1 Introduction
The human functions are controlled through different ways among which both the central and peripheral nervous systems (CNS, PNS) play a major part. Movement control and sensory input processing are the most obvious functions that use the neural network to propagate and process information. As regards muscle activation, skeletal striated muscles are controlled through the somatic nervous system allowing voluntary contractions, whereas other active organs are actuated by smooth muscles controlled through the autonomous neural system, as for the cardiac striated muscle. Thus, numerous physiological functions are more or less modulated by efferent signals coming from the CNS that is informed through afferent signals coming from various sensors. Within this complex network, the information code is based on Action Potential (AP) generation. Besides, muscles themselves, in particular cardiac and skeletal muscles, go into contraction when an AP is produced on the membrane of their fiber. Then, when either a sensory or a motor function is deficient, inducing APs may provide for a partial restoration.
Electrical Stimulation (ES) induces APs by depolarization of the membrane of the targeted cell, i.e., axons or muscle fibers. From the 1950s, ES has been successfully used in a growing set of applications linked to motor and sensory impairments including pain management. The most popular devices are the pacemakers that originally allow one to trigger directly the cardiac muscle through ES with implanted electrode and external stimulator [1], next implanted [2]. A few decades later [3], defibrillators gained the same level of popularity as they efficiently save life. In the sensory area, cochlear implants allow one to recover sound perception for deeply deaf persons. The principle is basically the same with a set of electrodes that increased from one to more than 20 over the 30 past years, located in the cochlea in order to activate the remaining auditory neural circuits [4]. It allowed equipped deaf people to understand speech and in the best case speak as healthy subjects. More recently, deep brain stimulation, with a simple stimulation scheme applied to deep brain areas allows for instance, to limit or even suppress tremor in Parkinson disease [5].
Attempts to use this approach have been made in movement rehabilitation, such as drop foot syndrome for hemiplegic patients [6] and more complex movements or functions for para- and quadriplegic patients [7–12]. In the latter case, the functional results could prove to be substantial, including, for instance, recovery of the grasp function for quadriplegic patients, who might then be able to grab hold of objects, eat and even, in the best cases, write with a pen [13]. The results for standing and walking restoration remain less functional in paraplegic patients. Due to insufficient functional benefits, most of the research carried out stopped for almost ten years. Although not optimal, Functional ES (FES) systems remain the only way for movement restoration in a daily use context. The main drawbacks of the technique are well known and include insufficient reliability, the complexity of the surgery, limited stimulation selectivity and efficiency, the non-physiological recruitment of motor units and muscle control. However, quite recently, researches for movement restoration of the lower limb in particular, regained interest through mainly new surgical approaches and stimulation targets [14,15]. The method described in [14] provided no improved functional benefits however, the new surgical approach – laparoscopy – gave access to new motor nerve targets that allowed less invasive surgery. In [15] the authors described the abilities of the spinal cord to generate some useful pattern of activation that may provide standing and even walking patterns. In this study, the patient had a complete motor and incomplete sensitive spinal cord lesion at C7-T1 level. The strategy consisted in stimulating the afferent pathways close the spinal cord at the lumbo sacral level close to the dura. The patient experienced much exercising for months and empirical optimal stimulation patterns were found in order to provide lower limb muscle contractions through modulation of the afferent pathways. The patient could even produce rhythmic but non functional gait-like movements. Even though the involved mechanisms are still not well described and the functional benefits quite low, the results are clearly important because they show that the spinal cord circuits can help to provide for functional movements.
However, available implanted stimulators dedicated to humans, remained too limited to explore widely all the possibilities that these techniques could provide. Besides, functional movement modeling and advanced movement control in particular in a closed-loop way, are still unused although it is known that the human nervous system is controlling movement through complex multilevel closed-loops. Even though the last study tried to use the spinal cord network including its natural closed-loops between afferent and efferent signals, they are neither controlled nor explicitly used by the stimulators so that the activation, from a global system point of view, remained open loop.
On one hand, a more physiological stimulation that provides functional benefits, implies that the activation of motor units is accurately controlled in order to focus the activation on the desired target with as less as possible side effects (fatigue, unwanted diffusion to other muscles, activation of reflexes). On the other hand, an efficient functional movement would need for closed-loop control (balance control, fatigue compensation) or optimized synthesized patterns (sit-to stand movement, grasping). The off-line synthesis may limit the duration and the number of clinical sessions needed to tune the neuroprosthesis, otherwise tuned empirically. To take up these two challenges for the future, we proposed both a theoretical approach of movement restoration based on control theory and an innovative technological platform that foreshadows new designs of neuroprotheses.
