1 Introduction
Many groups of animals use the advertisement display such as colorful phenotype, maneuvers, or call characteristics to communicate [1]. Intersexual and intrasexual differences are observed in animals that utilize acoustic displays [2–4]. Cicadas are a classic example of animals producing acoustic signals. The male cicada produces a sexual signal as a calling song that constitutes the first step of pairing, attracting conspecific females at long distance [5]. The acoustic properties of the sound pulses are determined by the physical properties of timbal vibration, which is located in the first segment of the abdomen and are modified by several body parts [6–12]. The calling song may be modified to a continuous series of distinctly amplitude-modulated phrases. Each phrase consists of three distinct components or sections that are always repeated in the same sequence [8,13].
Tibicen (= Lyristes) plebejus (Scopoli) is the largest cicada in the Mediterranean region [14], extending north to central Europe and east to Armenia, Georgia and Iran [15–19]. Adults are widely polyphagous and are seen on different trees and shrubs [13]. The phenology of the adults of T. plebejus is relatively short, starting in warm places in mid-June and extending about 40 days [14].
Several studies have shown that the morphology of the caller influences some aspects of the advertisement signal [20–27]. The frequency of the sound has been shown to correlate with the weight and length of males in some species of cicada [28,29], and frequency is also more important in long-range communication, whereas the temporal parameter of the calling song is more important in short-range communication or species recognition by females [30].
This article tries to correlate the morphology of 17 individuals of cicada of T. plebejus with nine parameter of the calling song. We hypothesize that females select males according to morphological characters. As result of that, we try to determine the correlation between the features of the calling song and the morphological characters in males of T. plebejus.
2 Material and methods
2.1 Location and climate condition
This research was conducted in Babol in Mazandaran province, which is located in northern Iran (52°41′ eastern longitude and 36°33′ northern latitude) from 5 to 28 July 2010. The ambient temperature and relative humidity were 28–30 °C and 70–80%, respectively, during the investigation period.
2.2 Collection of specimens
In the natural environment, the calling songs of cicadas were recorded. After recording, cicadas were caught by net. A total of 17 males were collected. The cicada was dried at room temperature for 72 h and these dried specimens were used to measure morphological characteristics.
2.3 Sound recording and analysis
The recordings were performed with a stereo-cassette recorder (Panasonic RQ-A320A) with a capacitor external microphone (bandwidth = 70–16,000 Hz) using SONY C-60EFB cassette tapes (Sony Crop. Tokyo, Japan). The signals were digitized from the analogue output of the cassette recorder at a sampling rate of 44.1 kHz by a sound card on a laptop (Acer, TravelMate 2480). The calling songs of cicadas were analyzed by Cool Record Edit Deluxe Ver. 7.8.6 and Sound Ruler Ver. 0.9.6.0 on a desktop PC. Each calling song was recorded for 15 min. The microphone was placed at a distance of 10–20 cm from the cicadas in the warmest hours of the day at noon, when more cicadas were observed.
To find a correlation between morphology and calling song, the maximum, minimum and average values of the acoustic variables of the calling song were analyzed: phrase duration, phrase part 1, phrase part 2, number of phrases/min, echeme duration, echeme period, interecheme interval, number of echemes/s, echeme/interecheme ratio and dominant frequency. Other variables of the calling song of T. plebejus like bandwidth and dominant frequency were calculated to describe the calling song. They were measured with a 0.001-s precision on signal oscillograms. The description of acoustic variables is given in Table 1.
Descriptions of the calling song variables analyzed in Tibicen plebejus.
| Variables | Description |
| Phrase | The calling song consists of regular repeats of long periods of time (s) [73] |
| Phrase duration (s) | Duration from the start of a phrase to the beginning of the following one |
| Phrase part 1 (s) | Duration of the first part of phrase, i.e. from the start of a phrase to the instant when there are no more echeme and interecheme intervals or to the beginning of part 2 of the phrase |
| Phrase part 2 (s) | Duration of the second part of phrase, i.e. from the end of part 1 of the phrase to end the of phrase and the beginning of the following one |
| Number of phrase/min | The number of phrases per minute |
| Echeme | Each phrase consisting of echemes or short periods of time (hundredth of seconds or tenths of milliseconds) when a sound is produced [73] |
| Echeme duration (ms) | Duration of each echeme from its start to its end [74] |
| Echeme period (ms) | Duration between the start of one echeme and the beginning of the following one [74] |
| Interecheme interval (ms) | Interval between the end of one echeme and the beginning of the next one [74] |
| Number of echemes/s | The number of echemes per second [74] |
| Echeme/interecheme interval ratio | Ratio between the echeme duration and the interecheme interval [74] |
| Dominant frequency | The frequency of the maximum amplitude on the spectrogram [74] |
| Bandwidth | Difference between maximum and minimum frequencies (at −20 dB) [74] |
2.4 Morphological characters
Males were measured for two morphological characters: body length using an electronic caliper (resolution = 0.01 mm) (INSIZE Co., LTD, China), and dry weight using a monopan balance (GM 152 model, Sartorius AG, Göttingen, Germany). The morphological characters are explained in Table 2.
Morphology variation for Tibicen plebejus males (n = 17).
| Variable | Mean | Standard deviation | Minimum | Maximum |
| Body length (mm) | 32.93 | 1.30 | 30.16 | 34.40 |
| Weight (g) | 0.55 | 0.10 | 0.30 | 0.70 |
2.5 Statistical analysis
The data were tested for normality by the Kolmogorov–Smirnov test, and then the Pearson Product Moment test was used for finding correlations between morphological and acoustic characteristics. The equations of correlations were explained by simple linear regression. Statistical analyses were performed using SPSS version 15 (SPSS Inc., Chicago, IL, USA).
