1 Introduction
Fire is a frequent disturbance in Mediterranean areas, with major direct, indirect and sometimes delayed effects on ecosystems [1–3]. Because of recent land-use changes (essentially shrubland encroachment of abandoned rural landscapes) and forecasted climate change (greater seasonal drought and precipitation), fires are predicted to increase in number and/or become more severe in the next few decades [4–6]. Thus, vegetation patterns may be modified as a result of the predicted fire regime changes [7]. Quercus suber L. (cork-oak) forest systems in particular are likely to become regionally endangered if the current trend continues [5,8,9], showing that a multidisciplinary survey is needed [10]. Fire regime [11] refers to the nature, recurrence (frequency, i.e. number of fires, and fire return, i.e. interval of time between two successive fires in a given area), intensity (energy released), season, and size of fires.
Many species of Mediterranean forests and shrublands, as well as open lands dominated by scrubs or perennial grasses, are able to resprout or regenerate from seeds after fire. A long history of fire in Mediterranean areas has strongly selected against fire-sensitive species and promoted a wide range of resistant species, including resprouter and/or recruiter species (seeders) [12]. For instance, Q. suber can quickly and effectively resprout from basal lignotuber but to a greater extent from epicormic stem buds [13]. In general, there is no evidence that fire decreases or eliminates oak-tree populations and the current decline in cork oak is believed to be primarily related to land management systems, pests and diseases, and climate change rather than fire [12,14].
However, few authors have differentiated their sampling design according to fire regime, and particularly fire recurrence, owing to difficulties in accurately determining fire history of different ecosystems, especially in Mediterranean areas, such as in France [15], Spain [2,3,16] and California [17,18].
Plant communities in fire-prone ecosystems have mainly been studied in Mediterranean-climate regions over a range of timescales from a few years (1–3 years) to about two decades [15,19,20]. Knowledge of the effects over longer periods is very limited [21,22]. An increasing wildfire regime or frequently recurring fires may significantly affect ecosystem functions, plant-storage amount, bud-bank availability, etc. and subsequently weaken its ability to return to a pre-fire state [23,24]. Thus, the resilience of vegetation [7,25–27] affects the dominant regional pattern of forest versus shrubland vegetation, i.e. their persistence [5]. Indeed, some species – even favoured species – may decrease in number as a result of recurrent fires because of the time required to build up reserves, or reach maturity and produce seeds [28].
Herbaceous annual sub-communities inserted within perennial communities are particularly resilient; they re-colonize two or three years after fire [15,19] following an “auto-succession” model [2,15,29]. Enhanced “auto-succession” [16] is mediated by efficient recovery strategies following fire, such as resprouting, enhanced seeding and fire resistance (e.g. Q. suber), which influence populations [30,31]. All these processes, with adaptative responses, favour the dominance and coexistence of fire-resilient, resistant or persistent species [32].
In southeastern France, the Var district has been repeatedly affected by large and intense wildfires in recent decades. In particular, two large and intense wildfires (1990 and 2003) burned areas of the Maures massif with acidic soils. Woodlands dominated by Q. suber are contractually protected in this Natura 2000 area, owing to their ecological and patrimonial importance (European Habitat directive 92/43/EEC). Because fires in the Maures massif have been fairly well documented since 1959 in the form of archives, aerial photographs and remote sensing images, we used these data combined with vegetation sampling and measurements of environment conditions to study vegetation patterns in response to abiotic parameters and historical events. Bond and van Wilgen [33] have proposed three hypotheses concerning important fire variables in post-fire dynamics, which are not necessarily exclusive. According to the first hypothesis, referred to as the event-dependent hypothesis, community (richness, biomass, etc.) depends on the number of fires and/or a specific fire event. In the second hypothesis, called the fire-interval hypothesis, community depends on the fire-return interval. Finally, the third hypothesis, known as the self-regulatory hypothesis, assumes that a community depends on its own dynamics with or without a specific event. Keeley et al. [34] extended these hypotheses with a fourth called the environmental filter hypothesis, which considers that environmental variables, such as slope, insolation, precipitation and soil characteristics (structure, texture, nutrient availability, etc.), can also regulate post-fire patterns.
Based on these hypotheses, we examined the effects of fire recurrence on vegetation patterns, i.e. plant composition, richness, and species diversity (taking into account the expressed vegetation, and excluding the seed bank), and their relationship with environmental variables.
2 Materials and methods
2.1 Study area
The study area was located in the Maures massif in southeastern France (43°3′ N; 6°3′ E) (Fig. 1). The annual mean temperature in this region varies between 11 and 14 °C (depending on altitude and distance from littoral) and precipitation is recorded as between 900 and 1100 mm (1960–2008). The altitude of the study plots varied between 50 and 450 m, and they were all characterized by acidic soils developed on magmatic and metamorphic bedrock. All plots were located in the eastern part of the massif, covering an area of around 30,000 ha on a homogeneous granitic and gneissic basement. Over the period 1959–2009, a total of 217 fires larger than 3 ha have burned 59,606 ha in the Maures massif (Fig. 2), a but few large and intense summer wildfires have recurrently burned the eastern part of the massif, including those in 1964, 1970, 1985, 1990 (25,000 ha) and 2003 (14,000 ha). All plots were similar in terms of soils (medium rich and average depth about 25 cm) and topography. Massif ridges and steep valleys were excluded to avoid azonal conditions.
