1 Introduction
The genus Artemisia (Anthemidae tribe), small herbs and shrubs, is one of the largest and most widely distributed genera of the Asteraceae family. Plants of this genus grow in temperate climates of both Northern and Southern Hemisphere, usually in dry or semi-dry habitats. Among this genus, some species are known as aromatic plants and used as food, others have toxic activity and may be used as medicine [1,2], and since the middle of the 1980s, there has been an increasing interest in species of the genus Artemisia since the discovery and successful clinical trials of the antimalarial sesquiterpene artemisinin that was obtained from the ancient Chinese medicinal plant Artemisia annua [3]. Our study focused on Artemisia species growing wild in the Mediterranean part of France and particularly on two species with different ecological features. The first is A. campestris L. var. glutinosa (Gay) Batt. (syn. A. glutinosa Gay ex Bess; A. campestris ssp. glutinosa Gay ex Bess.), a perennial undershrub widespread all over Europe, from Siberia to Great Britain and in Northern Africa. Traditional use of A. campestris is regularly cited in studies and activity of crude methanolic leaves extract of A. campestris against Gram-positive species has been recently proven [4–6]. However, morphological identification of Artemisia herbs is very confusing due to similarities in the shapes of young leaves and reports of traditional uses may contain some taxonomical confusion within this genus. The second species, A. molinieri Quézel, Barbero & Loisel, is an endemic species from two temporary ponds located in Southern France (Var department, Redon and Gavoty ponds). To our knowledge, no past or recent ethnobotanical study mentioned this latter species. In 2003, antimicrobial activity of its essential oil was reported for the first time [7]. The interest of studying a species with a restricted ecological range is that it may lead to specific adaptations and specific metabolites that may have particular activities such as insecticidal ones.
The house mosquito Culex pipiens L., 1758 (Diptera, Culicidae) is the most widely distributed mosquito in the Northern Hemisphere and is worldwide distributed except in Antarctica. It plays an important role in diseases transmission. This species is known to carry arboviruses (arthropod-bone viruses) and is recognized as the primary vector of St. Louis encephalitis and West Nile Virus in the Eastern US [8,9]. In this context, this study was carried out consecutively to a preoccupant and important increase in C. pipiens population in the French Mediterranean region during autumn 2005 (rainy season). This remarkable increase has led to the massive use of pesticides by authorities to eradicate this pest mosquito. Such peculiar events lead to considerable spread of insecticides in the environment that possess strong secondary effects on the non-target aquatic fauna [10–13]. In this context, there is a search for new mosquito larvicidal agents with minimal toxic effects on the environment and human health. Phytoproducts are choice candidates for such studies on account of minimal hazardous effects on the environment and wide range of availability. Compared to synthetic chemicals, the high degree of biodegradation exhibited by most phytochemicals makes them ‘eco-friendly and attractive’. Moreover, the use of chemical biocide leads to multiple resistance mechanisms. As insecticide resistance is an inherited characteristic involving changes in one or more insect gene, synthetic chemical biocide's widespread use in the environment represents a threat for their efficacy in the future [14–19]. Many phytochemicals have revealed larvicidal potential (see [20] for a review). Among these latter, endemic species extracts must retained peculiar attention as they certainly have developed specific unknown characteristics.
Therefore, in the present study, A. campestris var. glutinosa and A. molinieri were compared for their larvicidal efficiency. Artemisia extracts contain artemisinin that has been reported to reduce Plasmodium falciparum gametocyte development, thus reducing transmission of malaria, this fact being especially significant in preventing the spread of resistant strains [21]. However, to our knowledge, the mosquito larvicidal activity of Artemisia extracts has been little investigated [22]. A. campestris methanol extracts gave a LD50 of 23 ppm against Culex quinquefasciatus [23]. Lastly, extraction of biologically active compounds often needs a number of technological operations and the use of large amounts of environmentally unfriendly solvents. In the present article, the Artemisia leaf extracts were produced only with ethanol, an alternative solvent to the generally-used methanol, carbon tetrachloride or petroleum ones, avoiding toxic substance hazardous wastes. This choice is consistent with the proposition by Pavela et al. [24] to use the supercritical fluid extraction method with carbon dioxide for plant active compound extractions, a method that also used a polar entrainer such as methanol or ethanol. This study aimed at testing the larvicidal activity of A. molinieri extracts compared to a more common species, A. campestris, for both test a new candidate for phytoproduct investigations against C. pipiens and support a conservation approach of this rare plant species and its habitat.