3 Results
3.1 Acoustic signal
3.1.1 Song pattern
The signal of the calling song of T. plebejus can last without interruption for some minutes, including repeated phrases of some echemes (Fig. 1b). The phrase can be divided into two parts or sections according to echeme and interecheme intervals (Fig. 1c). In the first part of the phrase (phrase part 1), it consists of echeme sequences and intervals between echemes. Thus, the echemes and the intervals between them are clearly visible and measurable (Fig. 1d). The amplitude of the signals maximizes in the middle of the phrase and then decreases at the end. Interecheme intervals have very low amplitude. There cannot be any stop or pause between echemes. In the second part of the phrase (phrase part 2), the song changes to fused echemes, so there cannot be any echeme or interecheme interval. At this stage, intensity significantly decreases (Fig. 1e). Measurements of temporal parameters are summarized in Table 3.
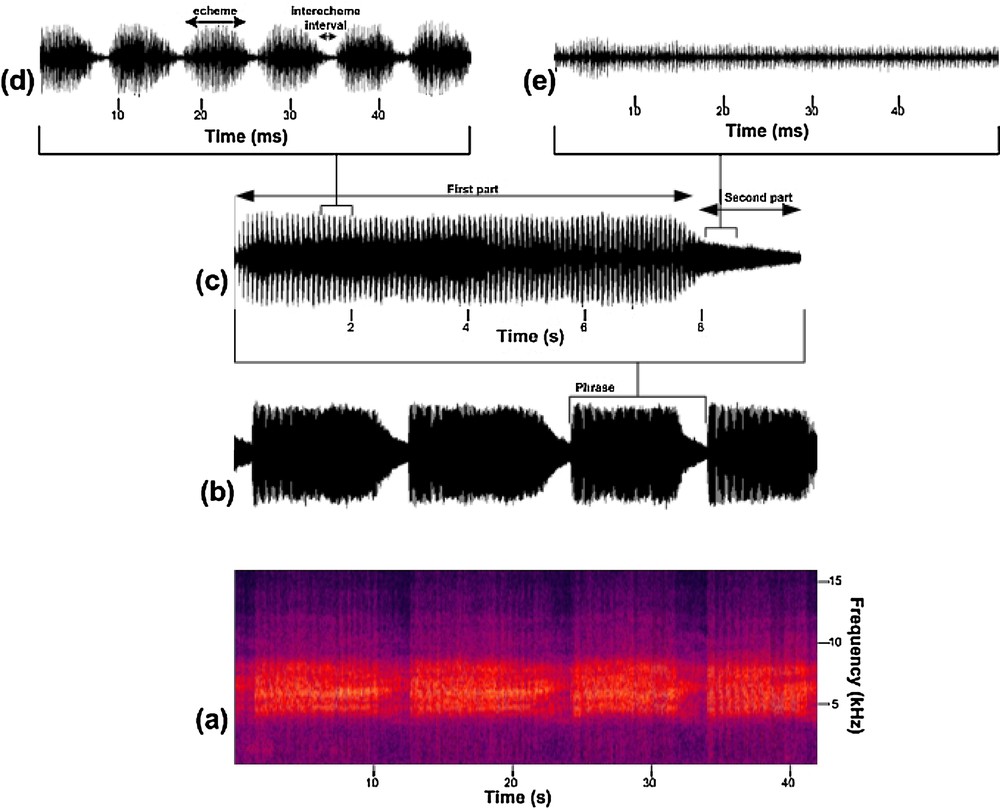
(Color online.) Calling song of Tibicen plebejus; a: spectrum of the calling song; b: spectrum of the continuous phrase; c: a phrase is divided into two parts; d: first part of the phrase: echeme/interecheme interval; e: second part of the phrase: there is no echeme.
Calling song variation for Tibicen plebejus males.
| Variables | Length | Weight | ||||||
| Pearson correlation | Sig. | Equation | r 2 | Pearson correlation | Sig. | Equation | r 2 | |
| Mean echeme duration | 0.51a | 0.03 | Y = −7.1 + 1.8x | 0.26 | – | – | – | – |
| Mean echeme/interecheme ratio | 0.54a | 0.02 | Y = −5.2 + 0.2x | 0.29 | – | – | – | – |
| Maximum echeme/interecheme ratio | 0.51a | 0.03 | Y = −10.04 + 0.4x | 0.26 | – | – | – | – |
| Minimum interecheme intervals | −0.52a | 0.03 | Y = 62.5–1.3x | 0.27 | −0.51a | 0.03 | Y = 26.7–17.2x | 0.26 |
| Mean dominant frequency | −0.48a | 0.04 | Y = 9.1–0.9x | 0.23 | −0.66b | 0.00 | Y = 7.02–1.5x | 0.43 |
| Minimum dominant frequency | −0.69b | 0.00 | Y = 12.4–0.2x | 0.48 | −0.61b | 0.00 | Y = 6.8–2.3x | 0.37 |
| Maximum dominant frequency | −0.49a | 0.04 | Y = 9.8–0.09x | 0.24 | −0.49a | 0.04 | Y = 7.2–1.2x | 0.24 |
a Correlation significant at the 0.05 level (2-tailed).
b Correlation significant at the 0.01 level (2-tailed).
3.1.2 Frequency range
The peak frequency for all the specimens analyzed varied from 4900 to 7200 Hz (Fig. 2). The bandwidth (−20 dB) ranged from 4000 to 8000 Hz.
