. Changes in the hydrocarbon quantities need to be more explained. It is not known, for example, if an increase in the thickness of the layer of hydrocarbons changes the protective properties of the cuticle.
The effects of melanization and protection by hydrocarbons are probably linked as melanization is also correlated to desiccation in Drosophila [29,30]. It has been shown that some epicuticular lipids also act as an antiseptic [11], thus, an increase in the amount of hydrocarbons may better protect the ant from pathogens.
It now is necessary to determine if this is provoked by fewer Blochmannia per se, or if this is a side effect of the antibiotic. Rifampicin is widely used in similar studies involving insects and symbionts and no physiological changes to the insects have been attributed to the toxicity of the antibiotic [2,6,31,32]. Moreover, rifampicin selectively eliminates endosymbionts in the pea aphid [33]. A recent study demonstrated that commensal bacteria (mainly Wolbachia and Lactobacillus) play a role in the mating choices of Drosophila mediated by cuticular hydrocarbons [34]. The authors tested three different antibiotics with the same results. The antibiotic probably also affects bacteria in the gut which are not well known. A major, recent survey [35] showed that gut symbionts are very important to herbivorous ants, but much less so to other ants.
To conclude, the increase in hydrocarbon quantity and melanization in antibiotic-treated C. fellah workers may enhance the protection the cuticle provides from desiccation and also from invasions by pathogens and parasites while nestmate recognition is not modified.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
We would like to thank Danielle Mersch and Stephane Dorsaz from Lausanne University and Abraham Hefetz from Tel-Aviv University for collecting the mated queen ants, Guy Bourdais for his help in taking care of the ants, Raphael Boulay for his help in data analysis. We are also grateful to Alain Dejean for his constructive comments and Andrea Yockey-Dejean for proofreading the manuscript.