Thus, the article describes first an advanced technological implantable system and how we deal with movement control and synthesis and then how it can be implemented with such an advanced technology.
2 Materials
The most common way to induce movements remains efferent pathway stimulation even though promising results recently showed that afferent pathways stimulation may do so [15]. Neural stimulation is clearly the most accurate and efficient method, but the classical tripolar cuff associated with a single rectangular current pulse generator allows no control of fiber type firing or localization of the fired axons within the nerve. Indeed, the tripolar cuff geometry is unable to focus currents within the nerve; thus firing zones that may relate to specific targets can not be selected. As well, a single current source with a rectangular waveform can not provide a way to selectively activate thin or thick axons. Therefore, if one considers that fatigue and reflex avoidance are the most critical points, selective stimulations may improve drastically the effectiveness and accuracy of movements. We design new concepts of neuroprotheses that support such advanced multipolar stimulation patterns. Indeed, limiting fatigue could be achieved by selectively firing thin axons that are linked to fatigue resistant fibers (although a rectangular pulse activates thick fibers first), and focusing currents could constrain the way of propagation (avoiding afferent pathway).
Selectivity can be obtained through multipolar cuff or intrafascicular electrodes in order to focus the current lines where expected, together with specific stimulation waveforms that can block the propagation of AP based on fiber diameter [16–18]. For the stimulus generator, these approaches lead to complex synchronous outputs to deliver the current simultaneously to the poles of the electrode. Besides, the number of poles increases together with the number of wires between the stimulus generator and the electrode. Furthermore, the waveform has to be sampled to provide for non rectangular shapes in order to produce time-selective activation and efficient activation to charge injection ratios. But, due to the complexity of such system design, up to now, no device for humans is available for chronic use. On the contrary, all the solutions tested on humans have been based on centralized implants from which the wires go through the body to the monopolar or bipolar electrodes [10,12,19–21]. One exception can be noted: the Bion technology for which bipolar stimulation is provided by injectable units [22,23]. But the Bion is limited by the power source, the rectangular stimulation shape, the use of bipolar electrode and in some cases, the migration of the implant itself. Multiplexing poles close to the electrode was proposed [24], but only to limit the number of wires and without the possibility to provide for multipolar stimulation. Finally, some electronic devices have been proposed [25–28] to solve the problem of multipolar and complex waveform stimulation, but no application was derived from these studies.
We propose a new architecture of neuroprostheses called Stimulation Electrique Neurale dIStribuée (SENIS). This concept is based on Distributed Stimulation Units (DSU) and Distributed Measurement Units (DMU) connected through a 2-wire bus that transmits bidirectional data and power supply [29]. The principle of the architecture is to provide for small units close to the multipolar electrodes. It limits the numerous wires needed between electrode's poles and DSU on a short distance without connector. It avoids inefficient deeply implanted radio frequency link and thus the DSU can be very small and allows for minimally invasive surgical procedures. Moreover, the number of DSU can be adapted to each patient's needs. A single bridge between the implanted and the external world is needed, located in a convenient area to allow for both antennas of the inductive link to be well aligned. To fulfill the challenges of this architecture, we work on two fundamental technological aspects:
- • the embedded electronics able to generate complex stimuli;
- • efficient network protocol able to ensure safety and closed-loop control over network with a guaranteed quality of service.
2.1 Embedded electronic design
The DSU was designed to optimize the size and power consumption, the safety, the data transmission through the network and the power of programming as regards control of the multipolar currents. To achieve these goals, we split the functional components into two elements: an analog Application Specific Integrated Circuit (ASIC) that can deliver currents to multiple poles and a digital Field Programmable Gate Array (FPGA) based processing system.
The first challenge is to provide an accurate generation and sharing of the current flows through the different poles of the electrode to control the localization of the activation volume within the nerve. The basic idea consists in providing synchronous current sources for each pole. Doing so, with n-bit current controlled sources, both for sinking or sourcing, we get for each pole j:
| (1) |
cj is the digital command, Iref the scale factor. However, one important feature of such a system, considering that the milieu is linear, is to provide for a constant current ratio between poles whatever the global current is. Let us consider a 4-cathode with one anode electrode configuration, the global injected current is given by:
| (2) |
if at least one current source is not 0, the ratio of the current of the pole j versus the global current is:
| (3) |
All the ratios are fractional numbers and if we want to maintain strict constant ratio we must write:
| (4) |
| (5) |
This currently used approach is simple to implement as regard the hardware but it has its main drawbacks:
- • all the commands cj have to be changed either I or Rj is changed;
- • it needs for four current sources;
- • the current amplitude step is depending on the ratios and is getting worst with close but not equal ratios;
- • it leads to variable dynamics;
- • the maximum available global current is dependent on the ratios.
