1 Introduction
In many fish species, aggressive interactions lead to dominance hierarchies where individuals usually fight for priority access to food, mate or space [1]. In adult fishes, sound production has been extensively described in these social contexts [2–4], which suggests that sounds may be useful to send information to competitors. Aggressive behaviour usually appears in the first days of life due to competition for food and space. It is thus possible that acoustic signals play a role during agonistic interactions between juvenile fish [5,6]. However, and while numerous studies have examined developmental changes in vocalizations in mammals and birds [7], few studies have focused on the ontogenesis of acoustic communication in fish, and little has been done in parallel as concerns the development of aggression. Ontogenetic development of vocalization and the occurrence of agonistic behaviour have been described in the croaking gourami Trichopsis vittata (Cuvier 1831) [8] in which first interactions between individuals relate to feeding context and consist in a series of approaches and flights. More aggressive displays, like lateral displays, occur after 3 weeks and first sounds, consisting in single pulses, are recorded after 8 weeks. The most aggressive behaviour, e.g. biting, occurs at the age of 10 weeks. During this period of time, sound characteristics evolve. Besides the transition from single to double pulses, the pulse period, the number of pulses and their intensity increase, while their dominant frequency decreases. The same changes in acoustic features were found in the grey gurnard Eutrigla gurnardus (L. 1758) [9] and in the squeaker catfish Synodontis schoutedeni (David 1936) [10]. In the Lusitanian toadfish Halobatrachus didactylus (Bloch & Schneider 1801), sound production appears in small individuals after a few months of age (4 cm in size) [11]. Sounds are mainly single grunts while adult sounds consist in series of grunts. As in T. vittata, E. gurnardus and S. schoutedeni, pulse period and intensity increased while pulse dominant frequency decreases with size. Conversely, the duration and number of pulses within a grunt increase with growth.
The present study focuses on Metriaclima (formerly Pseudotropheus) zebra (Boulenger 1899), a sound-producing rock-dwelling cichlid fish living in the sediment-free rocky coasts of Lake Malawi, in which sounds are produced during both aggressive and courtship interactions [12–14]. However, little is known about the occurrence and role of vocalization in juvenile fish, especially when reared in groups. The aim of this work is: (1) to describe the evolution of sounds production in a group of juvenile M. zebra from hatching to 4 months old; (2) to test the link between vocalizations and agonistic behaviour in this fish by setting dyadic interactions at three different ages: 40-day old, 200-day old and adult fish.
2 Material and methods
2.1 Fish
Adult M. zebra were purchased from N’Guyen International (Kingersheim, France) and stored in heterosexual groups in two holding tanks (60 cm × 120 cm × 50 cm) each containing 10–12 mature individuals (from 1 to 3 years old), with a male:female sex-ratio of 1:2. Each tank was equipped with an external filter (Rena Filstar xP3, Rena France; www.rena.fr), aeration, sand substrate, and terracotta pots and bricks as shelters. The temperature was maintained at 25, range ± 2 °C (Internal heater RenaCal 200, Rena France), and the pH at 8.0 on a 12L:12D cycle. The fish were daily fed with commercial cichlid food (JBL NovoRift, JBL GmbH & Co. KG; www.jbl.de/en) complemented with a frozen mixture of mussels, shrimps and spinach once a week. Every week during 2 months, about one third of the water was changed for colder water (c. 20 °C). These changes of temperature would promote reproduction, and make females begin to spawn. M. zebra is a mouthbreeding cichlid in which females keep eggs in their mouth once a male fertilized them. When detected, mouthbreeding females were isolated in a net in order to incubate eggs. After 21 days, females released tens of juveniles with a standard length (LS, from the tip of the head to the caudal peduncle) of about 1 cm in the water.
