1 Introduction
Among the fossil fuels, coal plays a crucial role in satisfying the energy demands of various countries worldwide. Combustion of coal in thermal power plants and other industries produce a variety of residues called fly ash. Fly ash is composed of predominately small glassy, hollow particles with low-to-medium bulk density ranging from 2.1 to 2.6 cm3 [1]. These particles have an average diameter of less than 10 μm, and a high surface area. Light textured fly ash particles are aggregates of micron- and sub-micron-sized spherical particles with sizes ranging from 0.01 to 100 μm [2].
Generally, fly ash application influences the physical properties of the soil such as water-holding capacity, bulk density, and soil structure [3–7]. Application of fly ash to the nutrient-stressed soils has been shown to improve plant growth and productivity [8,9]. Therefore, fly ash has often been advocated as a promising material for reclaiming wastelands or mine spoils [10,11]. Millions of hectares of fertile land that were transformed into waste lands due to strip mining for coal have been effectively reclaimed and stabilized using fly ash [12]. However, fly ash and some of the resuspended ash from ash ponds can deposit in the nearby environments (5–10 km) of coal-based power plants, causing great ecological damage. In addition to requiring large tracts of land for their disposal, the fugitive dust of fly ash also pollutes air and water [13]. The production of fly ash in India is projected to be 900 Mg per year in 2031–2032 [14]. Therefore, development of proper technology for disposal of this hazardous waste in an eco-friendly manner becomes mandatory to derive a maximum benefit from this nutrient-rich waste. It has been proposed that fly ash with organic manures and microbial inoculants could be used to formulate a soil benefaction strategy, which would help to improve the properties of the soil and enrich its nutrient status [8,15,16].
Mycorrhizae are the most common and widespread symbiosis-formed between plants and fungi. The fungi benefit the plants with the soil resources and the plants in turn provide carbon to the fungi, thereby forming a link between the biotic and geochemical portions of the environment [17]. Studies have clearly shown that the mycorrhizal fungi, especially the arbuscular mycorrhizal (AM) fungi, not only affect the nutrient uptake by plants, but also influence their nutrient-use efficiencies [18,19]. In addition to the mutual benefits to the symbionts, the extensive extraradical fungal hyphae found in the soil play an important role in maintaining the soil structure by binding the soil particles together [20]. Recently, there has been considerable interest in the possible utilization of AM fungi in promoting plant growth in agriculture, horticulture and forestry as well as in the restoration of degraded lands [9,21]. Much of the interest on the practical value of AM fungi in land restoration has stemmed from the experimental evidence that the AM fungi can improve the survival and growth of plants by alleviating the nutrient deficiencies encountered by plants during their establishment in stressed soils [22–25]. Recently, Piñeiro et al. [26] showed that inoculation with AM fungi alone or in combination with organic amendments can enhance both the survival and growth of plants in stressed environments. Nevertheless, such a positive effect does not hold true always, as Oliveira et al. [27] failed to find any significant influence of mycorrhization on plant performance in three native late-successional shrub species (Ceratonia siliqua, Olea europea, and Pistacia lentiscus). Arbuscular mycorrhizal colonization and spores in the rhizosphere of plants growing naturally on soils contaminated with fly ash or from areas used to store fly ash have been reported [28–31]. A few studies have also examined the influence of fly ash on AM formation and function in crop and tree species [8,32,33]. The results of these studies indicated that the mycorrhizal benefit to plants could substantially differ from the concentrations and the composition of the fly ash.
Millet, belonging to the family Poaceae, is a staple food supplying a major portion of calories and protein to large segments of populations in the semi-arid tropical regions of Africa and Asia [34]. Among all the millets, Kodo millet (Paspalum scrobiculatum L.) is very rare and it is very good host for mycorrhizal fungi. Kodo millet has been grown as a cereal crop exclusively in India for at least 3000 years, but is widespread as a tropical weed in other areas. Kodo millet has an indefinite storage life. The grains of kodo millet are nutritionally superior to rice and wheat, provide cheap proteins, minerals and vitamins to the poorest among the poor, where the need is essential. In this study, we evaluated the effect of different concentrations of fly ash amendment with or without AM fungus inoculation on plant growth, nutrient-use efficiencies and yield of kodo millet.
2 Materials and methods
2.1 Experimental design
Red sandy loam soil (0–20 cm) collected from Agricultural Research Station, Hanumanamatti (located at latitude 14° 40′ 06.12“ N and Longitude 75° 34′ 22.00′′ E) of Haveri district, Karnataka, India, was used in the study. The soil was air-dried, sieved (2 mm) and stored in gunny bags until used. The physicochemical properties of the soil, determined following standard procedures [35], were: 2.3 mg/kg of total P, 63.02 mg/kg of total N, 7.50 mg/kg of exchangeable K, 21.01 mg/kg of Na, 33.00 mg/kg of Mg and 72.02 mg/kg of Ca. The soil had an organic carbon content of 0.31%. Soil pH (1/2.5 soil: water ratio, v/v) and electrical conductivity (EC, dS/m) was 8.42 and 0.42 respectively. The AM fungal spore number in the native soil was 98 spores/10 g soils (determined according to [36]). A majority of these spores were devoid of contents and were only spore cases [37]. Prior to inoculation, spores from native AM fungal flora isolated from the soil sample were identified following the current taxonomic criteria [38,39] and information of INVAM (http://www.invam.caf.wdu.edu/). The studied soil contained AM fungal spores belonging to Acaulospora laevis Gerd and Trappe, Acaulospora spinosa Walker and Trappe, Archeospora trappei (Ames and Linderman) Morton and Redecker, Funneliformis caledonium (Nicolson and Gerd) Walker and Schüßler, Gigaspora rosea Nicolson and Schenck, Glomus aggregatum Schenck and Sm., Glomus albidum Walker and Rhodes, Glomus delhiense Mukerji, Bhattacharjee and Tewari, Glomus globiferum Koske and Walker, Glomus macrocarpum Tul. and Tul., Redeckera fulvum (Berk. and Broome) Walker and Schüßler, Rhizophagus clarus (Nicolson and Schenck) Walker and Schüßler, R. fasciculatus (Thaxt.) Walker and Schüßler, Sclerocystis dussii (Pat.) Hohn., Sclerocystis pubescens (Sacc. and Ellis) Hohn., and Scutellospora calospora (Nicolson and Gerd.) Walker and Sanders. Among all the isolated species, Rhizophagus fasciculatus was found to be the predominant fungus based on abundance (number of spores of a particular morphotype/total number of spores in the sample × 100).