2 Materials and methods
2.1 Plant materials
Aerial parts of A. campestris var. glutinosa (Gay ex Bess.) Y.R. Ling were harvested at Ponteau (20 km west of Marseille, Southern France), in October 2005 (while plants were flowering). The plants were gathered on a sandy soil at sea level. A voucher specimen (MARS-2000.4) has been deposited in the herbarium of the University of Provence (Marseille, France).
Aerial parts of the patrimonial endemic plant of Var (Southern France), A. molinieri Quézel, Barbero et Loisel, 1966 (protected species “Arrêté du 9 mai 1994 relatif à la liste des espèces végétales protégées en région Provence-Alpes-Côte d’Azur NOR: ENVN9430087A”) were harvested in the University greenhouse, in October 2005 (flowering plants). Seeds had previously been harvested in the temporary pond Redon Lake (Var, France) with the authorization of the “Conservatoire Botanique de Porquerolles”. They were sown in individual pots on a commercial composted soil. Seedlings were grown during five months. A voucher specimen (MARS-2000.6) has been deposited in the herbarium of the University of Provence.
The leaves were separated from stems on numerous representative individuals, early in the morning, and the material was taken immediately to the laboratory to be extracted.
2.2 Phytochemical screening
General phytochemical analyses of both plant species were done to detect alkaloids, saponins, coumarins, and flavonoids, according to classical reported procedures [25] on air-dried plant material (leaves).
2.3 Mosquito larvae
Mosquito larvae were collected in a temporary pond located in the Palayson forest near Le Muy (Var, Southern France) on 18 October 2005. The electrical conductivity (C25) of the pond was 199 μS cm−1, water temperature 18.6 °C, and pH 8.11 (WTW® portable meters). Larvae were brought back to the laboratory and for each replicates, 10 randomly sampled fourth-instar larvae have been immediately placed, at 20 °C ± 1 °C, in vials of 50 mL distilled water adjusted with commercial mineral water (Volvic®) to a conductivity (C25) of 105 μS cm−1 in order to avoid osmotic shock with or without concentrations of ethanolic plant extracts.
2.4 Ethanolic extracts
Fresh leaves of A. campestris var. glutinosa and A. molinieri (80 g weight) were macerated with ethanol (200 mL, 99.0%) during 24 h. The macerate was then filtered. The yields (extracts dried using a rotary evaporator) were respectively 4.0 and 5.4%.
Volatile compounds yields were 4.0 and 3.2% of ethanolic extract, for A. campestris var. glutinosa and A. molinieri, respectively.
2.5 Gas chromatographic analysis
Capillary gas chromatography was carried out using a Varian® (Model 3900GC) chromatographic system with a flame ionisation detector (FID), equipped with a CP SIL 8CB fused silica capillary column (30 m × 0.25 mm, 0.25 μm film thickness). Oven temperature was programmed from 50 to 220 °C at 3 °C min−1, after an isothermal step at 50 °C for 2 min. The carrier gas was H, with a flow rate of 0.5 mL.min−1. Injector and detector were heated to 220 and 230 °C, respectively. The injection volume was 0.1 μL for each sample.
Component identification was carried out by comparing with authentic reference compounds, previously analyzed Artemisia extracts, and retention indices [26,27]. Quantitative analysis of each extract component (expressed in percent) was carried out by peak area normalization measurements.
2.6 Bioassays
All the bioassays were conducted in an incubator at 20 ± 2 °C, and 12 h light and 12 h dark photoperiod.
To test the mosquito larvicidal activity of Artemisia extracts, a slightly modified method of WHO [28] was used. Ten fourth-instar mosquito larvae were placed in a 50 mL flask containing 30 mL of the prepared water (C25 = 105 μS cm−1) and 500 μL ethanolic Artemisia extracts to final concentrations of 10, 50, 100 and 500 ppm. To ensure a homogeneous test solution, each flask was gently shaken and then left at 20 °C. Both controls were prepared with 50 mL of degassed distilled water and 50 mL of degassed distilled water containing 1000 ppm of ethanol. Each experiment was replicated five times.
Mortality was recorded after 48 h of exposure, during which no food was given to the larvae. Larvae were considered as dead if they did not move when prodded with a needle. Percent mortality was corrected for control mortality using Abbott's formula, and the results were plotted on log/probability paper using the method by Finney [29]. Toxicity and activity were reported as LC50, representing the concentration (expressed in ppm) that caused 50% larval mortality in 48 h.
