1 Introduction
Coastal dune ecosystems are severely threatened worldwide by human activities [1–4] and many plant species are known to be declining, especially in the Mediterranean area [5]. Coastal plant communities play an important role in dune system maintenance since they create an efficient protective barrier for backdune vegetation and for human infrastructures [6,7].
Several key factors are known to influence seed germination, including light, temperature, salinity and nitrogen availability [8–12]. Exposure to light is strongly tied to seed mass, limiting seed burial to a critical depth for seed germination and seedling emergence [13]. In coastal dune environments, it has been observed that sand burial strongly influences plant growth and survival [14–16]. In addition, temperature shows strong fluctuations both seasonal and daily, with soil often being subjected to overheating, particularly in summer [14]. High levels of salinity may also impede germination [10,17]. Coastal regions are particularly prone to high levels of salinity in the soil due to seawater in the aquifer and salt spray. Salinity levels are known to fluctuate seasonally and in relation to distance from the sea, with values ranging from 0.1 to 3% [5]. Moreover, nutrient availability could be an important factor in the germination process; several authors have highlighted the positive role of nitrogenous compounds in improving seed germination [8,11,18–20]. In coastal areas, soils are usually relatively poor in nutrients and nitrogen deficit is known to limit plant growth in coastal dune ecosystems [21,22]. KNO3 solutions with concentrations of 10 mM are known to successfully improve germination compared to other concentrations [19,23]. Finally, local adaptation and differences in conditions among sites, such as climate and seed mass, may cause intraspecific differences in germination patterns [24–27].
Crucianella maritima L. is a suffruticous chamaephytes belonging to the Rubiaceae family and a diagnostic species of the Habitat 2210 (Coastal fixed dunes–Crucianellion maritimae, Habitat Directive 92/43/EEC). Its distribution comprises coastal dune systems of Portugal, Spain, France and Italy [28], but it is currently very fragmented and this species is now considered gravely threatened [29,30]. Little is known about the germination requirements of C. maritima and other species of this genus [31].
In this study, germination requirements of C. maritima seeds were investigated in seeds belonging to three separate populations sampled in different areas along the species distribution, with the aim to:
- (1) characterize seed germination (light and temperature requirements);
- (2) identify seed germination responses to salt stress (NaCl) and nutrients availability (KNO3);
- (3) verify inter-population variability in germination requirements, salt stress and nutrient availability.
2 Materials and methods
2.1 Seed lot details
In all the investigated populations, Sardinia (Chia, Cagliari, IT), Latium (Passoscuro, Rome, IT) and Mallorca island (Playa de Muro, Palma De Mallorca, ES) Mediterranean Pluviseasonal Oceanic (MPO) bioclimate prevails [32]. According to the DIR 92/43/EEC, the habitat where the plants grow was “Crucianellion maritimae, fixed beach dunes” (Habitat code 2210). Achenes (hereafter seeds) were collected in the tree populations in summer 2009, directly from randomly selected plants at the time of natural dispersal (Table 1). Seeds were then manually extracted and kept in the laboratory under room conditions until experiments started. Average seed mass was calculated for each seed lot by weighing 10 replicates, each of 20 seeds (Table 1).
Population data and seed lot details. Temperature information was extracted by Worldclime database (5 km resolution grid), by overlaying the georeferenced site of collection to climatic data in ArcGis 9.2 (ESRI Inc., Redlands, CA, USA). Winter: mean temperature of January, February, March; Spring: April, May, June; Summer: July, August, September; Autumn: October, November, December.
| Provenance | Collecting | Coordinates (Datum WGS84) | Seed weight ± SD (mg) | Number of sampled individuals | Winter (°C) | Spring (°C) | Summer (°C) | Autumn (°C) |
| Sardinia (Chia, Cagliari) | 06/07/2009 | 38°53’22.07”N 8°52’15.60”E |
2.31 ± 0.50 | 164 | 10 | 17 | 23 | 14 |
| Latium (Passoscuro, Rome) | 23/07/2009 | 41°54’44.40”N 12°8’45.23”E |
2.47 ± 0.21 | 100 | 11 | 18 | 24 | 15 |
| Mallorca (Playa de Muro, Palma de Mallorca) | 14/09/2009 | 39°47’2.34”N 3°7’59.00”E |
1.65 ± 0.16 | 65 | 11 | 17 | 24 | 15 |
2.2 Germination tests
Germination tests were carried out by sowing three replicates of 20 seeds each in covered petri dishes with a substrate of 1% water agar.