Fly ash (FA) was obtained from Harihar Polyfibers and Grasilene Division, a pulp-manufacturing plant located in Kumarapatnam, Harihar, of Davanger District, Karnataka, India. Fly ash was sieved (2 mm) and stored in black polythene bags at room temperature until used. The fly ash contained 0.66% organic carbon, 1.7 mg/kg of total P, 1.58 of total N, 33.42 mg/kg of exchangeable K, 20.30 mg/kg of Na, 8.14 mg/kg of Mg, and 36.12 mg/kg of Ca as assessed according to Jackson [35]. The pH (1/2.5 ash: water ratio, v/v) and the electrical conductivity (EC, dS/m) were 7.90 and 0.20, respectively. A specific quantity of fly ash was added and thoroughly mixed to the soil.
The experiment involved a factorial combination of four concentrations of fly ash amendments (0%, 2%, 4%, 6%), two AM inoculation levels (uninoculated or inoculated) and two harvests [60 and 90 days after sowing (DAS)]. The treatments were as follows:
- • unamended and uninoculated native soil (control);
- • AM fungus inoculation;
- • 2% fly ash (20 g/kg soil) amendment;
- • 4% fly ash (40 g/kg soil) amendment;
- • 6% fly ash (60 g/kg soil) amendment;
- • 2% fly ash amendment with AM fungus inoculation;
- • 4% fly ash amendment with AM fungus inoculation;
- • 6% fly ash amendment with AM fungus inoculation.
These eight treatments were replicated three times, giving a total of 48 pots (4 × 2 × 2 × 3) that were arranged in the greenhouse in a randomized block design.
2.2 Seeds and AM fungal source
Seeds of Kodo millet were obtained from the Krishi Vigyan Kendra of Dharwad Agricultural University, Hanumanamatti, Haveri, Karnataka, India. The seeds were surface sterilized in 2% mercuric chloride solution for 2 min and washed free of sterilant using distilled water prior to sowing. The AM fungus (R. fasciculatus) inoculum was prepared from spores isolated from the field soil using sorghum (Sorghum bicolor) as the host in the Microbiology Laboratory, P.G. Department of Studies in Botany, Karnatak University, Dharwad (15° 26′ 28.5“N, 74° 59′ 2.1′′E), India. The AM fungal inoculum (25 g/pot) containing chopped mycorrhizal root bits, soil hyphal fragments and spores/spore clusters (315–375 spores/25 g) were layered 5 cm below the soil surface in each pot involving AM fungi. Twenty-five grams of sterilized (120 °C, for 45 min) AM fungal inoculum were added to uninoculated treatments. Four seeds of kodo millet were sown in 30-cm-diameter earthen pots containing 15 kg of unsterile field soil. The seedlings were thinned to two per pot one week after germination.
2.3 Growth conditions
Plants were grown under greenhouse conditions with temperatures ranging between 25 ± 3 °C daytime and 15 ± 3 °C night-time, a 16/8-h light/dark photoperiod, and a relative humidity of 80–90%. The plants were irrigated manually with distilled water as needed during the experiment (judged by weighing pots). Every two weeks, 10 mL of nutrient solution [44] without P was added to each pot.
2.4 Harvest and measurements
Plants were harvested at 60 and 90 DAS. At harvest, plant growth parameters like plant height, and average first-order root length (total root length by the number of roots) was measured. Root fresh weights were recorded, and a weighed portion of the roots were preserved in a formalin–acetic acid–70% alcohol (0.5/ 0.5: 9.0; v:v:v) solution for the determination of AM fungal colonization. Shoot and root dry weights were recorded after drying at 65 °C for 48 h in a forced-air oven. The root/shoot (R/S) ratio was calculated on the dry weight basis. The number of grains per panicle was counted manually, and 100-grain dry weights were determined after drying them at room temperature to a constant weight. The AM colonization was determined as per Giovannetti and Mosse [40] after clearing and staining the roots with 0.05% trypan blue [41]. The number of AM fungal spores per 50 g of soil sample was determined as mentioned earlier.
The plant shoots were digested with a nitric acid–perchloric acid (3/1) mixture as described by Page et al. [42]. These samples were analyzed for mineral nutrients such as K, Ca, Na, and Mg [35]. The total P content in the shoot tissues was determined by the colorimetric method as described by Kitson and Mellon [43]. Nutrient-use efficiencies were calculated according to Koide [45].