2.7 Statistical analyses
Average mortality values are expressed in percent ± S.E.M. Comparisons of mortality values between two groups were made using the non-parametric Mann-Whitney U test. For each extract, the mortality values as function of extract concentrations were compared by the non-parametric Kruskall-Wallis test followed by the Bonferroni test for paired comparisons.
3 Results and discussion
3.1 Chemical composition of the extracts
For both species, phytochemical screening showed presence of phenolic compounds and flavonoids, a small amount of saponins, and no alkaloids nor coumarins. These results are in agreement with known compositions of many Artemisia species [30,31]. Flavonoid patterns for Artemisia campestris are quite complex and can show great variations [32]. Saponins have only been previously found in A. argentea [33]; this is the first report of these compounds for both tested species.
Volatile composition of ethanolic extract from A. campestris var. glutinosa showed high amount of aromatic polyacetylenes (49% of capillene and 18% of its precursor phenylpenta-2,4-diyne), γ-terpinene (23%) and traces of methyl-eugenol, p-cymene, Z-ß-ocimene, and germacrene D. These compounds had previously been identified in the field wormwood essential oil [34], and their relative content is in agreement with flowering stage of the plants.
Previous analyzes on A. molinieri content concerned diethyl ether extract with mainly ascaridole and two bisabolol oxide derivatives contents [35]. Some of the major compounds of its essential oil have been identified as α-terpinene, ascaridole and p-cymene [36]. According to a previous analysis [7], volatile part of A. molinieri extract was mainly composed of ascaridole (57%) p-cymene (21%), 1,8-cineole (14%), and some minor compounds as sabinene, bornyl acetate, α-copaene, geranyl acetate (Table 1).
Volatile composition of Artemisia campestris var. glutinosa and A. molinieri ethanolic extracts tested in bioassays.
| Componentsa | Artemisia glutinosa b | Artemisia molinieri b |
| Ascaridole | – | 57.3 |
| Capillene | 49.1 | – |
| γ-terpinene | 23.0 | – |
| p-cymene | 2.8 | 20.9 |
| 1-phenylpenta-2,4-diyne | 18.1 | 0.2 |
| 1,8-cineole | Tr | 13.8 |
| α-copaene | 0.2 | 1.8 |
| Sabinene | 0.1 | 1.4 |
| Bornyl acetate | – | 1.2 |
| (Z)-β-ocimene | 1.2 | 0.6 |
| Germacrene D | 0.8 | 1.0 |
| Methyleugenol | 1.0 | – |
| Bicyclogermacrene | 0.6 | – |
| Spathulenol | 0.5 | 0.3 |
a Traces (< 0.1%) of (E)-β-ocimene, γ-terpinene, terpinen-4-ol, α-cadinol, α-bisabolol, α-terpinene, γ-muurolene, thymol, β-himachalene, α-curcumene, limonene, carvacrol, (E)-nerolidol, β-pinene, (E)-β-farnesene, α-pinene has been identified in both extracts.
b Relative percentage of volatile compounds.
3.2 Larvicidal activity of the extracts
According to Mann-Whitney U test, no significant differences were detected between water and ethanolic control values at P > 0.05 (average of 4.44 and 4.72%, respectively). Therefore, the mortality control value, mean of both previous values i.e. 4.58%, was used for comparisons.
Concerning the larvicidal activity of A. campestris var. glutinosa extracts, the Kruskall-Wallis test showed an overall significant difference (K = 9.49, DDL = 4, P < 0.01) (Fig. 1). The Bonferroni test revealed that only the most elevated A. campestris var. glutinosa extract concentration tested (500 ppm) showed a significantly different larvicidal activity from controls after 48 h of exposure (33.60 and 4.58%, respectively, P < 0.01). The mean larvae mortality values observed after 48 h of exposure to 10, 50, 100 ppm of A. campestris var. glutinosa extracts were not different from controls (5.72, 8.20 and 12.30%, respectively, NS).