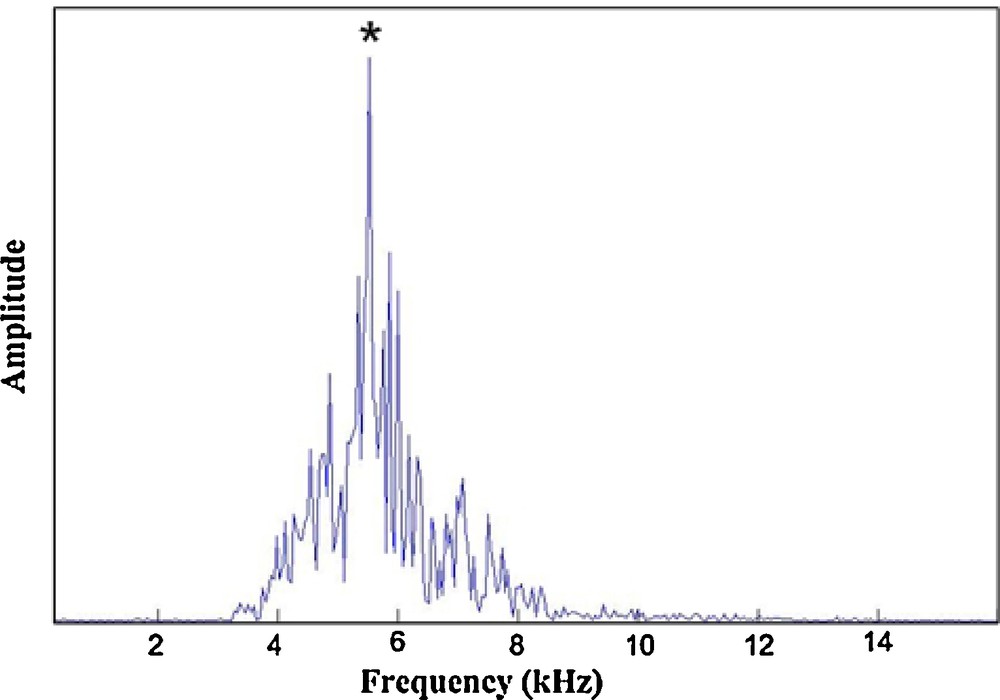
Example of peak frequency determination for the calling song of a Tibicen plebejus male in the range from 4900 to 7200 Hz.
3.2 Morphoacoustic relationships
Some relationships between morphology and acoustic parameters were found (Table 4). In terms of song structure, three significant positive correlations existed between length and (1) mean echeme duration (Pearson correlation = 0.51, P < 0.05, y = –7.1 + 1.8x, r2 = 0.26) (Fig. 3), (2) mean echeme/interecheme ratio (Pearson correlation = 0.54, P < 0.05, y = –5.2 + 0.2x, r2 = 0.29) (Fig. 4), (3) maximum echeme/interecheme ratio (Pearson correlation = 0.51, P < 0.05, y = –10.04 + 0.4x, r2 = 0.26) (Fig. 5). And there were also four significant negative correlations between length and (1) minimum interecheme intervals (Pearson correlation = –0.52, P < 0.05, y = 62.5–1.3x, r2 = 0.27) (Fig. 6), (2) mean dominant frequency (Pearson correlation = –0.48, P < 0.05, y = 9.1–0.9x, r2 = 0.23) (Fig. 7), (3) minimum dominant frequency (Pearson correlation = –0.69, P < 0.01, y = 12.4–0.2x, r2 = 0.48) (Fig. 8), (4) maximum dominant frequency (Pearson correlation = –0.49, P < 0.05, y = 9.8–0.09x, r2 = 0.24) (Fig. 9) and between weight and (1) minimum interecheme intervals (Pearson correlation = –0.51, P < 0.05, y = 26.7–17.2x, r2 = 0.26) (Fig. 10), (2) mean dominant frequency (Pearson correlation = –0.66, P < 0.01, y = 7.02–1.5x, r2 = 0.43) (Fig. 11), (3) minimum dominant frequency (Pearson correlation = –0.61, P < 0.01, y = 6.8–2.3x, r2 = 0.37) (Fig. 12), (4) maximum dominant frequency (Pearson correlation = –0.49, P < 0.05, y = 7.2–1.2x, r2 = 0.24) (Fig. 13). It is obvious that smaller males produce songs with higher dominant frequency. In addition, a minimum of interecheme intervals of males decrease with their length and weight, but the mean echeme duration, the mean echeme/interecheme ratio, and the maximum echeme/interecheme ratio increase with their length. Other parameters of the calling songs did not show any correlation.
Correlation between morphology and calling songs parameters of Tibicen plebejus. Correlation equations are explained by simple linear regression.
| Variable | Mean | Standard deviation | Minimum | Maximum |
| Phrase period (s) | 12.19 | 3.63 | 6.25 | 33.93 |
| Phrase part 1 (s) | 10.31 | 3.74 | 4.14 | 31.14 |
| Phrase part 2 (s) | 1.86 | 0.67 | 0.06 | 4.04 |
| Number of phrase/min | 5.29 | 1.03 | 3.5 | 8.5 |
| Echeme period (ms) | 80.15 | 7.01 | 65 | 102 |
| Echeme duration (ms) | 54.57 | 7.63 | 30 | 79 |
| Interecheme interval (ms) | 25.82 | 9.83 | 4 | 58 |
| Echeme/Interecheme ratio (ms) | 2.31 | 0.91 | 0.55 | 6.40 |
| Number of echeme/s | 12.24 | 0.89 | 10.5 | 12.24 |
| Dominant frequency (Hz) | 6126.96 | 446.13 | 4900 | 7200 |

Correlation analysis of the mean echeme duration of the calling songs vs. the body length of Tibicen plebejus males (Pearson correlation = 0.512, P < 0.05, y = –7.1 + 1.8x, r2 = 0.26).