2.5 Statistical analysis
Data on plant growth, yield, nutrient content, AM colonization and spore numbers were subjected to analysis of variance (ANOVA) using SPSS software (version 21.0). Means were compared by the Duncan's Multiple Range Test (DMRT). Statistical significance was determined at P < 0.05. The relationships between the variables were determined using Pearson's correlation coefficient.
3 Results
3.1 Effect of fly ash on AM colonization and spore numbers
Fly ash amendment and AM fungus inoculation significantly influenced the percentage of AM colonization in kodo millet roots. No AM fungal colonization of kodo millet roots was observed at 60 DAS and low colonization levels (16%) occurred at 90 DAS in uninoculated and unamended control soils (Fig. 1a). Though fly ash amendment stimulated the extent of AM colonization in kodo millet roots compared to control, AM inoculation further enhanced the extent of colonization. Maximum AM colonization was evident in treatments involving a low concentration (2%) of fly ash at both 60 and 90 DAS of harvests. Nevertheless, increasing concentrations of fly ash amendment decreased the extent of AM colonization, but the decrease was not proportional (r = 0.021; P > 0.05; n = 16).

(Colour online.) Influence of fly-ash (FA) amendment and arbuscular mycorrhizal (AM) fungus inoculation on root colonization (a) and spore numbers (b) of kodo millet at 60 and 90 days after sowing (DAS). Error bars indicate ± 1 S.E. Bars bearing same letter(s) are not significantly different (P > 0.05) according to DMRT.
Like AM colonization, spore numbers were also significantly influenced by fly ash amendment and AM fungus inoculation (Fig. 1b). Average spore numbers in soils inoculated with AM fungus were more than two folds higher than in uninoculated soils. Application of 2% fly ash improved the spore numbers, whereas a further increase in fly ash concentrations decreased spore numbers. The least spore numbers were observed in 6% fly-ash-amended soils irrespective of AM inoculation both at 60 and 90 DAS. The decrease in the spore numbers was not related to the concentration of fly ash amendment (r = –0.164; P > 0.05; n = 16). However, AM spore numbers were significantly and positively correlated to the extent of AM colonization in roots (r = 0.874; P < 0.01; n = 16).
3.2 Effect of fly ash on growth of kodo millet
Application of fly ash and AM fungus inoculation significantly influenced all the plant growth parameters both at 60 and 90 DAS (Table 1). In addition, all the interactions among factors were also highly significant. Generally, plants raised on soils amended with different rates of fly ash were significantly taller than those raised on unamended soils for both harvests, except for those raised on 6% fly-ash-amended soils at 60 DAS. Though kodo millet plants raised on 6% fly-ash-amended soils were 4% shorter than those raised on unamended soil at 60 DAS, plants in this treatment were taller by 6% than those raised on unamended soil at 90 DAS. Inoculation of AM fungus increased the seedling height by 12–51% at 60 DAS and by 61–98% at 90 DAS compared to their respective non-mycorrhizal conspecifics. Nevertheless, by 90 DAS, AM-inoculated plants grown in 6% fly-ash-amended soils were 7% shorter compared to the uninoculated plants grown on soil amended with a similar rate of fly ash.
Influence of fly ash (FA) amendment and arbuscular mycorrhizal (AM) fungus inoculation on growth of kodo millet at 60 and 90 days after sowing (DAS).
| Treatments | Plant height (cm/plant) | Root length (cm/plant) | Shoot dry weight (g/plant) | Root dry weight (g/plant) | R/S ratio | ||||||
| –AMa | +AM | –AM | +AM | –AM | +AM | –AM | +AM | –AM | +AM | ||
| 60 DAS | |||||||||||
| 0% FA | 21.51 ± 0.69 ab | 32.53 ± 0.46 fg | 8.35 ± 0.15 a | 14.70 ± 0.29 f | 8.81 ± 0.11 b | 11.50 ± 0.29 e | 1.39 ± 0.04 b | 1.98 ± 0.04 de | 0.16 ± 0.00 b | 0.17 ± 0.01 bc | |
| 2% FA | 30.49 ± 0.29 e | 44.07 ± 0.56 i | 10.33 ± 0.06 b | 22.57 ± 0.24k | 12.52 ± 0.09 f | 15.43 ± 0.16 h | 1.56 ± 0.05 bc | 4.22 ± 0.03k | 0.12 ± 0.00 a | 0.27 ± 0.00 fg | |
| 4% FA | 27.68 ± 1.36 c | 32.97 ± 0.56 g | 12.30 ± 0.14 d | 18.46 ± 0.28 h | 10.48 ± 0.14 d | 10.78 ± 0.15 d | 1.85 ± 0.07 de | 3.13 ± 0.06 gh | 0.18 ± 0.01 bc | 0.29 ± 0.00 g | |