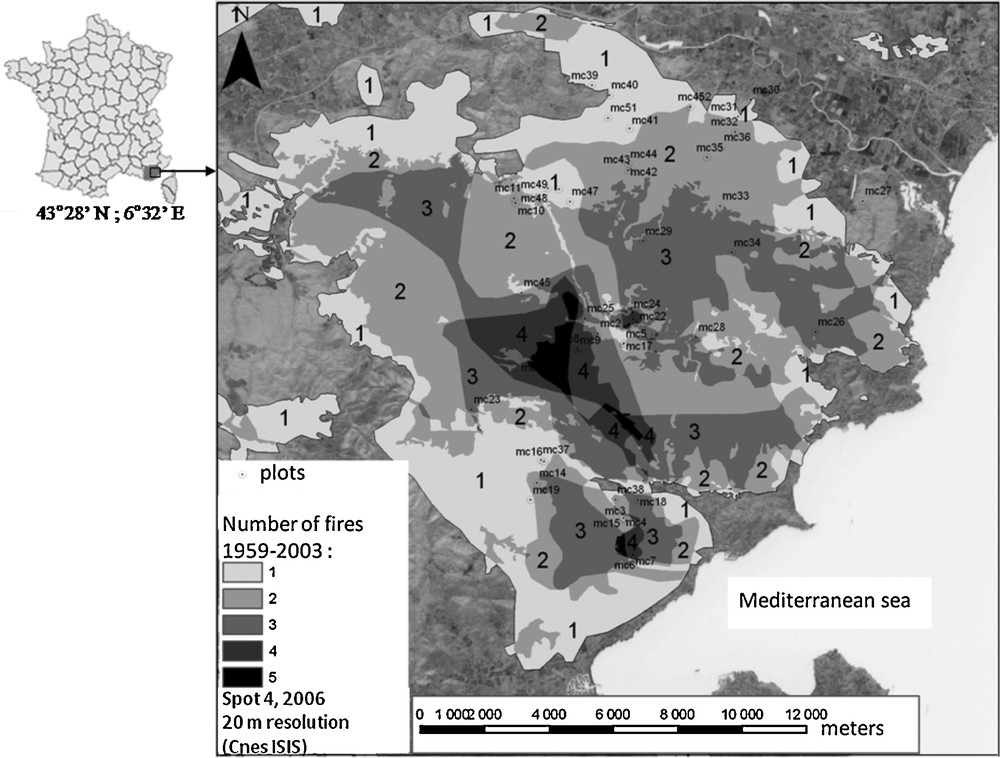
Study area with the number of fires since 1959 in the Eastern part of the Maures’ massif (Var, Southern France). Fire history is based on Mediterranean fire datasets, satellite images and aerial photographs recorded since 1959. The plots are designed by numbers after ‘mc’.
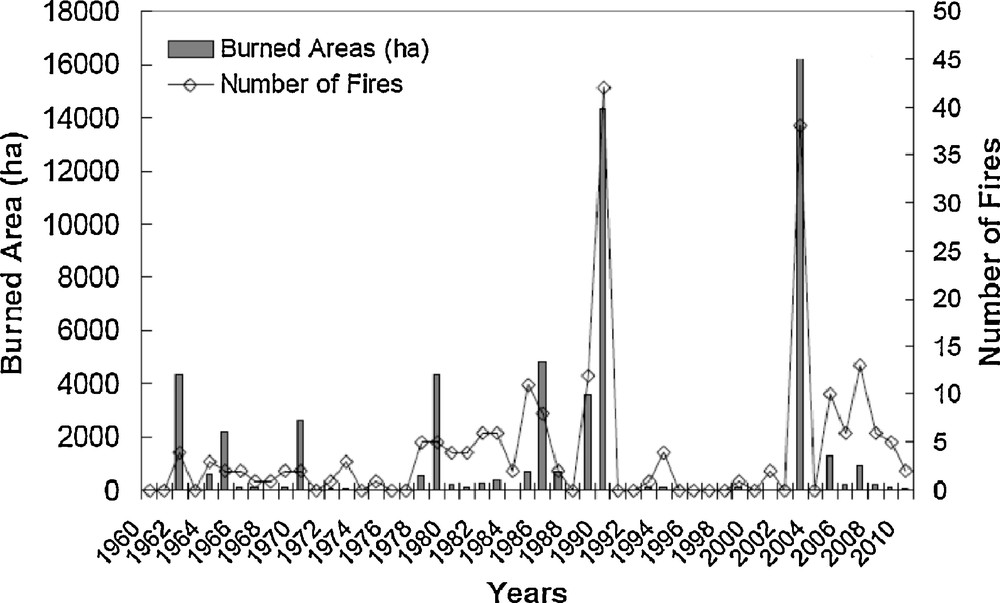
Timeline of fires in the Maures’ massif with burned area (ha) and number of fires.