To illustrate, let us set the following ratio configurations:
| (6) |
| (7) |
To avoid these problems, the new idea we introduced [30,31], is based on the decoupling of the ratio and the global current control. To do so, a unique digital to analog current converter (DACC) is used followed by a programmable current divider. In this case, the current is controlled in a completely different manner. The equations are then:
| (8) |
The most interesting benefits of such structure is that it allows:
- • a constant step size of the global current amplitude Iref and a constant maximum amplitude 2nIref, whatever the ratios are if their sum is constrained to 2p – 1;
- • an independent control of ratios and global amplitude.
However, equivalent structures between both solutions may be found but the cost is high. Let us compare the design of a stimulator for which we aim at 8-bit resolution for the global current and ratio configurations on 4-bit. In the second structure, the solution is directly n = 8, p = 4 that leads to 24-bit word command length for the whole structure. In the first solution, if we want to guarantee the specifications in the worst case, we have to provide for 4 DACC with 12-bit resolution that leads to 48-bits word length. The difference becomes even higher with more complex structures with up to 12-pole electrodes as we already developed. Moreover, DACC are much more power supply demanding than dividers. An ASIC for a 12-pole structure has been developed (Fig. 1). This structure is able to provide sinking or sourcing currents in order to allow each pole to be configured either as a cathode or an anode.
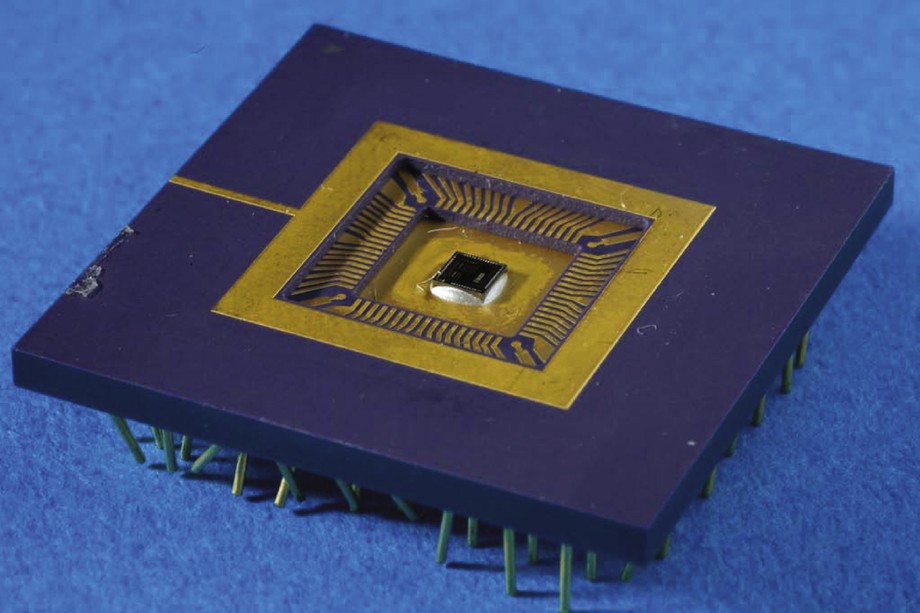
12-pole ASIC that can be seen in the center, about 4 mm by 4 mm.
Besides, this advanced output stage allows for a higher level of software abstraction. Indeed, from a functional point of view, both features mean different things. The ratio configuration determines on which zone of the nerve the current lines that induce APs are focused, whereas the global amplitude is linked to the extent of this area around the focus point. Thus, in most applications, changing the ratios is a long-term schedule compared to the amplitude control that may occur for each stimulus pulse. Our structure further optimized software design and data-flow. In our example, instead of transmitting 48-bits at each pulse generation, we only transmit 8-bits. Long-term configuration needs for 16-bits.
2.2 Dedicated Restricted Instruction Set Computer
Taking into account safety, power consumption minimization and time step accuracy, we designed a full dedicated digital core that includes: i) reference models that monitor the stimulation generation and stop the execution if programmed constraints are violated – maximum charge injection, for instance; ii) a communication module that allows the DSU to exchange data on the 2-wire network; iii) a dedicated, custom-made, RISC processor for the analog output stage control. Details can be found in [29], but let us focus on the design of the processor.