| 6% FA | 20.74 ± 0.83 a | 23.12 ± 0.50 b | 8.30 ± 0.09 a | 15.54 ± 0.22 g | 6.72 ± 0.15 a | 8.44 ± 0.11 b | 1.04 ± 0.03 a | 2.44 ± 0.10 f | 0.16 ± 0.00 b | 0.29 ± 0.01 g | |
| 90 DAS | |||||||||||
| 0% FA | 27.65 ± 1.05 c | 54.66 ± 1.12k | 11.79 ± 0.10 c | 19.23 ± 0.12 i | 12.48 ± 0.09 f | 16.30 ± 0.07 i | 2.06 ± 0.04 e | 3.46 ± 0.08 i | 0.17 ± 0.00 bc | 0.21 ± 0.00 d | |
| 2% FA | 35.26 ± 0.34 h | 65.18 ± 1.18l | 13.25 ± 0.12 e | 30.01 ± 0.18l | 14.74 ± 0.25 g | 21.44 ± 0.24k | 3.26 ± 0.09 hi | 7.04 ± 0.06 m | 0.22 ± 0.01 d | 0.33 ± 0.00 h | |
| 4% FA | 32.44 ± 0.84 fg | 52.27 ± 0.58 j | 18.26 ± 0.03 h | 21.60 ± 0.22 j | 11.32 ± 0.18 e | 18.48 ± 0.24 j | 3.34 ± 0.20 hi | 4.79 ± 0.02l | 0.30 ± 0.02 g | 0.26 ± 0.00 e | |
| 6% FA | 29.20 ± 0.18 de | 27.23 ± 0.64 c | 10.50 ± 0.22 b | 18.65 ± 0.22 h | 9.48 ± 0.27 c | 10.44 ± 0.11 d | 1.78 ± 0.11 cd | 3.85 ± 0.05 j | 0.19 ± 0.02 c | 0.37 ± 0.01 i | |
| F-statistics | df | ||||||||||
| Harvest (H) | 1.32 | 865.214*** | 1954.217*** | 1737.875*** | 1423.923*** | 160.931*** | |||||
| AM | 1.32 | 1203.20*** | 8347.556*** | 1332.969*** | 2127.958*** | 502.648*** | |||||
| FA | 3.32 | 397.845*** | 997.134*** | 1092.142*** | 474.316*** | 84.100*** | |||||
| H × AM | 1.32 | 189.711*** | 25.309*** | 235.155*** | 76.423*** | 13.017*** | |||||
| H × FA | 3.32 | 20.395*** | 33.757*** | 25.930*** | 49.927*** | 7.745*** | |||||
| AM × FA | 3.32 | 154.50*** | 517.742*** | 64.790*** | 151.506*** | 64.462*** | |||||
| H × AM × FA | 3.32 | 42.102*** | 65.598*** | 84.620*** | 6.159*** | 32.153*** |
a –AM and +AM indicates uninoculated and AM-inoculated, respectively.
*** Significant at P < 0.001.
Fly ash amendment at 2% and 4% significantly increased the length of first-order roots, but the application of 6% fly ash significantly reduced root lengths both at 60 and 90 DAS. Arbuscular mycorrhizal fungus inoculation significantly increased the root lengths at all concentrations of fly ash amendment. Maximum shoot and root dry weights were observed in plants raised on 2% fly-ash-amended soils and inoculated with the AM fungus for both harvests. On the contrary, plants raised on 6% fly ash amendment irrespective of AM fungus inoculation had the minimum shoot and root dry weights both at 60 DAS and 90 DAS. Fly ash amendment also significantly influenced the R/S ratios of plants at both the harvests. The R/S ratios of plants raised on fly-ash-amended soils was either similar or higher compared to control except for plants raised at 2% fly ash amendment at 60 DAS. Mycorrhizal inoculation increased the R/S ratios of plants raised on both fly-ash-amended and unamended soils. Plant growth parameters were significantly and positively correlated to AM fungal colonization (Table 2).
Pearson's correlation coefficients (r) for plant growth, tissue nutrient content and arbuscular mycorrhizal (AM) colonization.
| Plant growth | Tissue nutrients | AM colonization | ||||||||
| RL | SDW | RDW | R/S | P | K | Ca | Mg | Na | ||
| SL | 0.840*** | 0.951*** | 0.857*** | 0.365 | 0.915*** | 0.785*** | 0.904*** | 0.851*** | 0.884*** | 0.750** |
| RL | 0.785*** | 0.960*** | 0.766** | 0.811*** | 0.884*** | 0.864*** | 0.841*** | 0.809*** | 0.667** | |
| SDW | 0.849*** | 0.312 | 0.922*** | 0.820*** | 0.913*** | 0.887*** | 0.910*** | 0.774*** | ||
| RDW | 0.747*** | 0.868*** | 0.939*** | 0.901*** | 0.907*** | 0.876*** | 0.795*** | |||
| R/S | 0.423 | 0.721** | 0.522* | 0.560* | 0.477* | 0.514* | ||||
| P | 0.851*** | 0.949*** | 0.936*** | 0.978*** | 0.785*** | |||||
| K | 0.938*** | 0.950*** | 0.892*** | 0.759*** | ||||||
| Ca | 0.981*** | 0.966*** | 0.755*** | |||||||
| Mg | 0.974*** | 0.815*** | ||||||||
| Na | 0.842*** |
* Significant at P < 0.05.
** Significant at P < 0.01.
*** Significant at P < 0.001.
3.3 Effect of fly ash on grain number and yield
Fly ash amendment (F3,16 = 884.589) and AM inoculation (F1,16 = 4662.533) significantly (P < 0.001) influenced the number of grains per panicle (Fig. 2a). The interaction among these factors was also highly significant (F3,16 = 336.556; P < 0.001). The maximum grain production was observed in soils amended with 2% fly ash along with AM fungus inoculation, whereas the minimum grain production was observed in 6% fly-ash-amended soils. Mycorrhizal inoculation increased the average grain production by 55%.