The main vegetation types of the study region are Q. suber woodlands and shrublands known as ‘maquis’ on acidic soils, including intermediate stages such as the so-called “savannas” reported by Acácio et al. [5]. The most abundant tall shrub species are Erica arborea L. and Arbutus unedo L., whereas the main small shrub species is Cistus sp. pl.
2.2 Sampling
We first carried out a diachronic analysis based on images and aerial photographs taken since 1959 to map all areas that have burned since 1959. Fig. 1 shows the distribution of the major wildfires. Next, we selected plots according to the number and date of fires. Some fires in the area were very intense (summer conditions, strong wind), e.g. in 1959, 1990 and 2003, based on archival information and visual appraisal of aerial photographs and satellite images. Finally, the initial state of vegetation in the area was identified; since the early 1950s, it has comprised woodlands on maquis with Q. suber and E. arborea.
The sampling design was based on fire recurrence. Plots were selected according to the time since the last and penultimate fires. In addition to these two basic parameters, the number of fires was calculated for each plot and plots were classified according to combined criteria referred to as “fire class”, which was subsequently tested (Table 1). Two main reference wildfires were selected because they were similar in terms of fire intensity and covered a wide area in the Maures massif: the 1990 and 2003 fires were used as reference cases for regenerated shrubland vegetation at around 15 years after fire [35] and recently burned vegetation, respectively. Sampling was based on the following classes of fire recurrence and recentness: (i) control plots with no fire since at least 1959 (called ‘NoFire’); (ii) plots burned only once in 1990 (‘One1990’); (iii) plots burned twice in 1964 and 1990 (called ‘Long1990’ because having long fire intervals); (iv) plots burned 2 or 3 times with more recent fires (1970, 1985 and 1990) and short fire intervals (called ‘Short1990’; and (v) plots burned 3 or 4 times including in 2003 and having short fire intervals (called ‘Short2003’). The three central (or intermediate) modalities share the 1990 fire but have different old versus recent fires, and different intervals between fires, thus allowing to test both effects. Classes ‘Long1990’ and ‘Short1990’ were marked by different frequencies of fires; fires occurred at intervals of 26 to 31 years for the first class, compared with 5 to 16 years for the last. These two classes were used to test our hypothesis that the time interval between fires affects post-fire regeneration (old fires in the 1960s vs. more recent fires in the 1970s and 1980s, with a total of 2–3 fires).
Fire classes based on fire recurrence from analysis of images and aerial photographs of the study area taken since 1959.
| Fire class | Number of fires within the stand since 1959 | Time (years) since the last fire | Date of the last fire | Time (years) since the penultimate fire | Date of the penultimate fire |
| ‘No fire’ | None, control | 47 | 1959 at least | Unknown | Unknown |
| ‘One 1990’ | 1 | 16 | 1990 | 31 | 1959 at least |
| ‘Long 1990’ | 2 | 16 | 1990 | 26 | 1964 |
| ‘Short 1990’ | 2–3 (av. 2.8) | 16 | 1990 | 5 | 1985 |
| ‘Short 2003’ | 3–4 (av. 3.7) | 3 | 2003 | 13 | 1990 |
2.3 Field survey
Plant surveys were conducted according to the methodology of Godron [36], which involved assigning codes to data on plants and the environment [37]. We sampled 20 plots distributed throughout the study area per fire class (total: 102 plots, ‘No fire’ class was sampled with 22 replicates instead of 20). Vegetation analysis was performed in 2006 and then repeated in 2007 to avoid a bias due to a possibly exceptional meteorological year and increase the reliability of the data. Using the reference nomenclature of Kerguélen [38,39], plant inventories were made in square plots of area 400 m2 to sample communities in both open (i.e. low and sparse maquis) and rather closed environments (e.g. submature forest stands), which is comparable to most previous studies dealing with ecology and plant communities in forest-dominated contexts [40]. Surveys were made in late spring and all species found were identified. Vegetation composition was measured according to five vegetation layers, ranging from small herbaceous species to high trees as follows: (i) herb layer (species < 0.5 m high), (ii) small shrub layer (between 0.5–2 m high), (iii) shrub layer (2–4 m), and (iv) tree layer (4–10 m). The abundance (percent cover) of all vascular plant species and each layer was evaluated using a phytoecological degree scale modified from Braun-Blanquet [41], here referred to as the cover coefficient (Cov): 1 = species with a cover < 10%, 2 = cover between 10–24%, 3 = 25–49%, 4 = 50–74% and 5 = 75–100%.