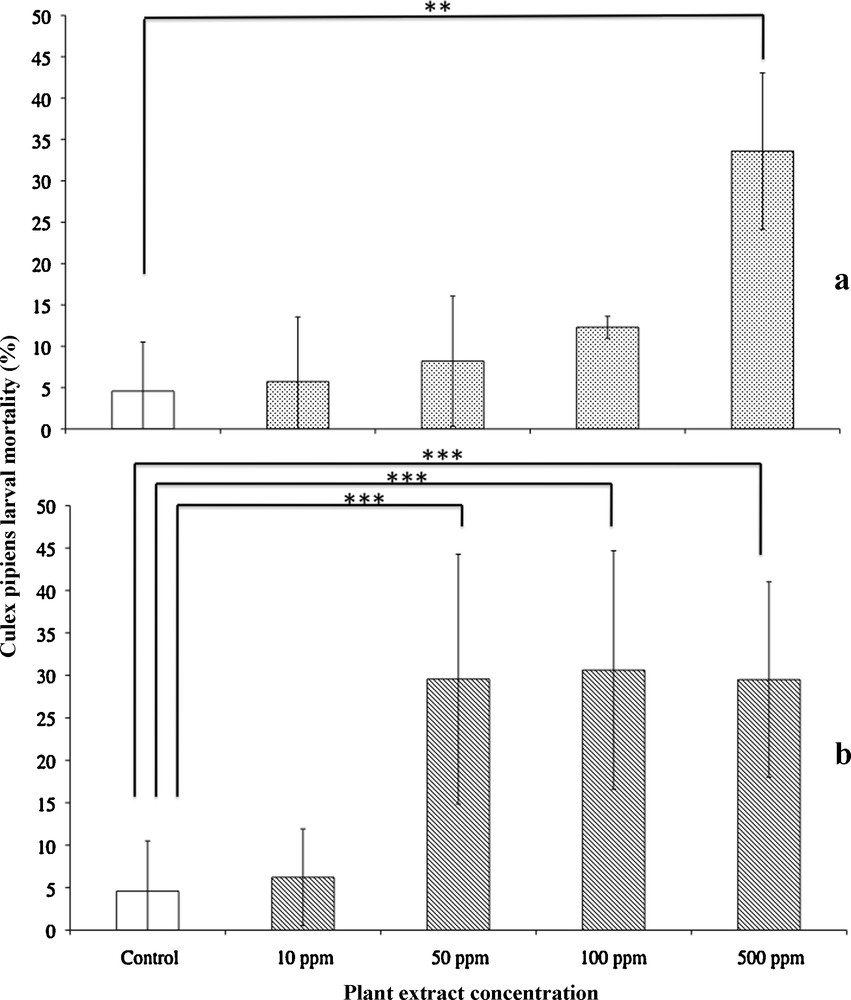
Mean larval mortality ± S.E.M. (in percent) of Culex pipiens larvae after 48 h exposure to gradual concentrations (10, 50, 100 and 500 ppm) of Artemisia campestris var. glutinosa extract (a) and Artemisia molinieri extract (b). Means were compared using the non-parametric Kruskall-Wallis test followed by the Bonferroni test (**P < 0.01).
Concerning the larvicidal activity of A. molinieri extracts, the Kruskall-Wallis test showed an overall significant difference (K = 9.488, DDL = 4, P < 0.001) (Fig. 1). The Bonferroni test revealed that the mean larvae mortality values observed after 48 h of exposure were significantly different from controls for 50, 100 and 500 ppm of A. molinieri extracts (29.56, 30.62 and 29.50%, respectively, P < 0.001). The mean larvae mortality value observed after 48 h of exposure to 10 ppm of A. molinieri extracts was not significantly different from controls (6.22% and 4.58%, respectively, NS).
When comparing the larvicidal activities of A. molinieri vs. A. campestris var. glutinosa extracts, the Mann-Whitney U test showed that the larvicidal activity to 50 ppm and 100 ppm of A. molinieri was significantly higher than that of A. campestris var. glutinosa (29.56 and 8.20%, respectively for 50 ppm, P < 0.05; 30.62 and 12.30%, respectively for 100 ppm, P < 0.05). There were no significant differences in larvae mortality values between A. molinieri and A. campestris var. glutinosa extracts for 10 ppm and 500 ppm (6.22 and 5.72% respectively for 10 ppm, NS; 29.50 and 33.60%, NS).
After 48 h exposure, LC50 have been calculated at 9091 ppm for A. molinieri extracts and at 9898 ppm for A. campestris var. glutinosa extracts, respectively.