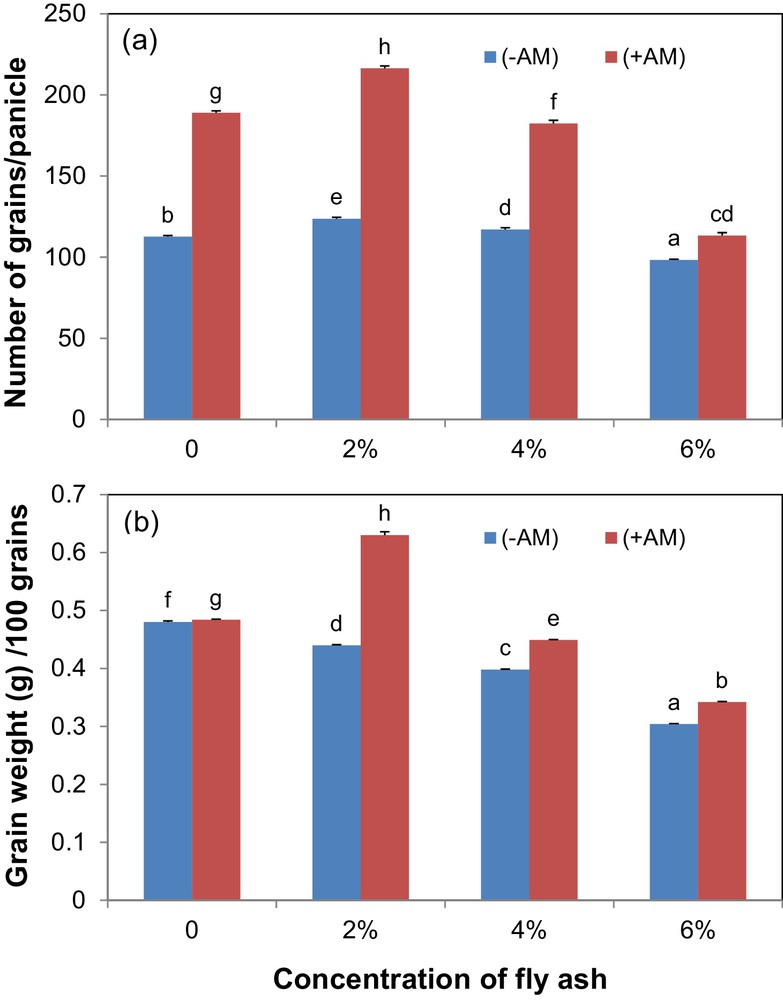
(Colour online.) Influence of fly-ash (FA) amendment and arbuscular mycorrhizal (AM) fungus inoculation on grain numbers (a) and grain weight (b) of kodo millet. Error bars indicate ± 1 S.E. Bars bearing same letter(s) are not significantly different (P > 0.05) according to DMRT.
Application of fly ash amendment (F3,16 = 2427.004) and AM inoculation (F1,16 = 2359.53) significantly (P < 0.001) influenced the grain weight (Fig. 2b). The interaction for fly ash amendment and AM fungus inoculation was also highly significant (F3,16 = 392.654; P < 0.001). The maximum grain weight was recorded in soils amended with 2% fly ash along with AM fungus inoculation, and the minimum grain production was observed in 6% fly-ash-amended soils with or without AM fungus inoculation.
3.4 Effect of fly ash on nutrient content of shoot and nutrient-use efficiencies
Nutrient concentrations in the shoots of kodo millet were significantly affected by fly ash amendment and AM fungus inoculation (Table 3). In addition, the interaction between the various factors was also highly significant for all the nutrients studied. Fly ash amendment reduced the concentration of P in kodo millet shoots at both the harvests, except for those raised on 2% fly-ash-amended soils at 60 DAS. Arbuscular mycorrhizal fungus inoculation improved the shoot P by 10% to 76% and 22% to 65% at 60 and 90 DAS respectively compared to non-mycorrhizal plants raised in soils amended with similar rates of fly ash. Application of 2% of fly ash significantly improved shoot K content in kodo millet both at 60 and 90 DAS, whereas a decline in shoot K content was observed at 4% and 6% fly-ash application rates. Inoculation of AM fungus significantly improved the shoot's K content at all fly-ash amendment rates. Although 2% of fly ash amendment significantly improved the concentrations of Ca, Mg and Na at 60 DAS, a further increase in fly-ash application rates decreased the nutrient contents. The Ca, Mg, and Na content of kodo millet shoots at 90 DAS were lower in all concentrations of fly ash amendment in uninoculated plants. However, Ca, Mg and Na contents in shoots at 90 DAS were significantly improved by AM fungus inoculation and were higher than those in uninoculated plants (Table 3). Shoot nutrient contents were significantly and positively correlated to the extent of AM fungal colonization as well as to plant-growth parameters (Table 2).