In each plot, we also estimated environmental factors such as elevation (m), slope (%), exposure (grades), soil depth (cm), presence of rocks (blocks > 20 cm and stones < 20 cm in size), and ground cover composition (no vascular cryptogams, bare soil, rocky outcrop cover) based on the same classes as the cover coefficient. We also included litter type (loose – made of fresh leaves, compact – made of decomposed leaves, or woody – made of dead woody debris such as twigs and bark fragments) when testing for the influence of vascular vegetation and fire recurrence variables on the likelihood of future fire ignition [42,43]. The variables were grouped into two datasets (FIRE and ENV for environment) (Table 2).
Variables measured on each plot and divided into two datasets: FIRE and ENVironment.
| Variable | Code | Unit | Initial type | Transformed type | Quantitative interval | Ordinal Interval | Group |
| Fire class | Modfire | O | O | [1;5] | FIRE | ||
| Number of firesa | Fires | QT | O | [0;4] | [1;4] | FIRE | |
| Time since the last firea | Tlf | Years | QT | O | [3;47] | [0;2] | FIRE |
| Time since the penultimate fire | Tplf | Years | QT | O | [5;31] | [0;2] | FIRE |
| Distance to fire edge | Edge | Meters | QT | O | [0;2050] | [0;2] | FIRE |
| Elevationa | Alti | Meters | QT | O | [30;450] | [1;4] | ENV |
| Topography | Topo | QL | O | [1;3] | ENV | ||
| Exposurea | Expo | Grades | QT | O | [0;400] | [1;4] | ENV |
| Soil depth | Soil | cm | QT | O | [0;100] | [1;5] | ENV |
| Slopea | Slope | % | QT | O | [0;55] | [1;5] | ENV |
| Flush rocks | Roc | Cov | O | O | [0;3] | ENV | |
| Blocks | Bloc | Cov | O | O | [0;3] | ENV | |
| Stones | Cail | Cov | O | O | [0;4] | ENV | |
| Cryptogamsa | Crypto | Cov | O | O | [0;4] | ENV | |
| Loose littera | Airlitt | Cov | O | O | [0;5] | ENV | |
| Dense litter | Denslitt | Cov | O | O | [0;5] | ENV | |
| Woody litter | Lignlitt | Cov | O | O | [0;4] | ENV |
a Indicates the most contributing factors in each dataset of explanatory variables (Akaike Information Criterion).
2.4 Ecological indices
In the analysis of vegetation patterns, three indices (species richness, diversity and equitability) were calculated to describe vegetation composition in each plot. Species richness was defined as the number of species per plot, while the Shannon diversity index [44,45] takes into account the relative abundance of each species present in the plot such that
The equitability index, E, is given by: , where N is the number of species in the plot.
2.5 Statistical analyses
We firstly performed a Canonical Correspondence Analysis (CCA) [46] to examine plant community ordination. We included the cover coefficient values for 120 species from all vegetation layers that were present at least four times in the whole database. Next, we performed a partial CCA to partition the effects of fire and environmental factors, as well as examine their potential interactions [47]. The Akaike Information Criterion (AIC) was used to identify the most contributing factors in each data set of explanatory variables (Table 2).
To explore the effectiveness of and time required for post-fire regeneration, we performed three series of multiple mean comparison tests (Kruskal-Wallis non-parametric tests, followed by Student-Newman-Keuls “SNK” tests when H0 was rejected). The Student-Newman-Keuls (SNK) method ranks all pairwise comparisons of the means from the smallest to the largest using a stepwise procedure. Within each series, we compared plots based on their species richness, species diversity, and equitability. First, we compared vegetation indices based on the five classes of fire recurrence to evaluate the overall combined effects of fire parameters (number, interval between fires, and time since the last or penultimate fires). Secondly, we analysed the pure effect of time since the last fire on vegetation. As the central fire classes all comprised 16-year-old plots, i.e. a total of 60 plots with the same length of time since the last fire, we randomly selected 20 of the 60 available. We compared these plots with both the 20 replicate plots of fire class ‘No fire’ (no fire since 1959), and the 22 replicate plots of fire class ‘Short 2003’ (last fire in 2003). Thirdly, to test the effect of time since the penultimate fire, we compared the three central fire classes which had all burned in 1990, but which had also burned 31, 26, and 5 years prior to 1990, respectively. Finally, fire variables were tested independently to verify the significance of any variations.
All statistical analyses were performed using the R statistical computing software (2.4.1., R Development Core Team, packages ade4 and vegan) and Statgraphics™ software (Centurion XV, version 15.2.06, Statpoint, Inc.).
3 Results
3.1 Vegetation patterns and common pool of species
Fig. 3 shows that the most contributing variables belonged to either of the two sets: i.e. the number of fires and the time since last fire, exposure, elevation and slope, and the cover by cryptogams and loose litter.