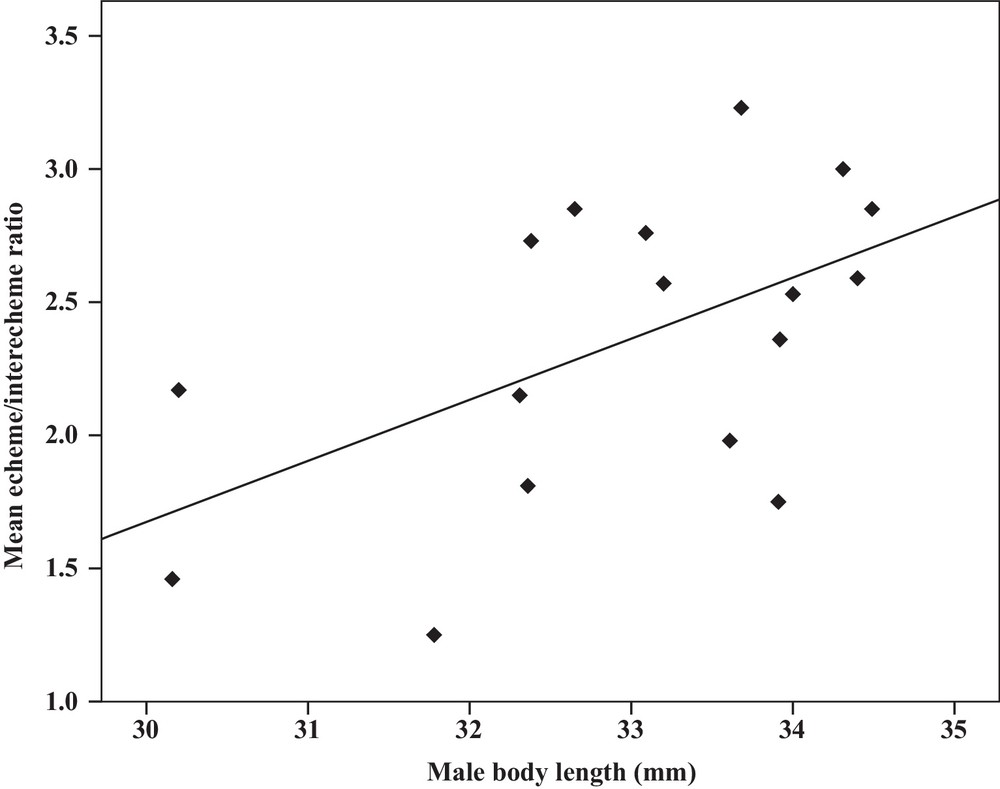
Correlation analysis of the mean echeme/interecheme ratio of the calling songs vs. the body length of Tibicen plebejus males (Pearson correlation = 0.54, P < 0.05, y = −5.2 + 0.2x, r2 = 0.29).
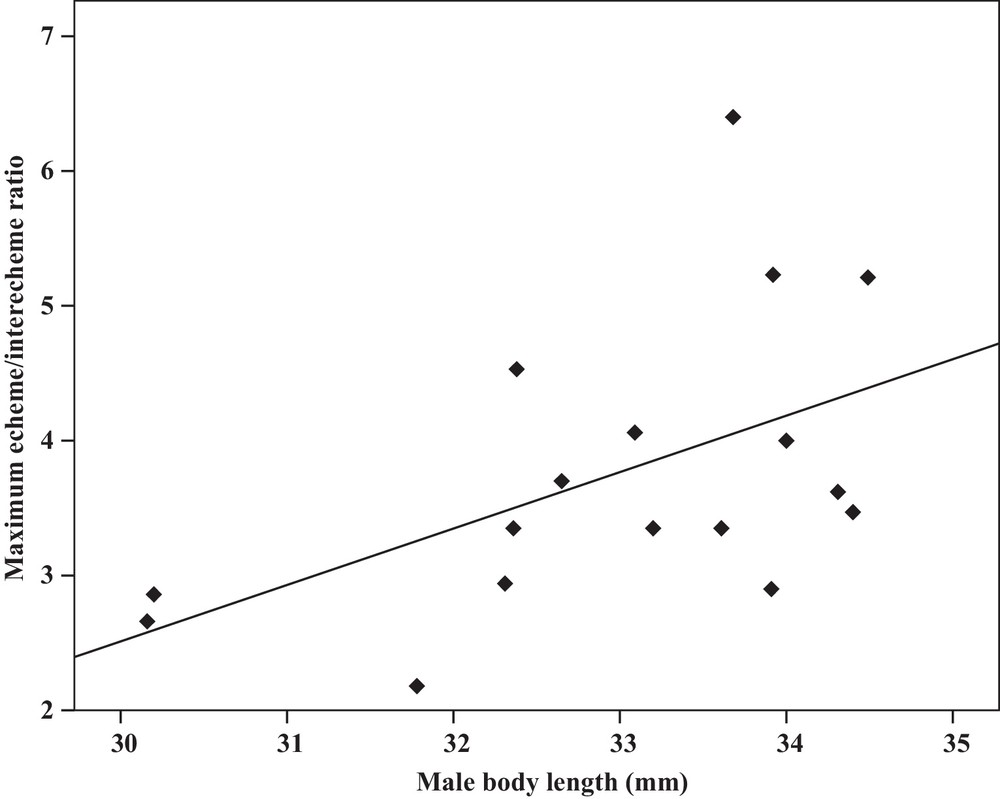
Correlation analysis of the maximum echeme/interecheme ratio of the calling songs vs. the body length of Tibicen plebejus males (Pearson correlation = 0.51, P < 0.05, y = −10.04 + 0.4x, r2 = 0.26).