2.2 Experimental set-ups
For the first experiment, five days after hatching, 15 juveniles coming from a single brood were put in an aquarium (25 cm × 25 cm × 25 cm). To improve the quality of sound recording, the aquarium was placed in a larger one (60 cm × 50 cm × 50 cm), and three internal walls of the aquaria were covered with acoustically absorbent material (high density foam) in order to reduce resonance and reflexion effects (Fig. 1). Besides, the experiment was performed in a sound proof chamber (PRIMO Silence-Box©, Tip Top Wood; www.tiptopwood.com) to minimize external background noise and electric devices were switched off to allow recordings of fish sound. A video camera (BUL520, Active Media Concept; www.amc-tec.com) was positioned in front of the set up and a hydrophone (Aquarian Audio Products, H2a-XLR, sensitivity: −180 dB re 1 V/μPa, flat frequency response: ±4 dB in the range: 20 Hz to 4.5 kHz; www.aquarianaudio.com) was positioned in the middle of the aquarium. Acoustic signals and interactions between individuals were recorded during 15 min before feeding, during 4 months at a rate of three times per week. Over the course of the experiment, subordinate individuals that received too much aggression from dominants were taken out of the set-up and returned to their storage tank. At the end of the experiment, the group was made of six juveniles. No regular biometry was conducted in order not to disturb the group structure. Fish were homogeneous in size, c. 1 cm, at the beginning of the experiment and heterogeneous, 1.5–3 cm, at the end.
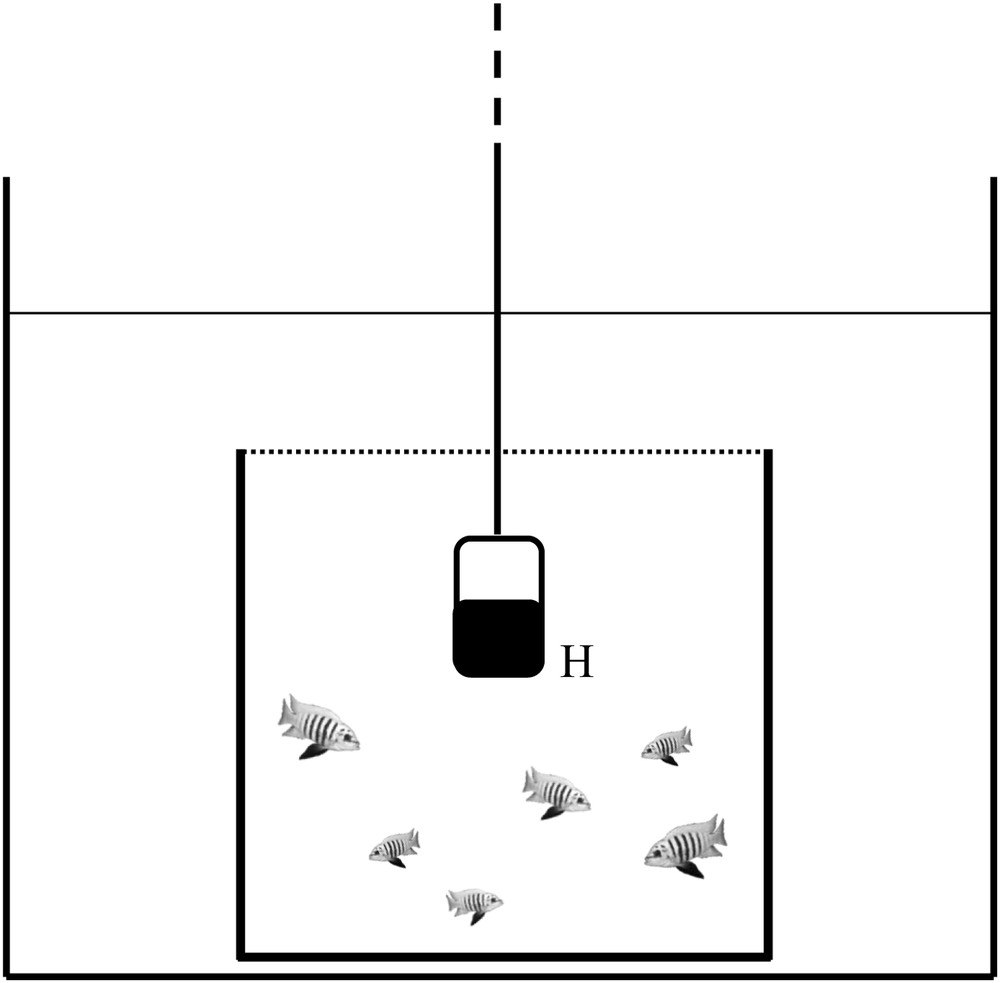
Experimental setup (not to scale) for the study of a group of juveniles (dashed line represents connection to the computer). The inner aquarium is covered with a net in order to constrain the fish (dotted line). H: hydrophone.