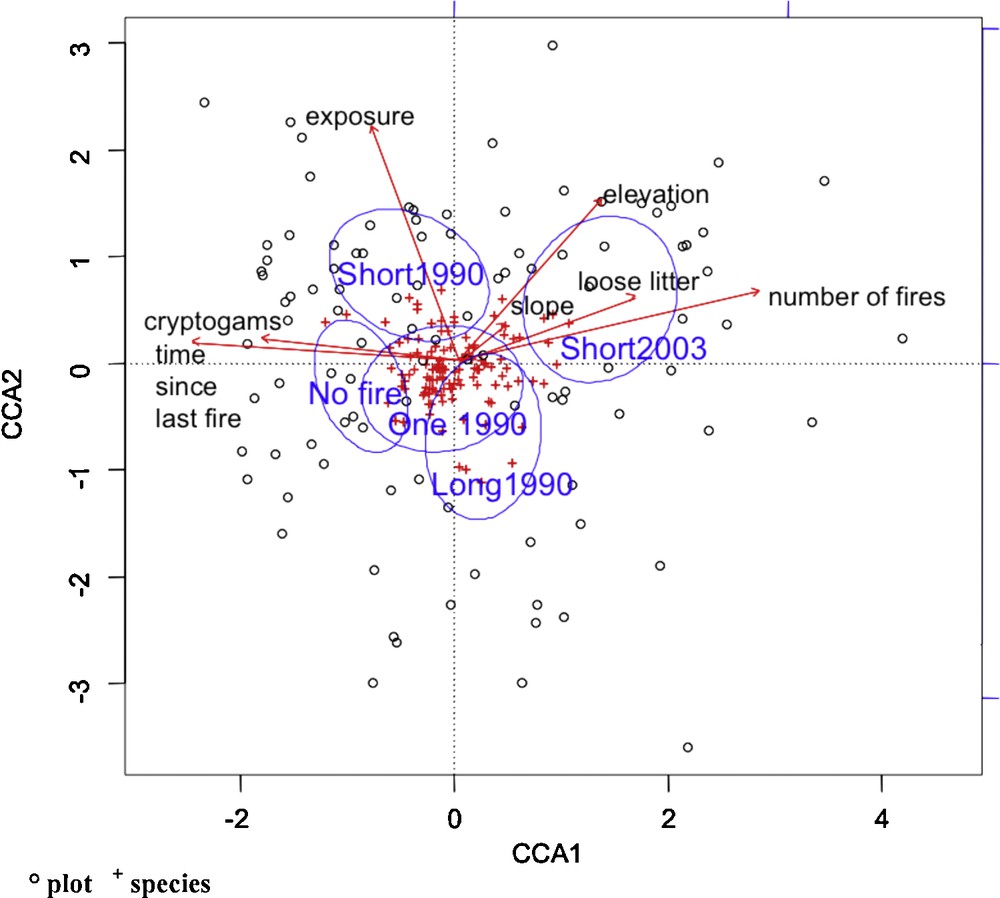
Canonical Correspondence Analysis (CCA) of species and plots showing clustering by fire recurrence class; ‘No fire’ (0 fire), ‘One1990’ (1 fire in 1990), ‘Long1990’ (2–3 fires, long interval between the two last fires, the last in 1990), ‘Short1990’ (2–3 fires, short interval between the two last fires, the last in 1990), ‘Short2003’ (3–4 fires, the last in 2003). Vectors represent explicative variables indicating the influence of the different fire and environmental factors. All species are not specified but the common pool of species is localised at the centre of the ordination. Its species are: Aira cupaniana, Andryala integrifolia, Anthoxantum odoratum s.l. Arbutus unedo, Asparagus acutifolius, Bituminaria bituminosa, Calicotome spinosa, Carduus litigiosus, Cistus albidus, Cistus monspeliensis, Cistus salviifolius, Daphne gnidium, Daucus carota subsp. carota, Erica arborea, Euphorbia segetalis, Geranium robertianum subsp. purpureum, Helichrysum stoechas, Hieracium murorum gr., Lavandula stoechas, Lonicera implexa, Phillyrea angustifolia, Pinus pinaster, Quercus ilex, Quercus pubescens s.l., Quercus suber, Rubia peregrina s.l., Smilax aspera, Trifolium campestre, Urospermum dalechampii, Vicia disperma.
The ordination analysis revealed little variability and only weakly explained variance (10.2% over the first 2 axes, 14.2% for the first 3 axes) due to the relative homogeneity of the sampling plots. Analysis of the species scores obtained from the CCA showed that many species had similar scores along each of two axes, clustering near the centre of the ordination plot. We identified 30 species present in all samples which exhibited the highest Cov values. We defined them as the “common pool of species” (listed in Fig. 3 legend), all of which were typical of Q. suber forests and maquis.
The species mainly lying on the positive side of the first axis were ruderal and/or pyrophile (i.e. Aira provincialis Jordan, Cynosurus echinatus L., Holcus lanatus L., Galactites elegans (Allioni) Soldano), whereas the sylvo-mesophile species (i.e. Hedera helix L., Asplenium onopteris L., Luzula forsteri (Sm.) D.C., Ruscus aculeatus L.) were preferentially distributed on the opposite side. This first axis was best explained by two opposing variables - number of fires (positive side) and time since last fire (negative side). The second axis was best explained by environmental variables, e.g. topography (southern exposure and high elevation at the positive side vs. northern exposure and low elevation at the negative side).
