1 Introduction
Oaks (Genus Quercus) are dominant woody species throughout the northern hemisphere and are key drivers of terrestrial biodiversity from tropical to boreal landscapes [1]. Their worldwide demographic success raises distinct questions regarding the evolutionary trajectories they adopted to cope with the drastic environmental crisis that occurred during the Pleistocene. The earth's climate has been dominated during the Pleistocene by the succession of more than fifteen glacial and interglacial periods. Glacial periods lasted on average from seventy thousand to hundred thousand years, while interglacial periods were much shorter (from ten to twenty thousand years). These repeated drastic environmental changes were followed by important alternating retractions and expansions of tree species distributions, placing them in different habitats over time and space. As for many other plant genera, some oak species got extinct at the onset of the first glacial periods [2], but remarkably several survived and some of them occupy today continental wide distributions (Quercus robur in Europe; Q. macrocarpa in North America; Q. acutissima in Asia). There has been no tree species extinction during the most recent glacial–interglacial periods, suggesting that extant tree species in Europe demonstrated an efficient ability to migrate and adapt to repeated climatic transitions. Quaternary evolutionary history suggests therefore that these species have developed mechanisms to help reduce the likelihood of extinction, despite the drastic environmental changes. This review aims at highlighting evolutionary mechanisms that triggered adaptive pathways of European temperate white oaks (Q. petraea and Q. robur) during major climatic transition since the Last Glacial Maximum (LGM). It builds on complementary insights provided by allochronic and synchronic approaches that were obtained during the past twenty years (Fig. 1). The review ultimately aims at suggesting hints for retracing the pace of microevolution under major environmental crisis, which is a timely concern in the context of ongoing climate change. It also explores how the lessons taken from past evolutionary scenarios may help to predict future responses of these long-lived tree species.

Evolutionary trajectories of oaks during the Holocene. At the end of the last glacial maximum, oaks, as many other trees, were restricted to three main genetically separated refuge areas (Iberian Peninsula, Spain, and the Balkan Peninsula) [4]. A. Illustrates the likely evolutionary trajectory of populations stemming from one refugial area (for example, Spain, orange circle). As the climate warmed, oaks migrated northwards (red arrows). Whether the migrating populations just tracked the climate change, without major changes in their fitness, is unknown. At the southern edge, populations were facing new climates and natural selection resulted in the extreme south to population extinction, while in other places populations adapted to the new climate (black arrow). However, extinction was limited during the post-glacial warming as the two temperate oaks are still present in Spain Italy and the Balkans, although in scattered populations. Overall, the whole scenario is more comparable to an expansion, rather than an entire shift of the distribution of the species from the south to the north. In this review, we illustrate some of the findings regarding the velocity and dynamics of migration, and the mechanics of local adaptation. The reconstruction of this scenario is based on paleobotany, phylogeography, and evolutionary inferences drawn from synchronic analysis of molecular or phenotypic data of modern populations (green circles). These investigations are currently being completed by ancient DNA analysis retrieved from fossil or archeological remains collected throughout Europe from ancient populations (blue circles). However today's oak forest in central Europe were colonized by different lineages stemming from two or more refugial areas (B), here represented by green and red circles. As migration developed from both source populations, both lineages met in central and northern European forests, followed by gene flow between both lineages (red and green arrows), thus generating substantial admixture between lineages within today's populations.
2 Tracking climate change by migration
The distribution of temperate white oaks in Europe has shifted repeatedly from the Mediterranean to the boreal regions and reciprocally during interglacial and glacial periods [3]. At the end of the Last Glacial Maximum, oak forests were restricted to the Iberian Peninsula, Italy, and the Balkan Peninsula (Greece and the western Coast of the Black Sea). A pan-European survey of oak pollen fossil remains showed that all refugial sites were located in mountainous areas (e.g., Sierra Nevada in Spain, the Southern Apennine chain in Italy, and the Pindos mountains in Greece) [4]. The extant distribution was approximately reached at 6000 BP (Fig. 1A). The velocity of oak migration during the post-glacial recolonization period (between 15,000 to 6000 BP) averaged 500 m/yr, reaching in some cases up to 1000 m/yr [4]. These figures inferred from pollen deposits maps exceed by far the dispersion by jays, squirrels, or rodents. To explain this paradox, occurrences of rare long-distance dispersion events were invoked. This mechanism has been tested by computer simulation and shows that dispersion events of a few tens of kilometres, even if very rare, would substantially accelerate the overall migration rate [5]. But no long-distance dispersion vector has received so far conclusive evidence. An alternative scenario has been proposed, suggesting that cryptic refugial populations persisted during the glacial period in more northern areas, and would have acted as source populations for recolonization [6]. It is likely that both dispersion mechanisms can be advocated in the case of oaks. A more recent intriguing hypothesis is that humans acted either as vector of long-distance dispersion, or contributed to the installation of northern cryptic refugia [7]. This hypothesis contends that early human populations, while invading Europe at the Upper Paleolithic period or while migrating in response to post-glacial warming, did actually contribute to the dispersion of oaks, as acorns were a major component of human diets. Acorns were not only used when other resources became exhausted, but also as dietary staple [8].