For the second experiment, two groups of juvenile fish (40-day and 200-day old individuals) and a group of adults (> 600-day old) were used to set dyadic interactions. Forty day old individuals (n = 20) were obtained from a single hatching and, just after the female released them into water, have been divided into two sub-groups of 10 individuals in two separate aquaria (20 cm × 30 cm × 30 cm) equipped with filter, aeration, sand substrate, and bricks as shelters. These two sub-groups had no possible visual, chemical, acoustic or tactile interactions for 20 days. Considering the mouthbreeding time and the subsequent isolation period, fish were 40-day old at the beginning of the experiment. Two hundred day old individuals (n = 30) were also obtained from a single hatching (of another female from a different tank) and have been separated into two sub-groups of 15 individuals. Again, the two sub-groups were maintained in two separate aquaria (20 cm × 30 cm × 30 cm) during 180 days. Considering the mouthbreeding time and the subsequent isolation period, fish were 200-day old at the beginning of the experiment. Dissection of the genital tracts showed that neither 40-day nor 200-day old fish were mature. The adult group was composed of six males from three different storage tanks. They were approximately 2 years old at the time of the experiment.
Dyadic interactions were set between unfamiliar individuals in an experimental setup consisting of (8 cm × 30 cm × 30 cm) aquaria for juveniles and (20 cm × 30 cm × 30 cm) for adults, separated in two compartments by two contiguous removable partitions, one opaque and one transparent. Each compartment contained a filter, aeration, an internal heater, a shelter and a sand substrate. The aquaria were placed on a vibration-insulated shelf and located in an acoustically insulated room in order to minimize background noise. A video camera (BUL520, Active Media Concept; www.amc-tec.com) was positioned in front of each aquarium and two hydrophones (Aquarian Audio Products H2a-XLR, sensitivity: −180 dB re 1 V/μPa, flat frequency response: ±4 dB in the range: 20 Hz to 4.5 kHz; www.aquarianaudio.com) were placed in the aquarium, one per compartment.
Twenty-four hours before the start of the experiment, two fish of the same age (40 days, 200 days or adults) were introduced in the experimental aquarium, one fish per compartment, and kept isolated. This procedure allowed individuals to acclimatize and become resident in their own compartment. At the beginning of the trials, all electric devices were switched off in order to reduce background noise. Audio and video recordings started with a 10 min control period during which both fish were still isolated. At the end of the control period, the opaque partition was removed, and the two individuals were allowed to interact visually for 10 min. For each dyadic interaction, two fish of two different stocking aquaria were chosen to form homogeneous pairs, i.e. with similar total length and weight.
Five dyadic interactions between 40-day old individuals (n = 10, with a mean ± SE LS of 1.51 ± 0.03 cm and a mass of 0.45 ± 0.03 g), five dyadic interactions between 200-day old individuals (n = 10, with a mean ± SE LS of 4.96 ± 0.41 cm and a mass of 2.46 ± 0.54 g), and three dyadic interactions between adult individuals (n = 6, with a mean ± SE LS of 7.27 ± 0.29 cm and a mass of 5.89 ± 0.11 g) were performed.
For both set of experiments, hydrophones were connected to a preamplifier (Yamaha MLA8, Yamaha Music France; www.yamaha.com) and then to the video capture card (Osprey-450e; www.viewcast.com/products/osprey-cards) that synchronizes audio and video signals.