Influence of fly ash (FA) amendment and arbuscular mycorrhizal (AM) fungus inoculation on shoot nutrient content of kodo millet at 60 and 90 days after sowing (DAS).
| Treatments | Phosphorus (%) | Potassium (%) | Calcium (%) | Magnesium (%) | Sodium (%) | ||||||
| a–AM | +AM | –AM | +AM | –AM | +AM | –AM | +AM | –AM | +AM | ||
| 60 DAS | |||||||||||
| 0% FA | 0.97 ± 0.009 d | 1.07 ± 0.015 f | 0.44 ± 0.007 d | 0.53 ± 0.01 f | 0.32 ± 0.007 c | 0.46 ± 0.019 f | 0.35 ± 0.012 b | 0.51 ± 0.009 d | 0.26 ± 0.010 bc | 0.37 ± 0.009 e | |
| 2% FA | 1.01 ± 0.038 e | 1.54 ± 0.013 k | 0.49 ± 0.012 e | 0.94 ± 0.012 j | 0.40 ± 0.010 de | 0.85 ± 0.010 j | 0.49 ± 0.009 d | 0.98 ± 0.007 i | 0.34 ± 0.015 d | 0.65 ± 0.013 i | |
| 4% FA | 0.74 ± 0.012 b | 1.30 ± 0.012 i | 0.31 ± 0.010 b | 0.83 ± 0.009 i | 0.27 ± 0.009 b | 0.75 ± 0.015 i | 0.32 ± 0.003 b | 0.84 ± 0.007 g | 0.24 ± 0.012 b | 0.53 ± 0.013 g | |
| 6% FA | 0.66 ± 0.012 a | 0.87 ± 0.009 c | 0.25 ± 0.012 a | 0.68 ± 0.003 h | 0.22 ± 0.010 a | 0.37 ± 0.007 d | 0.28 ± 0.015 a | 0.39 ± 0.012 c | 0.20 ± 0.017 a | 0.28 ± 0.007 c | |
| 90 DAS | |||||||||||
| 0% FA | 1.63 ± 0.009l | 1.99 ± 0.009 n | 0.67 ± 0.009 gh | 0.84 ± 0.007 i | 0.69 ± 0.010 h | 0.96 ± 0.009 k | 0.94 ± 0.009 h | 0.99 ± 0.015 i | 0.79 ± 0.009 j | 0.88 ± 0.007 k | |
| 2% FA | 1.39 ± 0.009 j | 2.74 ± 0.0120 | 0.84 ± 0.007 i | 1.42 ± 0.018 m | 0.64 ± 0.017 g | 1.34 ± 0.013 m | 0.80 ± 0.013 f | 1.64 ± 0.020 k | 0.60 ± 0.010 h | 1.26 ± 0.009 m | |
| 4% FA | 1.13 ± 0.015 g | 1.87 ± 0.017 m | 0.64 ± 0.015 g | 1.33 ± 0.015l | 0.43 ± 0.010 ef | 1.20 ± 0.010l | 0.55 ± 0.009 e | 1.44 ± 0.009 j | 0.48 ± 0.003 f | 1.00 ± 0.012l | |
| 6% FA | 0.98 ± 0.003 de | 1.25 ± 0.007 h | 0.40 ± 0.007 c | 1.06 ± 0.012 k | 0.30 ± 0.007 bc | 0.69 ± 0.012 h | 0.47 ± 0.003 d | 1.00 ± 0.017 i | 0.35 ± 0.010 de | 0.62 ± 0.010 h | |
| F-statistics | df | ||||||||||
| Harvest (H) | 1.32 | 6887.53*** | 3789.61*** | 3315.005*** | 6414.821*** | 5179.042*** | |||||
| AM | 1.32 | 5000.95*** | 6504.684*** | 5445.658*** | 6048.337*** | 2967.76*** | |||||
| FA | 3.32 | 1767.1*** | 722.807*** | 911.759*** | 1026.505*** | 714.427*** | |||||
| H × AM | 1.32 | 502.898*** | 189.203*** | 411.005*** | 492.821*** | 284.575*** | |||||
| H × FA | 3.32 | 241.091*** | 60.435*** | 77.686*** | 31.845*** | 120.184*** | |||||
| AM × FA | 3.32 | 562.301*** | 374.484*** | 357.672*** | 632.407*** | 287.952*** | |||||
| H × AM × FA | 3.32 | 135.301*** | 7.749*** | 9.455*** | 111.445*** | 49.669*** |
*** Significant at P < 0.001.
Fly ash amendment and AM fungus inoculation significantly affected the nutrient-use efficiencies of kodo millet (Table 4). Application of 6% fly ash in the absence of AM fungus increased P, K, Ca, Mg and Na use efficiencies of kodo millet by 46%, 58%, 210%, 149%, and 185% respectively. Inoculation of AM fungus reduced the P use efficiency of kodo millet plants by 9% to 43% compared to plants raised on soils amended with similar rates of fly ash. However, K, Ca, Mg and Na use efficiencies were respectively 137%, 31%, 50%, and 107% higher for kodo millet plants inoculated with the AM fungus compared to uninoculated plants when grown on soils amended with 4% fly ash. Potassium and Mg use efficiencies were 6% and 10% higher for AM-inoculated plants raised on 2% fly-ash-amended and unamended soils, respectively, compared to non-AM plants raised on same levels of fly ash amendment.