3.2 The contribution of fire and environment
Variance was partitioned according to fire and environmental factors by partial CCA. The difference between the environment and fire data sets was analysed by total variance partitioning: 10.77% due to total environment, 4.97% due to total fire, 9.43% due to environment only, 3.63% due to fire only, and thus 1.34% was the common part where both fire and environment influenced species, giving a total explained variance of 14.40%. Therefore, and despite these low explanatory values, our results indicate that environmental factors better explained the observed plant variation than fire factors (Fig. 3).
3.3 The effect of fire parameters on vegetation patterns
Fire classes with extremely contrasting characteristics (‘No fire’ vs. ‘Short2003’) were distinctly opposed along the main axis of the CCA plot (Fig. 3), corresponding to close forests that were unaffected by fire since 1959 with abundant cryptogamous vegetation (‘No fire’) or open shrublands affected by a large number of fires since 1959 with loose litter. Intermediate fire classes were not differentiated along the first axis but appeared to be relatively differentiated along the second. While class ‘One1990’ plots were located near the centre of the graph, indicating domination of standard local conditions and a common pool of species, class ‘Long1990’ plots were located on the negative side of axis 2, correlating with north exposure, and class ‘Short1990’ plots were located on the positive part of axis 2 correlating with south exposure. This result probably indicates a correlation between the penultimate fire (occurring in 1964 and 1985 for class ‘Long1990’ and ‘Short2003’, respectively) and topographic conditions rather than an ecological explicative process.
3.4 Fire parameters, vegetation richness and diversity
The overall combined effects of the fire parameters (Table 3 and Fig. 4) distinctively affected the richness and Shannon's diversity of trees, herbaceous species and shrubs. Equitability indices did not vary significantly (the distribution of plants between species was relatively stable), in contrast to Shannon's diversity index which mostly correlated with richness. The richness/diversity of trees was significantly less for the unburned compared to the most recently burned plots, with similar values for the intermediate fire classes, indicating a major influence of time since the last fire (classes ‘No fire’ vs. ‘Short2003’) and little influence of time since the penultimate fire (central classes). The species richness (but not Shannon's diversity) of herbaceous species was the only index which was significantly higher in unburned plots than in recently burned plots (fire class ‘Short2003’). The species richness/diversity of shrubs was lowest for the most recently burned class and highest for the intermediate classes.
Significant results of the mean comparison tests (Kruskal-Wallis non-parametric tests combined with Student-Neumann-Keuls “SNK” tests when we rejected H0) performed on the medians of species cover coefficients (Cov).
| Test Series | Layer | Indices | Khi2 | P | SNK homogeneous groups |
| Overall combined effects of fire parameters | Trees | Richness | 40.017 | 0.000 | 1c 2b 3b 4b 5a |
| Shrubs | Richness | 13.516 | 0.009 | 1b 2b 3b 4b 5a | |
| Herbs | Richness | 14.164 | 0.006 | 1b 2a 3ab 4ab 5a | |
| Number of fires | Trees | Richness | 39.093 | 0.000 | 1c 2b 3b 4b 5a |
| Shrubs | Richness | 8.571 | 0.015 | 1b 2b 3b 4b 5a | |
| Herbs | Richness | 8.832 | 0.010 | 1b 2a 3ab 4ab 5a | |
| Time since the last fire | Trees | Richness | 31.108 | 0.000 | 1c 234b 5a |
| Shrubs | Richness | 8.992 | 0.011 | 1b 234b 5a | |
| Small shrubs | Diversity | 6.317 | 0.042 | 1a 234ab 5b | |
| Herbs | Richness | 13.138 | 0.001 | 1b 234b 5a | |
| Time since the penultimate fire | Small shrubs | Richness | 6.263 | 0.043 | 2ab 3b 4a |

Richness (N, number of species) and diversity (Shannon index) for trees, shrubs, small shrubs and herbs (values are means per fire-recurrence class, with ‘standard-error’, SE). Fire-recurrence classes: ‘No fire’ (0 fire), ‘One1990’ (1 fire in 1990), ‘Long1990’ (2–3 fires, long interval between the two last fires, the last in 1990), ‘Short1990’ (2–3 fires, short interval between the two last fires, the last in 1990), ‘Short2003’ (3–4 fires, the last in 2003). The ** and *** indicate the P value < 0.01 and 0.001 (Kruskal-Wallis tests) and the letters a, b, c correspond to the homogeneous groups given by Student-Newman-Keuls tests.
The effect of the number of fires was independently tested using the same method (Kruskal-Wallis and Student-Newmann-Keuls) and gave similar variations for the three indices in each layer (Table 3). The effects of the time since last fire were also analysed: tree layer was the most affected showing significant differences for all classes. For herbaceous species and shrubs, species richness was significantly lower only during a period immediately following the fire (fire class ‘Short2003’). Finally, the small shrub layer was also affected, showing significant differences in the diversity indices between the most contrasting fire classes, with the highest values for fire class ‘Short2003’.