Behavioural data were collected from the videos recorded during the trials by means of the behavioural transcription software EthoLog 2.2.5 [15]. The total aggressiveness, defined as the sum of aggressive behaviour, i.e. lateral displays, quivers, charges and bite attempts performed either at the group level for the first experiment or by each individual of a pair in the dyadic interactions was then calculated.
Recorded sounds were digitized at 44.1 kHz (16 bit resolution). The total number of sounds (with clear starts and ends and a good signal-to-noise ratio) produced at the group level in the first experiment or by each individual in the second one was counted and the duration of pulses and their instantaneous frequency (measured as the inverse of the period of maximum amplitude) were measured from oscillograms with Praat software, v.5.0.35 (Boersma & Weenink, 1992–2008) (Fig. 2). Statistical analysis was carried with STATISTICA 6.0 (Statsoft Inc. 2004) and all tests were two-tailed.
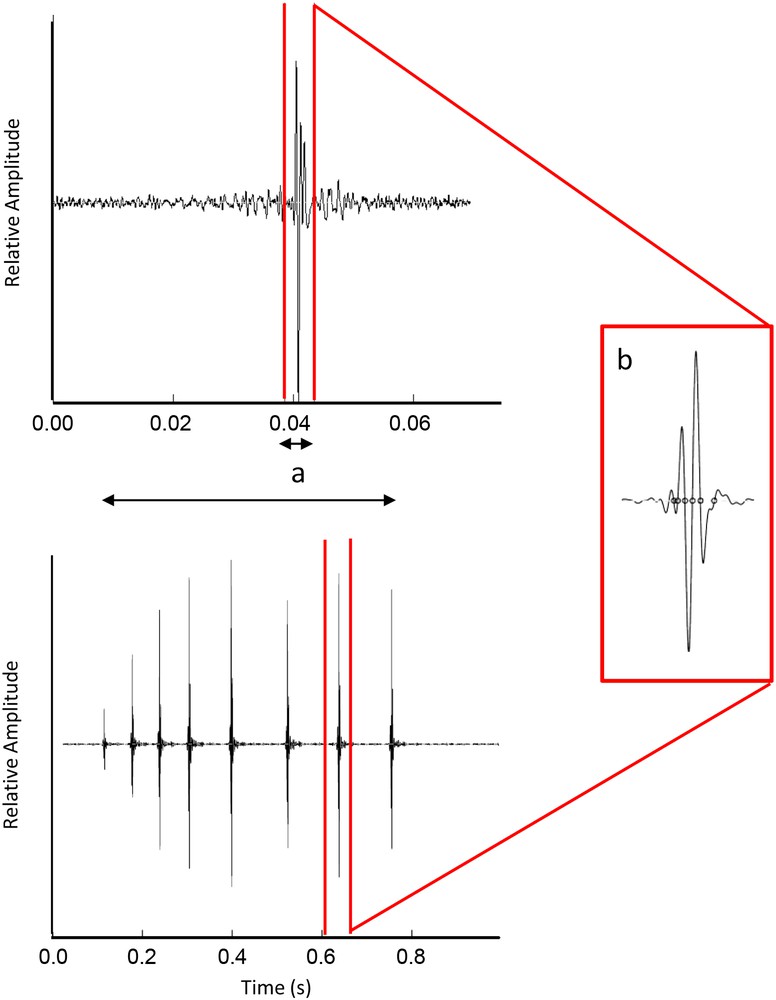
Oscillograms of a sound produced by a juvenile (top) and an adult (bottom) M. zebra in agonistic context. The measured parameters are represented: sound duration (a) and period of maximum amplitude used to calculate instantaneous pulse frequency (b).
3 Results
In the first experiment, no sound was recorded between day 26 and 43, probably due to the fact that fish of this age could not produce sound although we cannot exclude that sounds’ intensity was too low to be detected. From day 43, the number of sounds produced significantly increased until day 138 (simple linear regression analysis, n = 25, r = 0.45, P = 0.02) (Fig. 3).