Influence of fly-ash (FA) amendment and arbuscular mycorrhizal fungus (AM) inoculation on nutrient-use efficiencies of kodo millet.
| Treatmentsa | Nutrient use efficiency (mg/mg)b | |||||
| Phosphorus | Potassium | Calcium | Magnesium | Sodium | ||
| 0% FA,–AM | 103.46 ± 0.95 d | 81.63 ± 0.58 e | 63.66 ± 0.49 c | 42.35 ± 0.60 b | 48.27 ± 2.22 bc | |
| 2% FA,–AM | 98.96 ± 3.62 cd | 35.62 ± 4.30 b | 50.55 ± 6.34 ab | 38.23 ± 3.49 ab | 47.49 ± 4.68 bc | |
| 4% FA,–AM | 135.20 ± 2.11 f | 20.78 ± 2.18 a | 41.73 ± 3.58 a | 29.13 ± 3.01 a | 29.38 ± 4.28 a | |
| 6% FA,–AM | 150.85 ± 2.77 g | 129.30 ± 8.85 f | 197.53 ± 7.01 d | 105.28 ± 8.63 c | 137.76 ± 7.86 d | |
| 0% FA,+AM | 93.78 ± 1.28 c | 63.01 ± 1.98 d | 46.39 ± 2.15 ab | 46.62 ± 3.17 b | 47.50 ± 2.80 bc | |
| 2% FA,+AM | 64.80 ± 0.56 a | 37.82 ± 1.42 bc | 38.31 ± 0.11 a | 29.92 ± 0.56 a | 35.64 ± 0.43 ab | |
| 4% FA,+AM | 76.74 ± 0.71 b | 49.17 ± 1.68 c | 54.50 ± 0.70 bc | 43.78 ± 0.55 b | 60.74 ± 1.12 c | |
| 6% FA,+AM | 114.53 ± 1.15 e | 37.72 ± 3.12 b | 49.73 ± 3.44 ab | 28.21 ± 2.20 a | 48.98 ± 3.83 bc | |
| F-statistics | df | |||||
| FA | 3.16 | 239.228*** | 81.217*** | 188.319*** | 31.738*** | 72.799*** |
| AM | 1.16 | 641.924*** | 52.631*** | 226.873*** | 89.539*** | 37.479*** |
| FA × AM | 3.16 | 53.160*** | 88.143*** | 175.281*** | 61.317*** | 79.257*** |
a –AM and +AM, uninoculated and AM fungus-inoculated, respectively.
b Means ± S.E. in a column followed by the same alphabet letter(s) are not significantly (P > 0.05) different according to DMRT.
*** Significant at P < 0.001.
4 Discussion
Like most of the fly ash produced in India, the fly ash used in the present study was alkaline in its nature [46]. Our study demonstrated that fly ash could promote plant growth and yield of kodo millet, which is in line with the studies where fly ash application to soil has resulted in better yield in terms of various parameters like plant height, biomass, and productivity in different plant species. The increased seed yield of kodo millet in fly-ash-amended soils is in congruence with studies where fly ash amendment has been shown to improve yields of crops like barley (Hordeum vulgare), sunflower (Helianthus annuus), rye grass (Secale cereale), brinjal (Solanum melongena), potato (Solanum tuberosum), and garden pea (Pisum sativum) (see 5 and references therein). The application of fly ash has been shown to influence soil pH and provide plant-available nutrients, resulting in increased plant growth and productivity [46]. In addition, Babu and Reddy [33] also showed that the inoculation of AM fungi increased the organic carbon content of the fly ash, resulting in an increased growth of bamboo (Dendrocalamus strictus). Nevertheless, the plant benefits were dependent on the concentration of the amending fly ash. Usually, at 2% fly ash stimulated plant growth, nutrient uptake and yield, whereas a further increase in amendment rates (4% and 6%) was found to be inhibitory. Singh and Siddiqui [47] also reported that fly ash up to 5.0 g/plant was beneficial for plant growth and yield in all the cultivars of wheat (Triticum aestivum) varieties (HD-2009, HD-2329, and Lok-1), but above this concentration fly ash had an adverse effect on these parameters. Growth and yield of tomato (Solanum lycopersicum) were mostly enhanced in response to amendment with 50% or 60% of fly ash added to the field's soil. Even so, an increase above 60% tended to reduce plant growth and yield considerably [4]. It has also been observed that the addition of smaller doses of fly ash improves the ability of the soil to support plant growth through an increase in the biomass and diversity of the soil microbiota [5]. The inhibitory effect of fly ash at high concentrations has often been attributed to the presence of high concentrations of Ca, K, and Na and trace elements such as As, B, Mo, Se, and Sr. Therefore, application of unweathered fly ash to soil may cause a potential increase in soil salinity and build-up of the potentially toxic elements, including heavy metals [5].
It is interesting to note the absence of AM colonization in millet roots at 60 DAS, suggesting the low levels of infective propagule of AM fungi in the studied soils. Nevertheless, the application of fly ash and fungal inoculation increased AM colonization in millet roots. Limited studies have explored the effect of fly ash on AM colonization and sporulation. Root colonization by AM fungi and spore numbers were enhanced by application of low levels (2%) of fly ash amendment. However, root colonization and spore numbers tended to decline with increasing concentrations of fly ash. This is in accordance with the surveys reporting low levels of AM colonization and spore numbers in the rhizosphere of plants colonizing fly-ash-contaminated soils [28,29]. Selvam and Mahadevan [31] also showed that the application of fly pond soil at 1/1 or 1/2 ratios to red soil reduced AM colonization and spore numbers of native AM fungi. The reduction in AM colonization and spore numbers in response to increasing concentrations of fly ash in the soil can be attributed to changes in soil conditions brought about by the fly ash. It is well known that fly ash application could bring in changes in soil nutrient levels, reactivity and microbial populations and their activity, all of which are known to affect mycorrhization and sporulation of AM fungi. Though previous studies have demonstrated that the AM fungal colonization of plant roots could be affected by soil P availability [48–50], this could not be the reason behind the low AM fungal colonization in the present study as the P content in the fly ash was lower than that in the native soil. Another phenomenon observed in the present study was the existence of a strong positive correlation between AM colonization and spore numbers in the soil. This suggests that both the fungal variables respond to the existing or changing conditions similarly. Selvam and Mahadevan [31] also reported the existence of a positive correlation between AM fungal colonization and spore numbers in onion (Allium cepa) growing on fly ash soils.