Results of multiple comparison tests based on the time since the penultimate fire (Table 3) confirmed that this fire variable only affected small shrubs. Their species richness/diversity indices were lowest in recently burned plots and highest in the class affected by old fires before 1990.
4 Discussion
4.1 Common pool of species
The identified common pool of species was typical of both open maquis and closed forest. Based on their characteristics, we grouped species into five categories as follows:
- • sclerophyllous species of thickets (Quercus ilex L., A. unedo L., Smilax aspera L., Rubia peregrina L., Asparagus acutifolius L., and E. arborea L.);
- • heliophilous and pyrophilic species of the low maquis (Cistus monspeliensis L., Calicotome spinosa Link, Cistus albidus L., Cistus salviifolius L., and Lavandula stoechas L.);
- • herbaceous species among which some are ruderal (Bituminaria bituminosa (L.) Stirton, Andryala integrifolia L., Vicia disperma D.C., Urospermum dalechampii (L.) Scopoli ex Schmidt, Carduus litigiosus Nocca & Balbis and Euphorbia segetalis L.);
- • semi-forest herbaceous species typical of humic soils and shaded environments (Hieracium murorum L. and Geranium robertianum subsp. purpureum (Villars) Nyman);
- • and the main forest tree species (Quercus pubescens Will. and Q. suber L.).
Some of these species are resprouters (e.g. Quercus sp. pl.), while others are seeders (e.g. Cistus sp. pl.). Many of these species are also commonly found in cork-oak woodlands in other Mediterranean regions, such as Algeria [48]. Most species were closely associated with areas of frequent fire recurrence, such as Q. suber L., A. unedo L., C. spinosa Link, Cistus sp. pl. and some semi-ruderal herbaceous species, e.g. E. segetalis L. or B. bituminosa (L.) Stirton.
The heterogeneous common pool of species indicates global adaptation of vegetation to recurrent fires rather than merely select species (e.g. heliophilous/pyrophilous, or sclerophyllous maquis/forest), in agreement with the initial floristic model of Egler [29].
4.2 Effects of fire recurrence on vegetation and environmental filter
The number of fires since 1959 affected trees, shrubs and herbaceous species, but with significant differences (Fig. 4). Vegetation affected by the 1990 fire exhibited similar patterns of richness regardless of the number and date of previous fires. This suggests that the time since the last fire has a stronger effect than the number of fires, provided the former is sufficiently long to allow some vegetation to recover. This finding was confirmed by the mean comparison tests: more vegetation layers were affected. In plots with a short time since the last fire, there was a negative effect on trees and positive effect on small shrubs with respect to species richness/diversity. The effect of fire on herbaceous species was only slight, probably because most post-fire annuals are transitory in these ecosystems [15,16,19]. Finally, small shrubs reacted to the penultimate fire; their richness index took a longer time to recover.
Fire recurrence affected plant composition, but this effect was limited and/or temporary. The herbaceous layer exhibited a strong reaction to the number of fires but recovered rapidly after fire. The impact on tree overstory was low among intermediate fire classes because the dominant tree species (Q. suber L.) have especially high fire resistance and resilience. Vegetation type is thus a strong factor in post-fire dynamics in addition to dryness [31,49,50]. Similarly, most shrub species, such as E. arborea, strongly resprout after fire and have persisted since the early Holocene [51]. Even in fire-driven ecosystems, environmental or landscape factors can act as strong filters during successional processes [22,27].
The results of variance partitioning, despite being low, suggest that environmental factors had a globally greater impact than fire factors (double in terms of variance), even though we selected plots with similar soil conditions and topography. The CCA plot indicates the main contributory environmental factors, such as exposure (mainly south for the plots affected by high fire recurrence and recent fires, see also Acácio et al. [5]) and slope, which is an important factor in fire behaviour, governing fuel-moisture content, vegetation structure and wind [52–54].
Indeed, the results clearly show that past fires affect future fire behaviour and the most fire-prone areas are spatially explained by the proximity of wildland-urban interfaces and self-regulation in north exposures [55].
The resulting variables of fire-vegetation interplay show that the more the stands have been burned, the more the litter is loose, as confirmed by a flammability study on the same site [56]. Similarly, the cryptogam cover was higher in plots that had not burned for a long time, which is a good indication of ecosystem restoration following fire. Nevertheless, the same results show that fire classes ‘No fire’ and ‘Short1990’ have similarities, as confirmed by plant trait and structure analyses [50,57], for which we hypothesized a signal against the general trend. According to these analyses, higher values of structural and functional richness as well as diversity were observed for classes ‘No fire’ and ‘Short1990’ plots, implying a high frequency of fire recurrence led to tree, shrub and herb resilience. We could cite the Intermediate Disturbance Hypothesis for which a maximal diversity can be observed, but emphasizing that our sampling is based on a fire history of fifty years, which is not really sufficient for talking about an intermediate level of disturbance. Nevertheless, the relationship between plant composition and fire recurrence observed in our study seems to follow the same patterns as vegetation structure and subsequent flammability. Indeed, previous studies [50,56] have shown a Gaussian-like relationship between biomass (dominated first by shrubs then later trees) and fire risk. Finally, the vegetation structure analyses indicated there were other differences that make it difficult for many heliophile species to establish than plant composition alone, which while complementary, are needed to explain the lower diversity in high maquis.