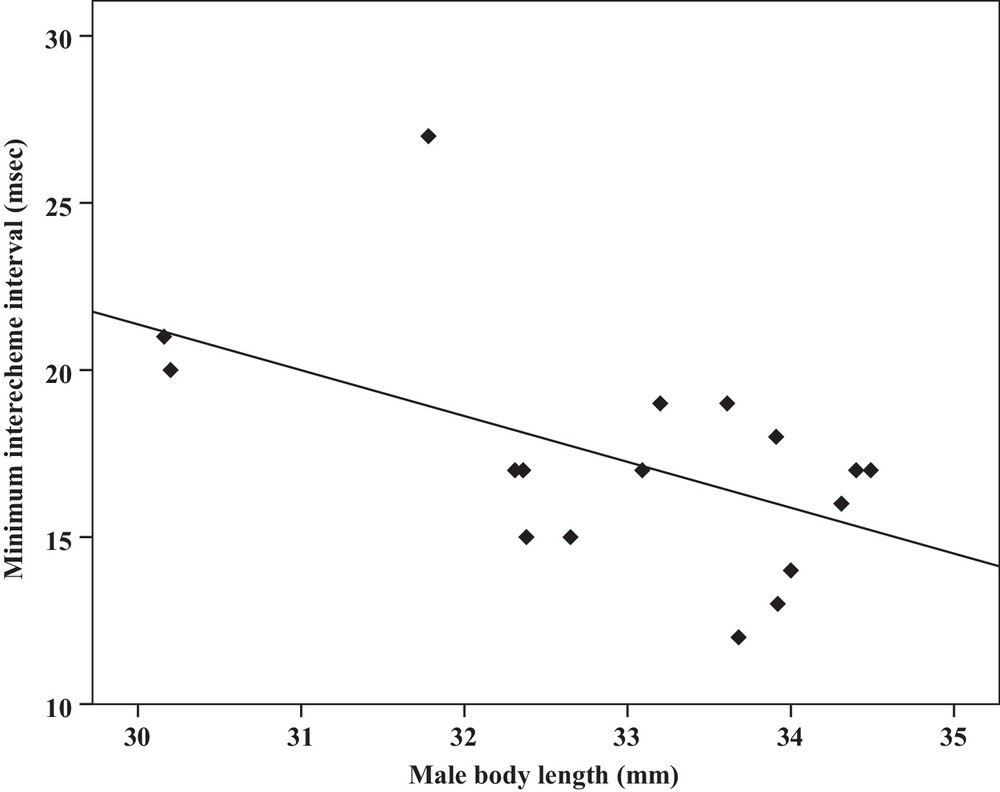
Correlation analysis of the minimum interecheme intervals of the calling songs vs. the body length of Tibicen plebejus males (Pearson correlation = −0.52, P < 0.05, y = 62.5–1.3x, r2 = 0.27).

Correlation analysis of the mean dominant frequency of the calling songs vs. the body length of Tibicen plebejus males (Pearson correlation = −0.48, P < 0.05, y = 9.1–0.9x, r2 = 0.23).

Correlation analysis of the minimum dominant frequency of the calling songs vs. the body length of Tibicen plebejus males (Pearson correlation = −0.69, P < 0.01, y = 12.4–0.2x, r2 = 0.48).
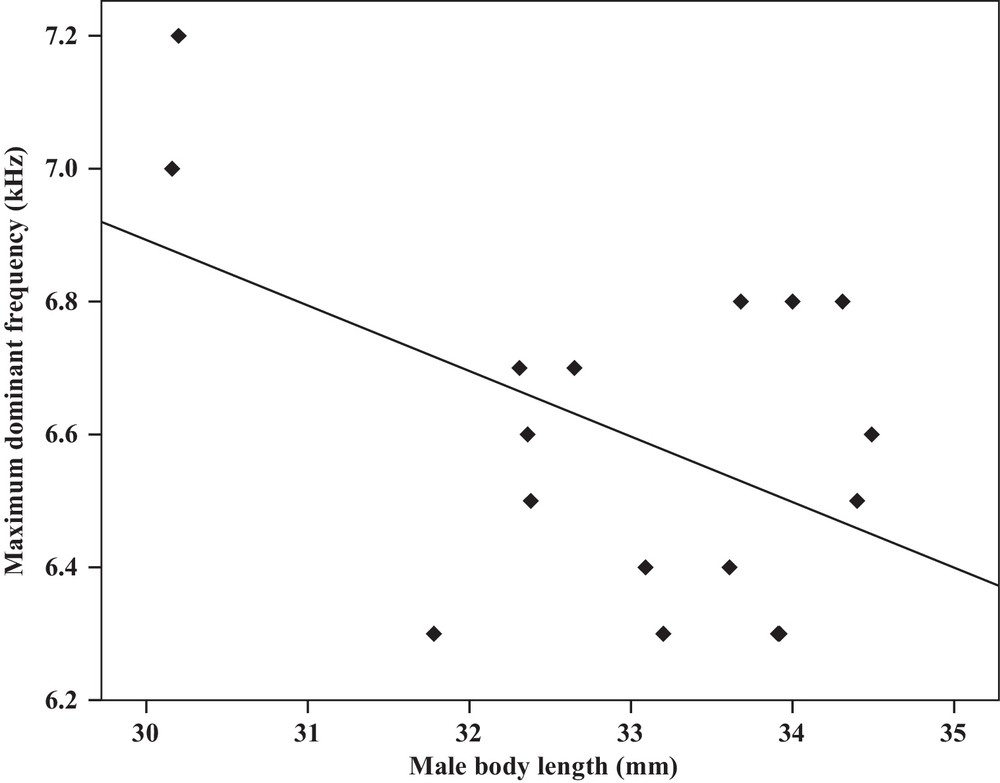
Correlation analysis of the maximum dominant frequency of the calling songs vs. the body length of Tibicen plebejus males (Pearson correlation = −0.49, P < 0.05, y = 9.8–0.09x, r2 = 0.24).