How much migration is actually going on at a contemporaneous times scale, due to global warming? There is scarce data available on the subject, as migration of trees by natural means is hardly tractable over of few decades. However, recent investigations based on past documentary description of forest composition at the north western margin of the Q. ilex distribution indicated that this Mediterranean oak species is indeed migrating at an average rate of less than 60 m/yr [9]. These figures however do not take into account the occurrences of large distance rare dispersion events that can hardly be detected today unless very detailed inventories are implemented. Finally migration by natural means may be severely constrained by land fragmentation and human impacts. Overall, one may conclude from these speculations that migration by natural means will not be sufficient to reach the shift of the oak distribution predicted by niche-based or process-based models [10].
3 Accelerating migration and species succession via hybridization
A pan-European genetic survey of chloroplast DNA diversity indicated that the two temperate oak species (Q. petraea and Q. robur) share systematically the same haplotypes when they occupy the same forest. The almost complete sharing of chloroplast variants among the two species occupying the same stand was observed across the natural distribution of the oaks, suggesting that hybridization was common and widespread during post-glacial colonization [11]. Hybridisation may have played an important role in the colonisation process, since repeated hybridization events accelerate species migration and succession rates. This scenario has been described in detail in the case of Q. petraea and Q. robur by Petit et al. [12]. Because Q. robur tends to be a more pioneering species than Q. petraea, it was most likely present at the northern edge of the oak migration front, while Q. petraea was somewhat behind it, but still capable of hybridising with Q. robur through pollen flow. If the first generation hybridization is followed by later backcrosses (e.g., between hybrids and parents) with Q. petraea as male parent, then successive introgression will lead to the restoration of Q. petraea trees within the Q. robur stand. The end result is that Q. petraea becomes established within the Q. robur stand, consequently enhancing species succession [12]. The peculiar role of hybridization shows that colonization of newly available territories by oaks (for instance following successive ice ages) is facilitated by interspecific gene exchanges, allowing some species to bypass colonization by seed to rapidly invade new sites. Incidently, continuous recent interspecific gene flow was confirmed by evolutionary investigations using ABC (Approximate Bayesian Computation) simulations (Leroy et al., unpublished results) based on genome wide SNP polymorphism in Q. petraea and Q. robur. Indeed genomic differentiation among the two species has been scanned by complete genome sequencing, thus allowing one to test different evolutionary scenarios resulting in such divergence patterns. Comparison of different contrasting speciation scenarios (strict isolation, ancient migration, constant migration, and secondary contact) showed that posterior probabilities for secondary and recent contacts were much larger than for any other scenario. We may further extrapolate these results in the context of present ongoing shifts of species distribution. Range overlaps between Mediterranean and temperate white oaks can be anticipated more frequently, hence triggering hybridization and facilitating species migration via introgression, in a similar manner as during the post-glacial.
4 Local adaptation during past climate change
The comparison of today's distribution of temperate oaks with their past post-glacial distribution surprisingly indicates a clear overlap of their past and present range at the southern extant margin [4]. This observation clearly suggests that local adaptation has occurred, as some populations stayed in place over the past 15,000 years (Fig. 1A). These considerations are supported by experimental results obtained in large-scale common garden plantations of Q. petraea established along large-scale geographical gradients. There is now evidence that the genetic divergence among extant populations resulted from adaptive evolution due to local selection from the time of establishment of populations on new sites [13]. Assuming that the Spanish and Italian-Balkan refugial populations were genetically separated for more than 100,000 years during the last glacial period, genetic differentiation may have accumulated between these two source populations and may be partly responsible for the differentiation between extant populations in central Northern Europe that originated from different refugial origin. Genetic variation of phenotypic traits assessed in common garden experiments did however not support the persistence of a footprint due to the maternal origin of today's populations. Indeed most phenotypic traits show large population differentiation, with clinal patterns of variation that differ from historical patterns [13] (Fig. 2). A demonstrative example comes from phenological traits that exhibited consistent continuous variation across temperature gradients. Interestingly, some of the underlying genes that exhibit genetic associations with phenological traits exhibit also clinal variation along the same gradient [14]. There is little evidence in current populations of any phenotypic or adaptive trait differentiation relating to the refugial origin, even in neighbouring central European populations that are known to have originated (through migration) from different refugia. This is interpreted as the result of extensive gene flow during admixture followed by local adaptation [13] (Fig. 1B). One may conclude from these analyses that most variation in provenance tests is the result of recent adaptive evolution. Theoretical approaches have however shown that the interaction of extensive gene flow and strong diversifying selection will accelerate the divergence of complex multilocus traits [15] and achieve adaptation in less than 20 generations. The rapid process is generated by allelic associations among different loci that increase population differentiation of adaptive traits in response to diversifying selection. The mechanism is enhanced by gene flow that provides the diversity needed to create different allelic associations. The overall process is accelerated if the number of loci contributing to the trait of interest increases. To sum up, congruent results stemming from experimental results of trait and gene variation and from simulations suggest that evolutionary change can be substantial over a few generations leading to local adaptation. Recent sequencing of oak genomes showed indeed that standing genetic variation in oak population is extremely large, thus providing the necessary resource for adaptation over successive generations [16].