4.3 Forest persistence, resilience and auto-succession
The floristic similarity is consistent with the fact that in fire-prone Mediterranean ecosystems, plant communities have been shaped and selected by fires since the Neolithic period [51]. Thus, disturbance by fire can be considered as an internal variable in the dynamics, referred to as endogenous (sensu Rykiel [58]).
Vegetation richness and diversity were globally lowest in recently (and more recurrently) burned plant communities. This suggests that the resilience of vegetation could be affected by a high fire recurrence rate (here above 3–4 fires over the last 50 years), in agreement with recently reported findings from several studies at different scales [7,26,49]. In fact, a minimum amount of time is needed to recover typical vegetation after fire and then regain the overall intrinsic resilience capacity.
The post-fire dynamics were generally characterized by a decrease in the abundance of some species (e.g. Q. suber L.) and/or the positive selection of species highly adapted to recurrent fires (e.g. Cistus sp. pl.). However, small shrubs exhibited the highest diversity a short time after the last fire. This might be linked to the fact that repeated fires favour the establishment or persistence of various short-lived resprouting or seeder shrubs. In contrast, longer intervals between successive fires favour the persistence of long-lived resprouters, such as E. arborea L. or Quercus sp. pl., and the late resilience of cryptogams. This allows some species to grow and dominate, such as in high maquis where Erica dominates with high biomass rates and even high necromass rates following dominancy and then senescence. In southern Portugal Q. suber dominated systems, Acácio et al. [5] have shown that fire recurrence, environmental drought and erosion following intensive precipitation events are the main factors explaining persistence or transition of vegetation types: shrubland persistence positively correlated with wildfire occurrence, particularly on southern exposures, whereas forest persistence increased with slope steepness and decreased with wildfire occurrence.
4.4 A global scheme of post-fire plant succession
Based on our results, the four hypotheses on post-fire dynamics from Bond and van Wilgen [33] and Keeley et al. [34] could all apply because they are not necessarily exclusive. The first (event-dependent) hypothesis is supported by our results showing significant differences according to the number of fires. The second (fire-interval) hypothesis is consistent with both the influence of the interval since the last fire (only significant for the species richness/diversity of small shrubs) and also the significant fuel accumulation associated with formation of high maquis when there is a long period of recovery after an intense fire. The third (self-regulatory) hypothesis is supported by the marked similarity between communities of different fire classes and the inverse evolution of high and low vegetation layers (trees vs shrubs and herbs). Finally, the fourth (environmental filter) hypothesis agrees with our variance partitioning results, which suggested the importance of environmental filters such as slope or exposure, even though we focused on similar plots belonging to one geographical site and excluded sites with azonal vegetation.
5 Conclusion
This study shows that the effects of fire recurrence on a 50-year time interval in French Mediterranean ecosystems growing on acidic soils were only significant when comparing the most contrasting fire classes because of the dominant effect of time since the last fire and its (negative) correlation with the number of fires. However, the effects differed according to the vegetation layer: negative effects for trees and herbs and positive effects for small shrubs, according to fire variables. Overall, the observed strong species similarity was consistent with the long fire history of French Mediterranean ecosystems, as stated in the concept of endogenous disturbance of ecosystems sensu Rykiel [58]. The length of time since the last fire and number of fires were the most explanatory fire variables, but the whole set of environmental factors were the most explanatory factors overall. This is linked to the close interrelationship between fire, plants and environment; a high rate of fire recurrence can govern the future persistence or expansion of shrublands, as observed in other Mediterranean areas [5]. To examine the latter effects further, plant traits and structure were analysed [50,57] and indicated strong differences between the intermediate fire recurrence classes, as found for other disturbances, such as grazing or clearing [59]. These findings have important implications for both biodiversity conservation and fire-risk prevention.
Disclosure of interest
The authors state that the content has not been published or submitted for publication elsewhere in any language, that all authors have contributed significantly, that all authors are in agreement with the content of the manuscript and that no conflict of interest exists.
Acknowledgements
This work was financially supported by the ‘Conseil Régional Provence Alpes Côte d’Azur’ and the CEMAGREF through a PhD scholarship, and several other projects (IRISE, MEDDATT-DNP). We are grateful to Christelle Hély and Serge Payette who provided helpful comments on the manuscript and Véronique Bonnet, Clémentine Coiffait, Daniel Pavon, Arne Saatkamp and Mathieu Santonja for conducting field sampling and/or plant identification.