In a first experimental trial (Experiment 1), the effect of light on seed germination, at different temperatures, was investigated. Seeds from each provenance were incubated at a range of constant temperatures (10, 15, 20 and 25 °C) so as to simulate seasonally average temperatures (Table 1), as well as at an alternating temperature regime (25/10 °C), to simulate daily temperature fluctuation. Seeds were incubated with both a photoperiod of 12 h of irradiance per day (“light”) and in the dark (obtained by wrapping the dishes in two layers of aluminum foil). Germination was assessed on the basis of visible radicle protrusion. In the light, germinated seeds were counted and removed every two days, while, in the dark, seeds were scored only once, at the end of the experiment, to avoid any exposure to light. When, in the light treatment, no additional germination occurred for 15 days, both tests were ended. The viability of the remaining seeds was assessed by a cut test [33] and the final number of germinated seeds calculated on the basis of the total number of filled seeds.
In a second experimental trial (Experiment 2), seeds belonging to the three populations were incubated at the above mentioned temperature regimes in the dark (on the basis of the results achieved in the Experiment 1), on 1% water agar with different solutions (“treatments”). The treatments corresponded to increasing salt concentrations (NaCl: 125 mM, 250 mM and 500 mM, to simulate seawater at 25%, 50% and 100%; [34]) or a nitrate solution (KNO3: 10 mM) in the germination substrate. Seeds were scored only once, for a maximum of 96 days and seed viability assessed by the cut test.
2.3 Data analysis
The effect of light (Experiment 1) was determined considering the different temperatures and provenance and all interaction terms. The effect of treatments (five levels corresponding to the control, three concentrations of NaCl and one of KNO3; Experiment 2) was determined, taking into consideration the different temperatures and provenance including all interaction terms. In both experiments, seed germination responses were determined by fitting two separated Generalized Linear Mixed Models (GLMM), using Statistica v. 7.0, on arcsin transformed data, with provenance as random factor. This analysis made it possible to investigate whether germination responses of C. maritima varied across the plant's range. Finally, planned comparisons of least squares means were performed to investigate for differences in germination percentages between control and treatments (Experiment 2).
3 Results
3.1 Experiment 1 – Effects of light, temperature and seed provenance
Achieved results showed a significant effect of both light (F1,2 = 26.5; P = 0.036) and temperature (F4,8 = 20.5; P < 0.0001) on seed germination, while the seed provenance did not influence germination significantly (F2,1.37 = 3.5; P = 0.29). Crucianella maritima seeds from all populations showed considerably higher germination percentages when incubated in the dark in respect to when incubated in the light (Fig. 1). The temperature and light regimes did not interact significantly (F4,8 = 0.83; P = 0.54), as germination of dark-incubated seeds did not vary among the tested temperatures. However, germination of light-incubated seeds was generally affected by warmer temperatures (> 15 °C), while the alternating temperature regime (25/10 °C) did not improve germination (Fig. 1). Neither did temperature and provenance interact significantly (F8,8 = 0.21; P = 0.98). A significant two-way interaction was only found between light regime and provenance (F2,8 = 4.8; P = 0.04), due to the lower germination percentage of light-incubated seeds of Mallorca. Germination percentages in the light were always under 50% for Sardinia and Latium populations and under 15% for Mallorca (Fig. 1).

Plot of means of germination percentage in light and dark in the three populations. Data are the mean ± standard deviation of three replicates of 20 seeds each.
A significant three-way interactive effect was found (temperature × light × provenance; F8,60 = 6.2; P < 0.0001), highlighting the lower germination percentage of Mallorca seeds, both in light at all temperatures and in the dark at 25 °C and 25/10 °C. Dark-incubated seeds of Sardinia and Latium populations germinated at percentages higher than 50% at all the tested temperatures, reaching 78.3 ± 2.9% at 20 °C and 91.0 ± 7.6% at 15 °C for Sardinia and Latium, respectively. Seeds belonging to Mallorca population showed a lower overall germination than the other two populations also kept in the dark, with an average value of 40%, reaching 56.6 ± 18.9% at 15 °C (Fig. 1).
The cut test carried out at the end of the germination tests highlighted that seed viability was similar in all the three seed lots, with 76.0 ± 9.6%, 84.0 ± 8.2% and 65.7 ± 11.7% of viable seeds for Sardinia, Latium and Mallorca, respectively. Light-incubated seeds showed a high number (> 50%) of non-germinated viable seeds in all seedlots, while all dark-incubated non-germinated seeds had died, except at 25 °C and 25/10 °C, at which temperatures viable seeds were about a half of the non-germinated ones and then at 10 °C, at which temperature very few seeds (< 10%) remained viable.