4 Conclusion
Phytoproducts possess different bioactive components that can be used as general toxicants against various larval stages of mosquitoes [20]. The combined ovicidal, larvicidal and growth regulating effects displayed by many phytochemicals can produce impressive results. For example, extracts of Allium sativa and Citrus limon [37], Alnus glutinosa [38], Anthemis nobilis [39] and, Cassia obtusifolia [40] have proven efficiency on Culex pipiens larvae lethality. In the present work, both ethanolic extracts of A. molinieri and A. campestris var glutinosa showed larvicidal activity against the mosquito C. pipiens. However, extracts of A. molinieri revealed a higher larvicidal activity than those of A. campestris var glutinosa. The biocide differences found for the tested extracts can be explained by their different chemical compositions, with aromatic polyacetylene dominant fraction for A. campestris var glutinosa and ascaridole dominant fraction for A. molinieri ethanolic extracts. Polyacetylenes from another wormwood extract (A. borealis) have previously shown a larvicidal activity against the yellow fever mosquito Aedes aegypti [41]. These authors showed that the dichloromethane extract of A. borealis displayed a LC100 of 80 ppm at 24 h and that the main polyacetylene compound, the heptadeca-1,9(Z),16-trien-4,6-diyn-3,8-diol, contributes only in part to the larvicidal activity of the extract which seems to contain additional, more potent larvicidal compounds. In the present study, the larvicidal activity of both ethanolic Artemisia extracts is less efficient compared with the dichloromethane extract of A. borealis. It is not surprising since larvicidal potentialities depend on the extraction method. For example, recently, comparing the efficiency of phytoextracts of Ocimum basilicum on Culex quinquefasciatus larvicidal activity, it has been shown that the methanol extract has a lower larvicidal than carbon tetrachloride and petroleum ether leaf extracts with LC50 after 48 h of 53.77 ppm, 17.02 ppm and, 6.06 ppm, respectively [42]. Nevertheless, it is difficult to compare the larvicidal activity of extracts from different Artemisia species because in papers dealing with this, the chemical composition of the extracts is not always known [22]. However, in this study, phytoextracts of Artemisia sp. show effective larvicidal activity. For example, Sharma et al. [22] mentioned a LC50 of Artemisia annua methanol extract against Anopheles stephensi third instar larvae of 414.48 ppm after 48 h of exposure.
On another hand, the higher efficiency of A. molinieri ethanolic extract in our results may be due to the presence of ascaridole in this extract. This compound is always abundant in essential oils of full flowering A. molinieri, and is known to be strongly effective against Anopheles aegypti larvae [43] or also Culex quinquefasciatus larvae [24].
Even if our present results showed upper LC50 than many previous studies, there are four main reasons for the ongoing development of such biocides:
- • There are evidences to suggest that the activity of such extracts may be greater than that of the essential oil. This has been shown by Chen Liu Chiung-Sheue et al. [44] and Buhner [45] that whole extracts of A. annua, even those artemisinin-free possess effective anti-malaria effects.
- • Development of biological resistance to single actives is common, especially for synthetic chemicals [14,46], whereas resistance to whole or complex plant extracts is rare.
- • Botanical extracts offer a melt of many various active components and display a large range of biocide activity. For example, phytoextracts of Artemisia annua have effective larvicidal effects and significant influence on hatching and post-hatching development of the mosquito Anopheles stephensi [22]. Therefore, phytoextracts of Artemisia sp. could be used at different mosquito developmental periods for better result in field mosquitocidal application and, also tested on another culicid species, such as Aedes albopictus, actually reaching the South of France [47].
- • Rare and endangered narrow endemic species, such as A. molinieri, are desperately in need of economic value that is a strong argument for preservation of their habitats. Several options for development of A. molinieri larvicidal activity are conceivable: chemical synthesis, biotechnological production, and ecological engineering of local constructed wetlands. Further studies could evaluate the feasibility as well as the legal, ethical and ecological objections to these three options.
As a conclusion, the local plant species may be an important resource in phytobiocides adapted to local uses as it has already been showed in various studies from Argentina [48], Morroco [49], Eritrea [50] and Malia [51].
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
The authors are grateful to Pr. André Lavagne for helpful discussion on Artemisia particularities and to the “Conservatoire Botanique de Porquerolles” for providing authorization for seed collection of A. molinieri. Many thanks to Sophie Leroy (University of Provence) for optimizing the cultural conditions of A. molinieri under greenhouse conditions.