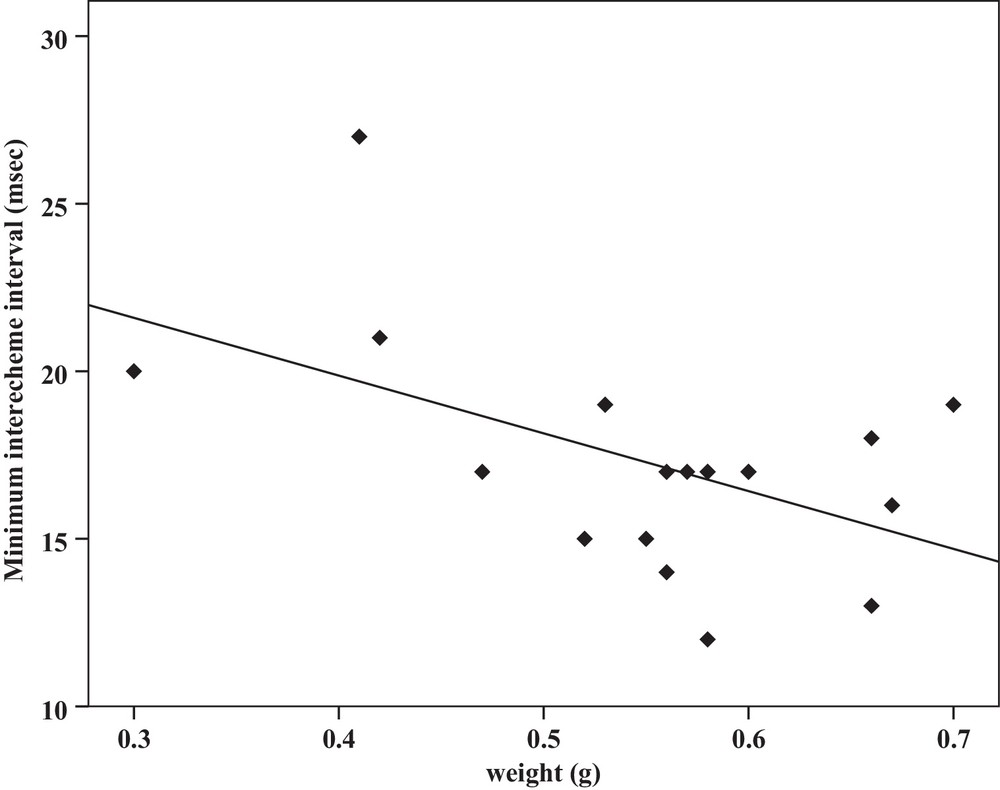
Correlation analysis of minimum interecheme intervals of the calling songs vs. the weight of Tibicen plebejus males (Pearson correlation = −0.51, P < 0.05, y = 26.7–17.2x, r2 = 0.26).
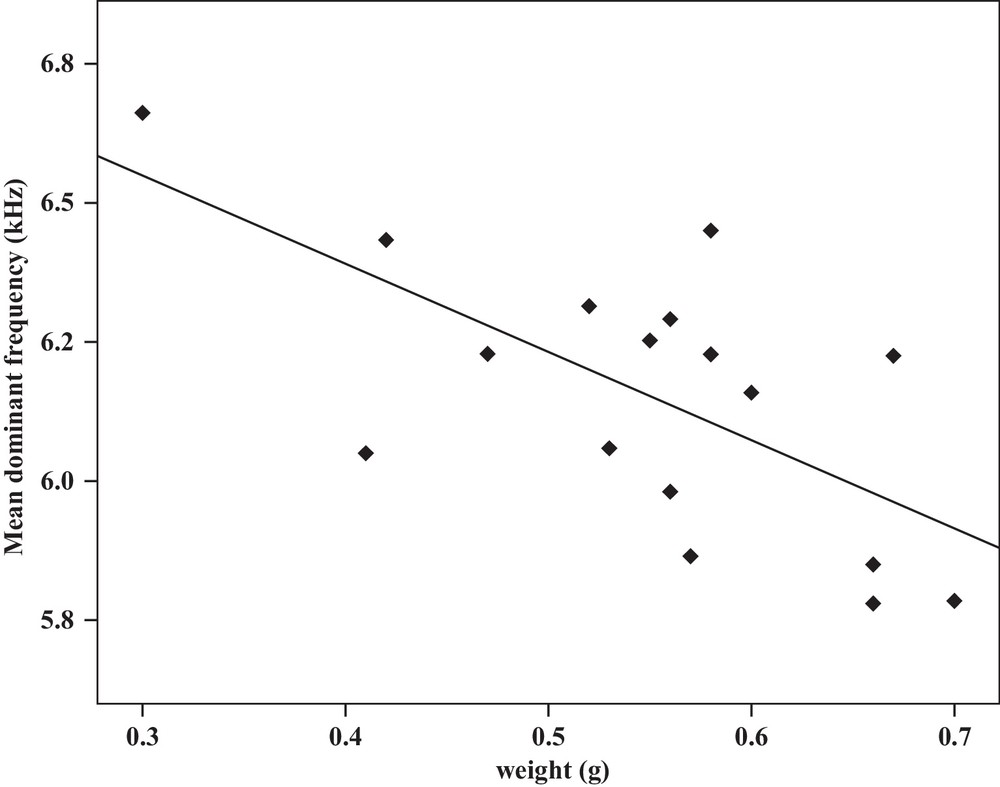
Correlation analysis of the mean dominant frequency of the calling songs vs. the weight of Tibicen plebejus males (Pearson correlation = −0.66, P < 0.01, y = 7.02–1.5x, r2 = 0.43).