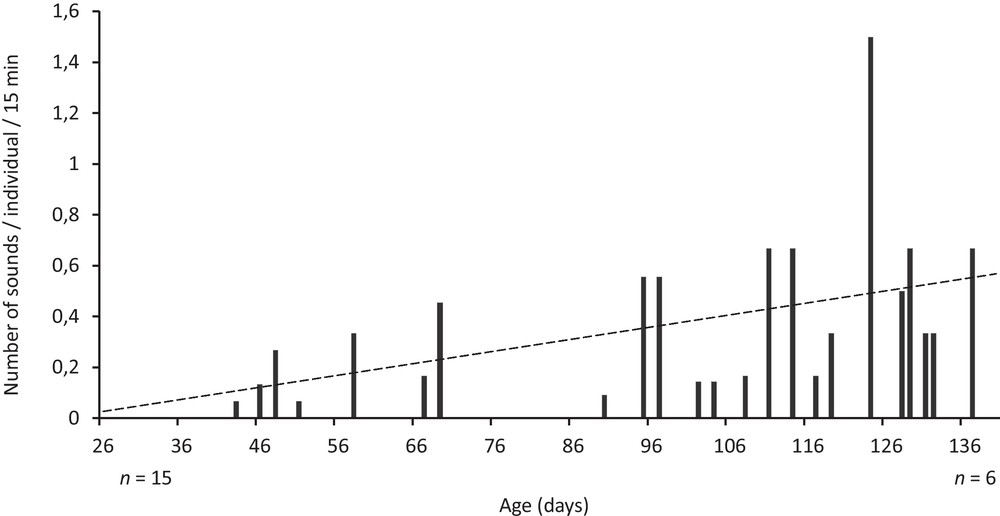
Number of sounds produced per individual over a period of 15 min within the group of juveniles from day 26 (n = 15) until day 138 (n = 6) (linear regression equation: number of sounds/individual/15 min = 0.005 × age [days] + 0.021).
During dyadic interactions, 40-day old individuals exhibited very little aggressiveness (Table 1), but all the possible aggressive behaviour generally encountered in adults [16] was observed. The two individuals mainly swam within their respective compartment and seldom displayed aggressive behaviour towards each other. Due to this low number of behaviours, winners and losers were hard to distinguish at the end of the interactions. Likewise, and in accordance with the group observation, few sounds were emitted.
Total number of sounds, total aggressiveness (number of aggressive behaviours), and status (winner/loser, defined from the total aggressiveness) recorded during dyadic interactions in 40-day old, 200-day old and adult fish.
| 40-day old | 200-day old | Adults | ||||||
| Total number of sounds | Total aggressiveness | Status | Total number of sounds | Total aggressiveness | Status | Total number of sounds | Total aggressiveness | Status |
| 1 | 7 | Equality | 0 | 64 | Loser | 22 | 234 | Winner |
| 0 | 5 | Equality | 0 | 119 | Winner | 2 | 34 | Loser |
| 0 | 1 | Loser | 2 | 52 | Winner | 14 | 187 | Winner |
| 0 | 7 | Winner | 0 | 43 | Loser | 3 | 47 | Loser |
| 0 | 3 | Equality | 1 | 13 | Loser | 16 | 187 | Winner |
| 1 | 1 | Equality | 0 | 19 | Winner | 5 | 67 | Loser |
| 0 | 1 | Equality | 2 | 44 | Equality | |||
| 0 | 3 | Equality | 0 | 42 | Equality | |||
| 2 | 1 | Equality | 0 | 110 | Loser | |||
| 0 | 0 | Equality | 2 | 127 | Winner |
Conversely, a larger amount of aggressive behaviour was observed during dyadic interactions in 200-day old individuals and adults with a significant increase of the total aggressiveness from 40-day to 200-day individuals and to adults (one-way ANOVA, F2,27 = 12.71, P < 0.001, the post-hoc Fisher tests were significant at P < 0.05). Likewise, the total aggressiveness of a pair (summed aggressiveness of both individuals) increased with age (simple linear regression analysis, n = 15, r2 = 0.90, P < 0.001). In 200-day old and adult individuals, it was possible to define a winner and a loser at the end of the interaction (Table 1). However, there were significant differences between the winners and the losers only for adults regarding the total aggressiveness (Wilcoxon paired test, n = 10, Z = −1.99, P < 0.05). In adults, winners emitted significantly more sounds than losers (Wilcoxon paired test, n = 10, Z = −1.96, P < 0.05).