Inoculation of AM fungi in the present study either enhanced the positive benefits or reduced the deleterious effect of fly ash on plant growth and yield. This is in line with the observations of Garampalli et al. [51], where three different concentrations of fly ash (10 g, 20 g and 30 g fly ash/kg soil) improved the infectivity and effectiveness of AM fungus (Glomus aggregatum) on pigeon pea (Cajanus cajan L.) cv Maruti. Babu and Reddy [33] also showed that dual inoculation of AM fungus and phosphate solubilizing fungus (Aspergillus tubingensis) in fly ash ponds could improve plant growth and nutrient uptake of bamboo (Dendrocalamus strictus). These observations suggested that AM fungal inoculation with fly ash application could successfully enhance plant growth through the increased availability of nutrients, especially P and minerals such as Zn, Cu, K and Ca, available in the soil and fly ash to the host plants [52]. In spite of the presence of many essential plant nutrients in fly ash, their availability to the plants may be limited, as reported by different authors [53,54]. Bryan et al. [55] have demonstrated that the contribution of mycorrhizae was highest in the survival of Withania somnifera in fly ash due to reduction of stresses, creating a congenial environment in the rhizosphere. Further, fly ash additions are also known to cause a reduction in some of the soil nutrients like N [4]. The dependence of kodo millet on AM fungi is evidenced by the presence of a strong positive correlation between plant growth and AM fungal variables.
The increase in root mass in response to AM fungus inoculation observed in the present study could be the result of plant hormones produced by AM fungi [56] or induced by AM fungi [57]. Changes in root morphology mediated by AM fungi resulting in increased root dry weights have been reported in Allium cepa [58], Casuarina equisetifolia [19], and Prunus cerasifera [59]. In contrast to the general assumption that AM association lowers the R/S ratio of plants, the R/S ratios of kodo millet plants inoculated with the AM fungus both in fly-ash-amended, and unamended soils were higher. This indicates that the plants invest more resources in roots to increase the capture of nutrients from the soil [60]. As R/S ratios are also altered by nutrient application, the effect of mycorrhizae on R/S ratios could probably be nutritional, in addition to the hormonal effect discussed earlier [61].
In our study, P concentrations in kodo millet shoots was lowered by 4% and 6% fly ash amendment to the soil, which is in accordance with the studies where fly ash application has been known to lower shoot P concentrations [62,63]. Similarly, plant availability of K in fly ash has also been reported to be low, in spite of its high concentration in fly ash [64]. In the present study, nutrient concentrations like P, K, Ca, Mg and Na were maximal in the shoot tissues of kodo millet inoculated with AM fungi and raised on soils amended with 2% fly ash. Further, nutrient concentrations in tissues of AM-inoculated plants were always higher than in uninoculated plants. This is in agreement with Bi et al. [32], who reported that maize (Zea mays) plants inoculated with AM fungi accumulated more nutrients in their shoots compared to non-mycorrhizal plants when grown on soils overlying coal fly ash. The increased concentration of tissue nutrients in different levels of fly ash amendments could be attributed to the contribution of the external hyphae of AM fungi in the uptake of available nutrients both from the soil and weathering of fly ash [32]. The existence of a positive correlation between shoot nutrient content and AM fungal colonization levels clearly suggests the role of AM fungi mediated nutrient uptake in kodo millet.
Kodo millet plants inoculated with AM fungus had lower nutrient-use efficiencies than uninoculated seedlings. This is similar to the observations made by Stribley et al. [65] and Koide [45], where mycorrhizal plants had low nutrient-use efficiencies compared to their non-mycorrhizal counterparts. Koide [45] suggested that any increase in nutrient content for plants with sufficient concentrations of nutrients would produce a depression in nutrient-use efficiency. However, plants could use such nutrient reserves later for continued growth, even if uptake from the soil was no longer possible under nutrient-stressed conditions [45].
In addition to plant nutrients, fly ash may also contain non-essential elements like Al, Fe, Cu, Pb, Cr, Ni, Zn, and Hg, as well as other elements that may be toxic at elevated concentrations [5]. The mechanisms involved in AM-mediated nutrient uptake and accumulation in plant tissues also includes the suppression of the toxic effects of elements affecting plant growth, sequestration of the toxic metals in the fungal structures, and the development of tolerance by the AM fungi [66].
5 Conclusions
This study clearly indicates that the mycorrhizal benefit to plants in fly-ash-amended soils is more concentration-dependent. Further, long-term studies carried out on the effect of fly ash on soil fertility and crop yields has clearly revealed that optimum concentrations of fly ash can be effectively used for improving the productivity of soils and increasing the yield of crops, vegetables, and cereals without affecting the food quality and soil fertility [67]. The present study, therefore, leads to practical agronomic application of fly ash along with AM fungi as a promising strategy to maximize crop growth and yield in low fertile soils. The results of the present study also emphasize the need to understand the response of AM colonized plants as they may behave differently depending on the fly ash concentrations. However, more studies in different soil types and with different AM fungal species are needed to further ascertain and understand the influence of fly ash amendment on AM symbiosis and its benefits.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.