3.2 Experiment 2 – Effect of treatments
The effect of treatments was found to be significant (F4,8 = 31.4; P < 0.0001). Comparison tests showed significant differences between control and NaCl concentrations of 125 mM (t = 29.2; P < 0.0001), 250 mM (t = 39.5; P < 0.0001) and 500 mM (t = 39.5; P <0.0001), while KNO3 had no a significant effect (t = –0.7; P = 0.4). NaCl affected germination with an overall decrement in germination of approx. 47% at 125 mM concentration, while no germination occurred at concentrations greater or equal to 250 mM. The significant effect of temperature (F4,8 = 15.6; P = 0.0007) highlighted the scarce germination at 25 °C, while the effect of provenance (F2,6.5 = 5.6; P = 0.038) brought out the poor germination of seeds from Mallorca. Significant interaction were found between both temperature and treatment (F16,32 = 2.4; P = 0.016) and also between treatment and provenance (F8,32 = 4.8; P = 0.0005). These interactions highlighted the scarce germination at temperatures greater than 20 °C and at 25/10 °C, in each of the three seed batches in saline conditions and the higher sensibility to salinity of Mallorca's seeds. The only non-significant interaction was temperature × provenance (F8,32 = 0.58; P = 0.79).
The significant three-way interactive effect (treatment × temperature × provenance: F32,150 = 5.43; P < 0.0001) revealed a general pattern of germination at temperatures lower than 20 °C and the lower germination of Mallorca seeds at all tested temperatures with 125 mM of NaCl. Indeed, at this salinity concentration C. maritima seeds reached their maximum germination at 15 °C (48.3 ± 5.8% and 68.3 ± 10.4% for Sardinia and Latium, respectively) and values of ca. 40% at 10 °C (Fig. 2). Seeds belonging to the Mallorca population germinated only at 15 °C (15.0 ± 8.7%).
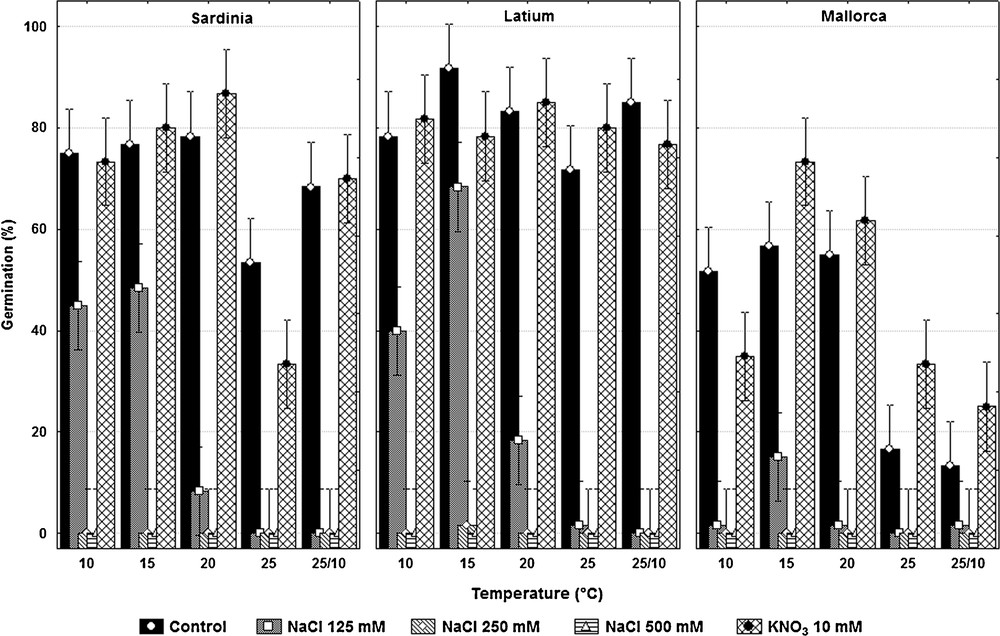
Plot of means of germination percentage in each treatment; data are the mean ± standard deviation of three replicates of 20 seeds each.
In terms of viability, the cut test carried out on non-germinated seeds highlighted an increasing proportion (from 50 to 60%) of non-germinated but viable seeds at higher levels of NaCl, whereas KNO3 treatment showed no difference with the control.