The requirements of such a stimulus generator are:
- • the focus zone is determined by the ratio configuration. It allows for activating part of the nerve, for instance, in a fasciculated area, to selectively activate different target muscles;
- • the progressive activation of the target through the global intensity or pulse duration modulation. Indeed, the more the injected charges are, the greater the number of fired axons is;
- • the ability to selectively activate thin or thick fibers, myelinated or not. To do so, techniques such as high frequency or anodal blocking can be used. To achieve this goal, non rectangular waveforms are needed;
- • optimized charge injection versus functional effect. In this case, waveform with exponential or trapezoidal rising/decay should be used;
- • typical time step should be around 1μs.
Achieving such features with a classical sequential processor architecture needs for a fine control of interrupts, timers with a much higher clocking than needed at the stimulus scale, typically at least 10 MHz. Moreover, preventing software failure is tricky, indeed impossible. Pure digital systems developed on FPGA even on ASIC, may bring neat solutions. We design a RISC processor in a formal way using Petri Nets. It provides a way to check properties of the global system such as possible deadlocks or conflicting conditions. A translator from Petri Nets to VHDL was then developed to implement solutions on FPGA keeping all the properties of the original Petri Net design (Fig. 2). The basic idea is that defining a stimulation waveform can be done using few conceptual instructions (Fig. 3):
- • SIT setting a current for a given duration;
- • NST setting the output in high impedance or short circuit without any current for a given duration;
- • LOOP repeating the whole waveform several times.
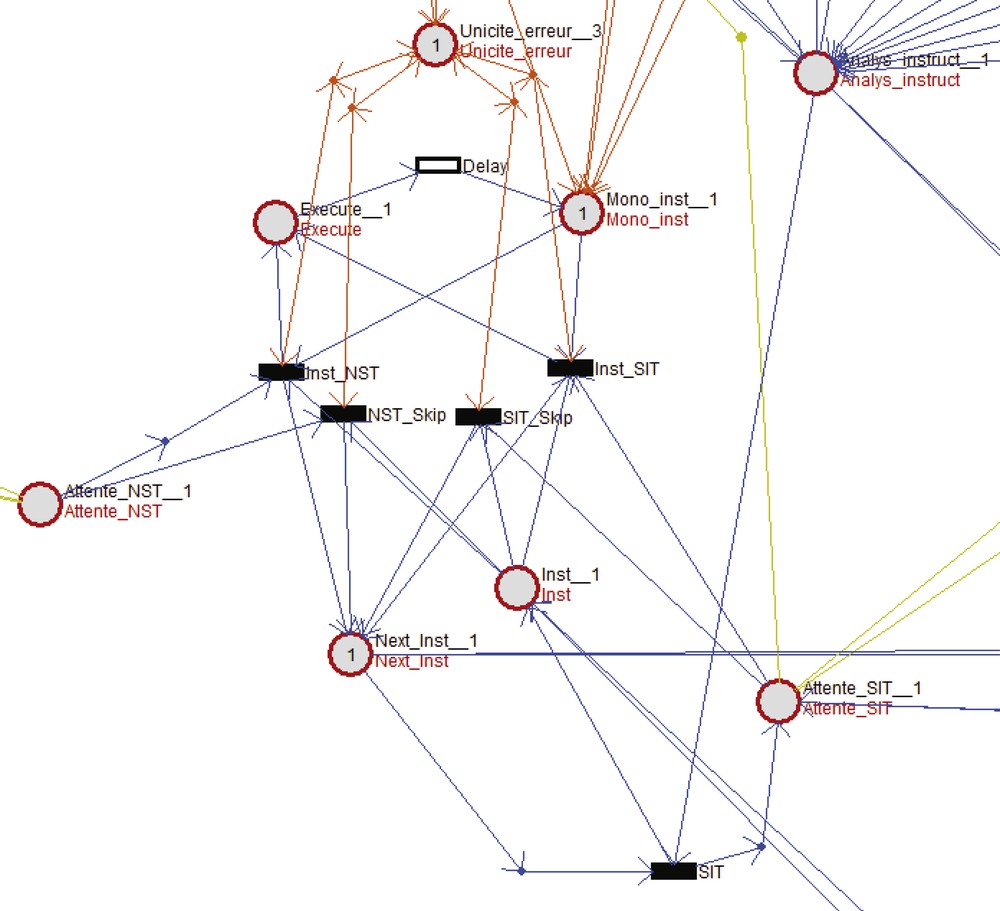
“Analyse-instruct” waits for an instruction and if it is an SIT opcode transition “SIT” is fired. “Execute”, “Mono-inst” and “Delay” with the four transitions below constitute the core of the execution of the instruction that configures the ASIC and counts the pulse width time.