In both experiments, sounds of juveniles were made of single pulses, while adult sounds were constituted of burst of pulses (5 to 10 pulses typically, [14,16]) (Fig. 2). The analysis of sound characteristics performed on sounds recorded in the group of juveniles (first experiment) showed that isolated pulses had a mean ± SE duration of 5.08 ± 0.25 ms, with a instantaneous frequency of 1610.53 ± 77.54 Hz and that these two parameters decreased from day 48 to day 138 [r = −0.33, P < 0.05 for instantaneous frequency, Fig. 4(a); r = −0.63, P < 0.001 for pulse duration, Fig. 4(b)]. Sounds were produced mainly during a chase (46.5%) or were associated to visual displays, i.e. lateral displays and quivers (36.6%).
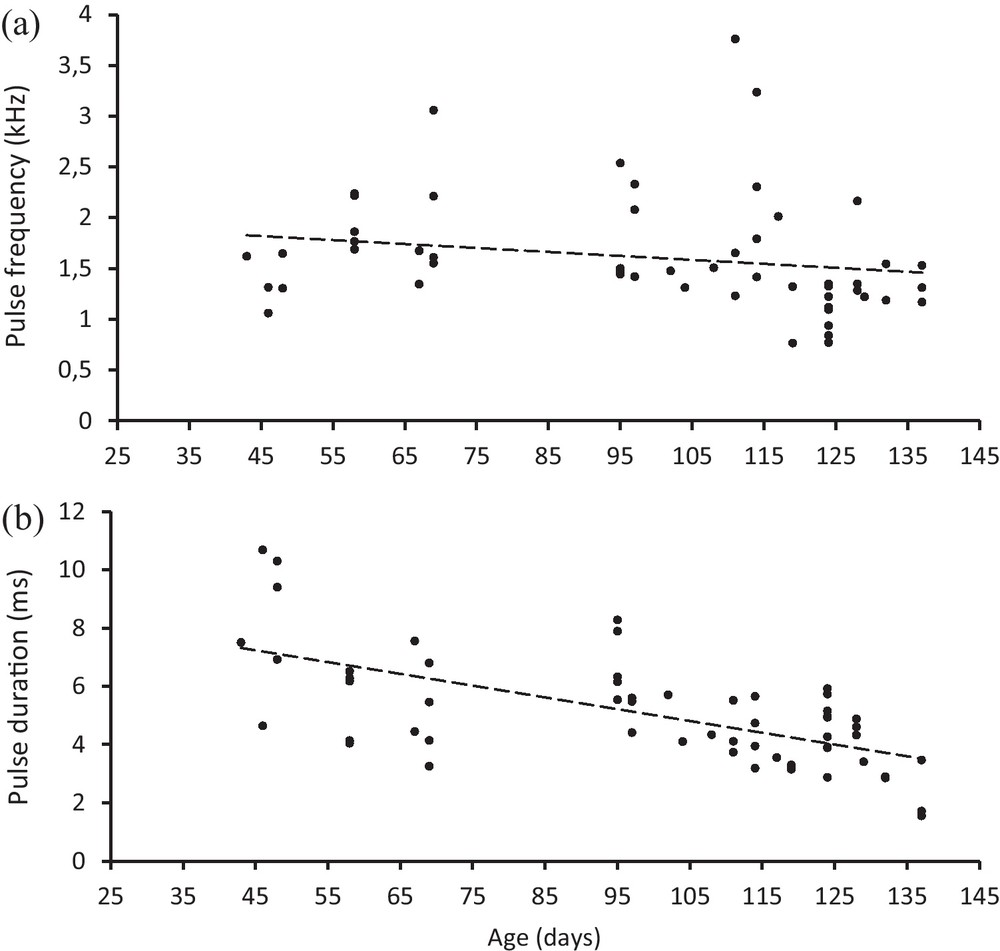
Relationship between (a) the age (x) and the instantaneous pulse frequency (y) (linear regression equation: pulse frequency [kHz] = −0.004 × age [days] + 1.99); (b) the age (x) and the pulse duration (y) (linear regression equation: pulse duration [ms] = −0.04 × age [days] + 9.05).