4 Discussion
4.1 Light and temperature requirements
Seed germination of C. maritima was highly inhibited by light. The dune environment where this species grows is strongly characterized by movements of the substrate and consequent burials by sand [5,14]. Thanos et al. [35] found that germination of several Mediterranean maritime species is photoinhibited, highlighting a surface avoiding mechanism, since light is only able to penetrate 4–5 mm into the soil in physiologically significant quantities [36]. As found in several studies, small seeds are more likely to require light for germination [13,37,38], while C. maritima seeds, with a seed mass of ca. 2.15 mg (see Table 1), are able to emerge when buried deeper than 5 mm. However, seed germination may be reduced or even prevented by sand movements due to insufficient soil coverage of seeds.
In the dark, C. maritima seeds can tolerate a wide range of temperatures, even though different germination optima could be obtained depending on seed provenance. They are therefore likely to be non-dormant (sensu Baskin and Baskin [39]), as the great majority of viable seeds germinated at high percentages in a wide range of conditions, without any pre-treatment. In the light, germination percentages mainly decreased at temperatures greater than 15 °C, as detected for other Mediterranean species [40–42], suggesting that germination is most likely to occur in late autumn, in order to prevent seedlings from facing the harsh summer conditions [43,44].
4.2 Salt stress and nutrient availability
C. maritima seeds were able to tolerate the NaCl solution with a concentration of 125 mM, although germination was significantly affected, while at higher concentrations germination was completely inhibited. NaCl is known to reduce germination in several dune species. Cakile maritima showed a lower germination percentage and rate when seeds were exposed at NaCl greater than 100 mM) [10], while germination of Pancratium maritimum was completely absent in seawater solutions (100%, 50%, 25% and 12.5%) [5]. NaCl at 200 mM also completely inhibited the germination process in both Crithmum maritimum [11] and Cyperus kalli [43]. Salinity concentrations did not affect seed viability of C. maritima, suggesting a salt-induced secondary dormancy. Many authors pointed out the ability of halophytes and coastal species to recover from saline conditions when transferred to distilled water [5,10,12,43,44]. The salt-induced dormancy and the increased sensibility to salt at high temperatures detected in this study for C. maritima might represent an advantage in harsh ecosystems, helping seed to germinate during the period which guarantees higher seedling survival and a successful seedling establishment. In general, salinity is considered one of the most important filtering factors that determine zonation in dune systems: C. maritima typically grows in the transition dune slack, behind the mobile dune system [16] where the harsh coastal environmental conditions are milder.
A significant influence of KNO3 on germination was not revealed, suggesting that nutrient availability is not a requirement for seed germination of this species. However, some authors have found that nitrogenous compounds can improve germination in Aster pilosus [23], or allow the process even in saline conditions in C. maritimum and Suaeda salsa [11,18,19]. The germination rate is also known to be improved when nitrogen is added [20,45]. Unfortunately, due to the experimental conditions of this study (dark; Experiment 2), it was not possible to quantify the germination rate. Therefore, further experiments are needed to investigate the effects of nitrogen compounds on other germination parameters, such as rate and delay.
4.3 Intraspecific variability
Intraspecific variability in germination patterns has been reported for several species [24,27,46]. Depending on seed provenance, C. maritima and Polypogon monspeliensis showed intraspecific variability in stress tolerance [25,26], while Atriplex halimus also showed different germination patterns in distilled water [47]. In this study, all seedlots showed the same pattern of response to light, temperature, salt stress and nitrogen availability. A lower overall germination percentage, however, was detected for Mallorca seeds. This difference on final germination can be related to the smaller seed mass of the lot (Table 1). Besides genetic factors, seed mass variability can depend on resource availability, drought, population size and on the rate and duration of seed growth [48–50]. Mallorca seeds were collected in September while seeds from Sardinia and Latium were collected in July. Thus, the low seed mass detected for this population cannot be related to an early harvesting of unripe seeds. The intraspecific effect of resource availability, drought and population size on seed mass of this species remains to be investigated.
5 Conclusions
Seed germination of C. maritima was characterized by photoinhibition, absence of primary dormancy and a secondary dormancy imposed by high salt concentrations, with no requirement for a substrate rich in nutrients (KNO3). Seed germination is likely to occur in autumn, when temperatures are low, rainfall is abundant and soil salinity is lower. Moreover, seeds should be buried at a depth where light cannot penetrate the soil in physiologically significant amounts. Depending on the seed provenance, intraspecific differences in final germination percentages are most probably due to a different seed mass. These results highlight several important traits of C. maritima seed germination which should be taken into account for population restoration or reintroduction of this threatened species.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We thank Roma Tre Vegetation Ecology Laboratory and IMEDEA (Instituto Mediterráneo de Estudios Avanzados) staff for helping with seed sampling in the field and the Sardinian Germplasm Bank (BG-SAR) staff for helping with seed cleaning.