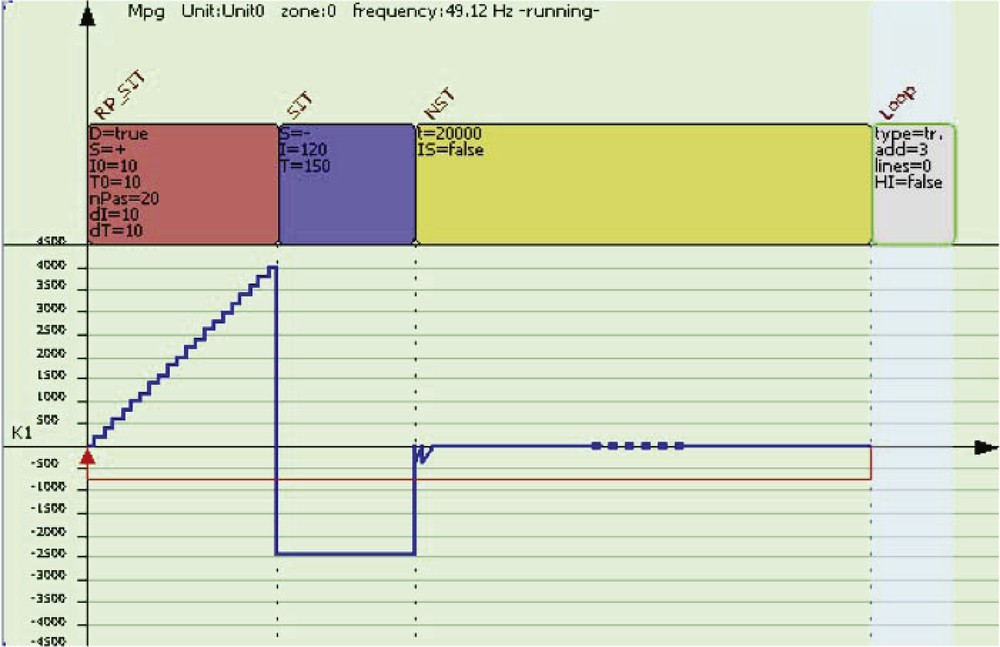
Example of stimulation waveform that needs only four instructions in the following micro-program: RP-SIT, SIT, NST, LOOP with due parameters.
On this very restricted instruction set, one can build few more abstract instructions such as one generating a ramp RP-SIT. Indeed, it is based on repetitive increasing current steps that SIT implements. The parameters of these instructions are: duration, intensity, number of steps, size of step and almost all can be remotely controlled through the network. A detailed description can be found in [29].
Thus, programming a DSU for a specific selective target muscle with less side effects is equivalent to designing and then uploading a micro-program into the DSU, whereas realtime remote control over the network of all the DSU generates the global function such as standing up for a patient with a spinal cord injury.
3 Models, simulations and controls
One efficient way to explore movement deficiencies and assess rehabilitation processes eventually using neuroprostheses, is to perform quantitative and subject-specific simulations. To do so, comprehensive models with meaningful parameters are developed. The challenge is to provide for computational effective models but with a high level of accuracy. Biomechanics of the skeleton is actually sufficiently accurate to use state-of-the-art modeling. However, one key point remains the muscle activation and so modeling. More precisely, controlled models with inputs related to either measured activation on subject - often derived from ElectroMyoGraphy (EMG) - or artificial induced contraction through ES are needed.
The recent advance in technology provided the means of embeding closed-loop control algorithms to adapt the delivery of ES depending on the current situation [32–34]. However, the scientific issues remain opened because of the great variety of non-linear phenomena related to the neuromusculo-skeletal system [35,36]: effects such as muscle fatigue and time-varying dynamics, remaining uncontrolled reflex loops and limited generated force complicate the control task. The use of mathematical models can improve and accelerate the design and the tuning of neuroprostheses for rehabilitation purposes.
A great variety of striated muscle models has been proposed since the 1930s [37], differing in mathematical complexity, level of physiological structures considered and fidelity to biological behavior. During the 1950s, the microscopic behavior of the muscle was investigated and modeled [38] for the first time. Since these articles, modeling never stopped evolving towards more and more detailed developments. We recently proposed a complete multiscale model with control inputs. This model fulfill the well-established laws among which the force-length, the force-velocity and the force-active stiffness relationships [39]. We extended this work to a specific smooth muscle: the detrusor [40] that constitutes the bladder wall and can be activated through neural ES when bladder voiding is deficient in some pathologies.