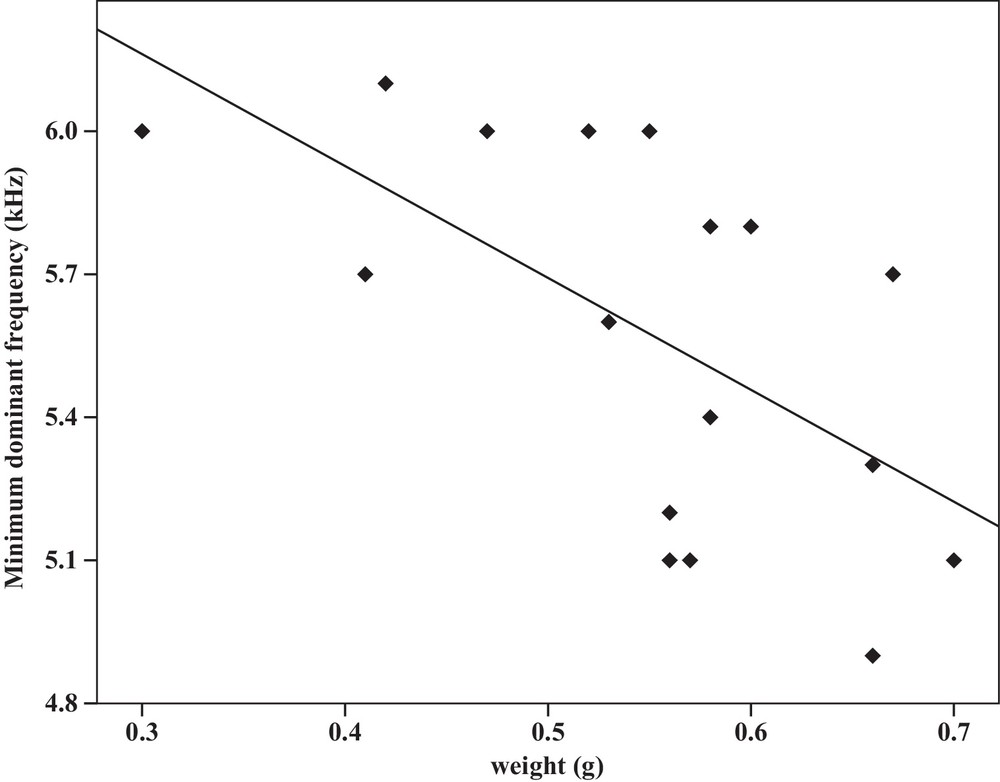
Correlation analysis of the minimum dominant frequency of the calling songs vs. the weight of Tibicen plebejus males (Pearson correlation = −0.61, P < 0.01, y = 6.8–2.3x, r2 = 0.37).
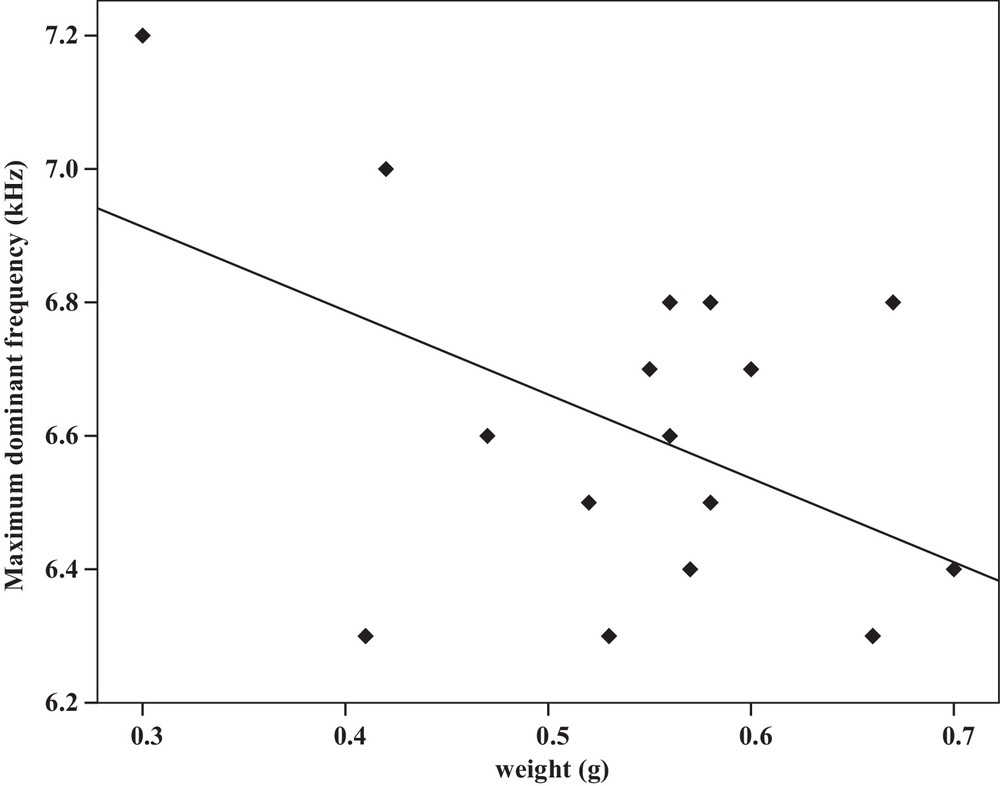
Correlation analysis of the maximum dominant frequency of the calling songs vs. the weight of Tibicen plebejus males (Pearson correlation = −0.49, P < 0.05, y = 7.2–1.2x, r2 = 0.24).
4 Discussion
4.1 Acoustic signal
The general patterns of the calling song were the same as those reported by Claridge et al. [13], Boulard [31], Gogala [32], Sueur et al. [33] and Puissant [34]. Claridge et al. [13] indicated that the phrase amplitude is divided into three parts. The first part of the signal begins from a low-amplitude level until it reaches its maximum level. In the second part, the amplitude remains maximal, whereas it decreases in the third part of the song. Indeed, in the present research, the phrase is classified into two parts according to the echemes: in the first part, the echeme/interecheme interval is the same as those described by Claridge et al. [13] in the first and second parts of the phrase. The second part, in which no interecheme interval can be observed and in which the amplitude of the phrase is low, is similar to the third part of the phrase described by Claridge et al. [13]. As far as the dominant frequency of T. plebejus is concerned, it was reported to be at 5.48 ± 0.77 kHz and around 6–7 kHz by Sueur et al. [35] and Puissant [34] respectively, which is almost equal to the present data.
4.2 Morphoacoustic relationships
Our results clearly demonstrated some correlation between some aspects of the calling song and the morphology of T. plebejus. Preliminary analyses of the results reveal some heterogeneity in temporal aspects and minor dissimilarities in the song structure and spectral characteristics of the calling song of T. plebejus. Nonetheless, the observed variability in these temporal parameters was not closely correlated with a variation in morphology. Correlation analyses revealed relationships between the dominant frequency, the interecheme intervals, the echeme duration and the echeme/interecheme ratio of the calling song and the size and mass of the male individual. Our hypothesis concerning the relationship between some morphological aspects and the features of calling song is supported.