4 Conclusion
The present study showed that the number of agonistic interactions increases with age in juvenile M. zebra. Number of sounds follows the same way. It is noticeable that juveniles start to vocalize very early, prior to the ability to develop complex agonistic behaviour, producing very few, mono-pulse sounds from 40 days of age.
Sound production has been studied during reproductive context, defence of a territory or when competing for food in representatives of approximately 30 families [3,4,17], notably in cichlid fish [2,18]. However, the emergence of aggressive behaviour during development has been studied in few species of teleost fish. Henglmuller and Ladich [8] noticed that most of the aggressive patterns observed in adult T. vittata during dyadic interactions were observed in fish of less than 3 months of age and considered the repertoire of agonistic behaviours fully developed at the age of 3–4 months. For example, lateral displays, generally regarded as late-occurring, ritualized aggressive acts, first occurred in the third week of life. Likewise, in dyadic interactions of 40-day old M. zebra, and if most of the aggressive acts consisted in charges, all the behaviours encountered in adults, i.e. lateral displays, quivers, charges, and bites were observed [14,16]. In accordance to those previous experiments, sounds consisted in one-pulse signals and were recorded in association to chases, lateral displays and quivers as observed by Henglmuller and Ladich [8], i.e. one-pulse sounds first accompanied lateral displays during week 8.
Even though it appears that the close association between visual and acoustic stimuli seems to be present early in the life of this cichlid and may unsurprisingly confirm the probably innate origin of these behaviours [19], studies focussing on the development of acoustic communication and hearing capacities have often demonstrated that smaller, juvenile fishes could hardly detect sounds of high frequencies since these sounds were usually below their hearing thresholds, e.g. in damselfish [20], croaking gouramis [21], toadfishes [11,22], clown fishes [23]. Acoustic communication might therefore be absent in the earliest stages of development in these species and start when sounds intensity are above hearing thresholds and increase with agonistic behaviours. In contrast, the squeaker catfish seems to be able to detect sounds of all their conspecifics at all stages [10]. This difference might be explained by a higher hearing sensitivity and sound intensity in early stages individuals of this fish [10].
While sounds of juvenile T. vittata became bi- then multi-pulses after 12 weeks, only changes in pulses instantaneous frequency and duration could be observed in M. zebra. The change from single pulse to multi-pulses sounds must therefore occur later, after 200 days, in this cichlid. Such modifications in the sound structure during ontogenesis are likely to be due to the development of sound generating structures and changes in individual's morphology. In particular, a decreased sound frequency with an increasing body size is a general phenomenon in adult fishes [14,24–27].
The studies focusing on the ontogenesis of vocalizations in fishes performed so far [8–11,20] and the results presented here may reveal general patterns in the changes of temporal and spectral characteristics observed during the development of juvenile fish. First sounds usually consist in single isolated units of the future adult sounds. Number of units then increases with age and sounds become longer with an increase in the pulse period. The dominant frequency of sounds decreases as the fish grow and their intensity (not measured here) increases. One difference appears in the duration of pulses which decreases with age in the toadfish and M. zebra while it increases in the grey gurnard.
In conclusion, this study participates to the understanding of ontogenetic development of aggression and the evolution of sound characteristics in juvenile fish and may serve future studies focusing on the role of acoustic signals in the social life of fishes.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
The authors would like to thank N. Boyer and C. Bouchut for their technical support and M. Dalmas for his help during data analysis. For this study, F.B. was supported by a PhD fellowship from the French Ministère de l’Enseignement Supérieur et de la Recherche. This work has been funded by the Institut Universitaire de France (NM), the Centre National de la Recherche Scientifique, and the University of Saint-Étienne.