In both cases, we proposed numerical methods to fit experimental data with models. This issue is often a critical one, in particular with humans protocols. Indeed, less measurements than on animals can be performed and the data obtained are less accurate. Our model is a trade off between mathematical complexity leading to accuracy and model's expressiveness and ability to estimate parameters.
3.1 A controlled muscle model
The model we proposed can be summed up through the following reduced equations:
| (9) |
This set of equations is a simplified version of a more detailed and accurate one that can be found in [39]. Active stiffness kc and force output Fc of the contractile part of the muscle depend on several parameters: Uc is the contraction rate while Ur is the relaxation rate, Lc0 is the length of the contractile element, kmax, Fmax are the maximum values that the muscle can deliver; they depend on the relative length of the contractile element -- relationship known as force-length relationship, a is a shape parameter that allows one to adjust the force-velocity relationship to the studied muscle. The most important is that these equations reflect the short term non linearities that allow for accurate computation when activation is quickly changing. On a long-term, neglecting the fast dynamics, considering isokinetic movement and tetanic contraction, the solution looks like first order differential equations with the following asymptotic values:
| (10) |
The force-velocity relationship appears through the variable and we show consistency with well-established muscle behavior. These equations indicate that the generated force depends on the recruitment α and allows for muscle force modulation. In isometric conditions we have the very simple relationship αFmax for the steady-state induced force. α depends on the current intensity injected in the electrode through a sigmoidal relationship. The higher the level of intensity, the deeper and the thinner the activated axons are, leading to increased number of activated Motor Units (MUs) and thus, of force. Then, simulations, movement synthesis and control theoretical studies become possible. Through geometrical relations at the different joints considered for the movement, forces’ composition coming from mono and bi-articular muscles can be achieved to simulate a global movement induced by ES. This mathematical modeling, close to the physiological reality, is a powerful tool that helps a lot in the design and the use of neuroprostheses. Fig. 4 shows how an open loop movement on a single joint can be simulated and then synthesized, given that the model fits the patient's features. Fig. 5 shows the result on a whole body movement with a patient with a complete spinal cord injury, whose muscles are stimulated. However, automatic control approaches remain complex thus rare, because on one hand formal equations need for simplification to obtain theoretical results such as stability, on the other hand simulations must remain close to the measured data to keep any interest.

Example of a comparison between synthesized and measured data of the knee joint under ES using a model fitting to the patient. Data are extracted from [41], horizontal line represents time in seconds.
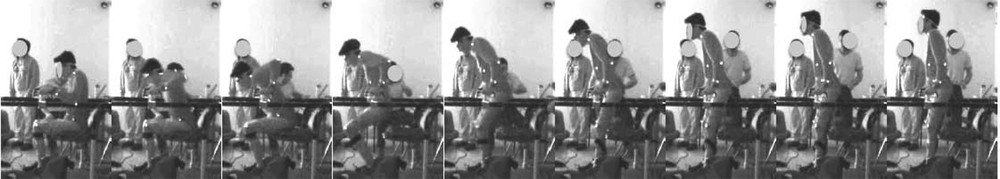
A paraplegic patient stands up with external ES applied on paralyzed muscles (quadriceps, gluteus max, hamstrings). Movement is captured with a MOCAP Vicon system (white dots are markers) and the measured trajectory could be compared to simulated ones.
4 Conclusion
Electrical stimulation is clearly one of the advanced technologies used in implantable systems to restore deeply deficient functions. The set of clinical applications is growing from year to year and nowadays, such complex devices such as neuroprotheses for blind people come slowly to reality. It brings much hope to patients with deficiencies that can not be treated at all such as movements of paralyzed limbs as shown on Fig. 5. However, technology concepts together with physiological studies and mathematical modeling have to be developed at the same time. Indeed, increasing complexity of both the materials and the clinical applications still need for theoretical researches. As regards movement restoration for instance, automatic control theory as used in robotics is increasingly introduced in the domain but needs for specific developments such as muscle modeling. We try to follow this goal that may provide for a major breakthrough but on a long-term research basis. The multidisciplinary aspect of the research becomes obvious and source of great challenges for the next decades.
Disclosure of interest
The author declares that he has no conflicts of interest concerning this article.