Previous studies on the effect of morphology on call parameters have given approximately similar results in singing insects (e.g., [28,29,36]). The characteristics of the advertisement song are limited by several factors such as the overall size of the individual, and the morphology and musculature of the sound-producing apparatus [22]. The dominant frequency of the calling songs of advertising males across insect taxa has been shown to correlate with some aspects of the body size [21,24,26,27,36–40]. The dominant frequency of cicada calls has an inverse relationship with body size [28,29]. In cicada groups, the resonant frequency of the timbals [6,7,11] and several body components [6,7,10] are crucial for determining the dominant frequency produced during the calling song. The present research clearly determined that the dominant frequency has a straight negative correlation with the morphology or size of males of T. plebejus. A negative correlation between dominant frequency and male's length was observed in the cricket Gryllotalpa major [36]. Morphology is considered as determinant in male mating success in singing insects such as some crickets [41–44]. Females preferentially mate with males that produce more sperm and the spermatophore increases with male mass [45–47]. Thus, the larger male crickets are most successful than others (e.g., [41,48,49]).
Behavioral characteristics, especially calling songs, may provide identification cues in the temporal signal. However, most likely it is the slight distinctions observed in the phenotypes of individual calling males that best explain the variation observed in the dominant frequencies, echeme duration, interecheme intervals and echeme/interecheme ratio produced in T. plebejus’ calling song. Moreover, it is the same phenotypic variation, specifically emerging as an individual variability in the plectrum. Acoustics may also contribute to explain the characters of the males to their rivals [50] and to females [30]. In either case, perhaps the question should focus now on any female detection or discriminatory response to these variations.
In addition, our observations report interindividual differences in the calling song of T. plebejus. Furthermore, these interindividual differences could support mate selection by females [51]. Females attracted to males at long range by their calling song while in flight evaluate species recognition cues based on some temporal and structural elements of the calling song [52,53]. Females may then preferentially isolate one of the calling males from the aggregation based on characteristics of the call as signal fitness, or conversely based solely on the proximity to the signaler. It is the first step in pair formation [53]. If females employ similar selection criteria, the variation in the temporal parameters observed in male calls could have pronounced effects on the way a male and a female would find to be acceptable mates. The important temporal parameter produced by an individual male is to make his song as attractive as possible [54]. Female field crickets could potentially distinguish older males from younger ones based on the shorter syllable durations and slower chirp rates produced by older males [55,56]. As regards to cicada, there have been two studies [30,57] that have investigated which acoustic parameters are considered by female cicadas for finding and selecting a mate. The data from these studies show that a female cicada is phonotactic toward a particular male based on the frequency or intensity of his call so as to mate with a male based on the temporal parameters of the call after she lands near the male [30]. Another potential problem in mate recognition could occur if the recognition system of the female cicada is temperature dependent as it has been reported for plant hoppers [58] and crickets [59,60].
It is hypothesized that during the approach at higher altitudes the females of T. plebejus passively select specificity and proximity. During the lower-level flight it is hypothesized that females may actively assess aspects of the calling song that, in addition to proximity, may reveal aspects of the male's fitness [36]. Whereas individual variation in both dominant frequency and in syllable structure can be correlated with aspects of male morphology, as a result of that, these three call parameters may be assessed by females as a component of the complex and poorly understood process of active mate selection in T. plebejus. It is supposed that females of T. plebejus prefer larger males, in a similar way to crickets (e.g., [41,48,49,61]). Larger males of T. plebejus produce lower-frequency-range signals than smaller males and the hearing sensitivity of females also is tuned to the calling song of the males [35]. Low frequencies correspond to longer wavelengths than high frequencies. So, low frequencies send information signals to greater distances [62]. So, a low-frequency signal provides larger males with an opportunity to attract females that are both close or far away, while smaller males only can attract females that are close. In addition, larger males produce calling songs that have shorter silences between echemes, and there is a positive correlation between length and echeme duration and echeme/interecheme ratio, and a negative correlation between morphology and interecheme also emphasizes it. In conclusion, the larger males of T. plebejus emit not only low frequencies that cover more distances, but also more continuous signals.
The sound pressure levels of both calling songs and alarm calls in some cicadas correlate with an increase in body mass [50,63,64]. A larger insect, therefore, has the potential to interact with a greater number of conspecific individuals without changing calling perches. Perhaps the relatively large body size of cicadas is related to their acoustic strategies of reproduction [50]. Nowadays, many researchers tend to find out relationships between morphology and calling song of singing insects. It will lead not only to improve the basic knowledge about their behavioral but also to find a taxonomic approach based on these insects’ acoustics, especially cicada. In other words, as we get a deeper insight into the basic behavior of cicadas, acoustical taxonomy studies of the special characters of the calling song of the male that are related to morphological characters [65] will be undertaken.
It is important to know that environmental characteristics such as temperature affect the acoustic properties of the songs [66]. Most cicadas are thermoregulators. In other words, most cicadas can maintain a thermal gradient from ambient temperature and can avoid any temperature effect on the call's structure [67]. For example, temporal parameters of Cicadetta tibialis [68], Pycna semiclara [67] and T. plebejus [8] are independent of ambient temperature, but temporal parameters of Cicada orni and Cicada barbara [69,70] have been correlated with temperature. On the other hand, the sound frequency of cicadas is independent of ambient temperatures such as in the Magicicada species [71], P. semiclara (Germar) [67], C. orni, and C. barbara [69,70]. Hence, in T. plebejus, not only the sound's frequency is independent of ambient temperature in the same way as in other species, but also the temporal parameters of this species are not affected by the ambient temperature [72].
Further studies are needed to improve our understanding of the features of the calls that are deployed to attract females and select mates as well as to classify and identify cicadas by their song characters, which could be helpful in delineating species boundaries among cicadas in a taxonomic approach.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We would like to thank Prof. Matija Gogala and Dr. Daniel Howard for their valuable advices.