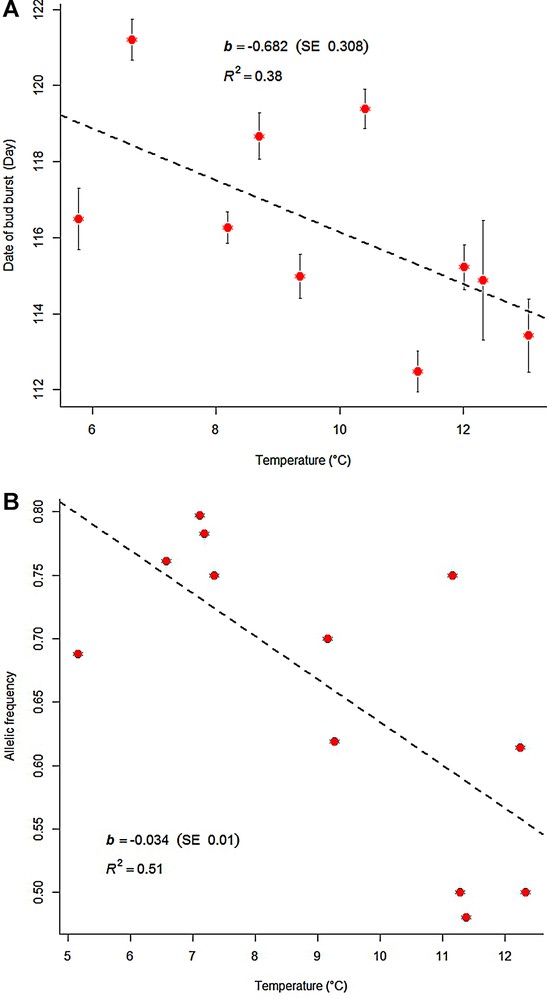
Illustration of local adaptation of bud burst in Oaks. The time when oaks flush in the spring is highly dependent on the local temperature. Under warm spring temperatures, trees will flush earlier, and when the climate is cooler trees will flush later. These in situ patterns can also be observed in common garden experiments, where trees originating from different geographic origins start flushing according to the temperature at their origin. Under common garden conditions, trees from lower elevation and altitude will flush earlier than trees from higher elevation. This is illustrated in (A), where the date of flushing (number of days since 21 January) is plotted versus the spring temperature at the place of origin of the trees [13]. The populations were sampled along altitude within two Pyrenean valleys. Similar patterns were observed when the trees were collected along latitudinal gradients, suggesting that temperature is the driving environmental factor of population differentiation. Similar clinal variation was also observed in candidate genes [13], as illustrated here by linear variation of allele frequencies of one SNP within gene APS (5′-adenylylsulfate reductase) along the temperature gradient (B). The evolutionary driving force may either be biotic as defoliating insect pressure or abiotic as freezing damage due to late frost. On a wide-range scale, the latitudinal clinal pattern of bud burst is orthogonal to the historical distribution of variation (East-West pattern) that was generated by the different post-glacial migration routes. Thus, the extant bud burst genetic variation is likely to result from selection pressures and local adaptation that occurred during the Holocene.
5 Conclusion
This brief review outlines peculiar microevolutionary strategies of long-lived tree species as oaks during past climatic transitions, and provides clues for understanding their current demographic success in terrestrial ecosystems. Inherent resources and capacities to oaks that facilitated migration and adaptation stem from generic life history and demographic features of trees as large population size, extensive gene flow, and high levels of genetic diversity. In addition, hybridization, which is usually considered as a nightmare in oak taxonomy, is seen here as a key evolutionary mechanism enhancing migration and succession, and contributing to adaptation by feeding standing genetic variation in mixed oak stands. Finally, this review suggests that human interferences may as well have contributed to the evolutionary success of oaks. While strong convincing evidence is still lacking, comparative archeobiological and genetic investigations indicate that post-glacial migration dynamics may have been accelerated by humans while feeding with acorns. It may well be that human interference may be again needed for transferring populations through assisted migration to cope with the rapid ongoing climate change.
Disclosure of interest
The author declares that he has no competing interest.
Acknowledgements
I acknowledge the financial support of the European Union through successive EU projects (OAK GENETICS, FAIROAK, OAKFLOW, EVOLTREE, FORESTTRAC, TREEPEACE) that allowed one to implement continental wide research on oak genetics and evolution during the past 20 years. National support was also obtained by ANR grants (QDIV, TRANSBIODIV, GENOAK) or grants by the Aquitaine Region. I am grateful to the numerous colleagues of partner labs from different European countries, and from INRA that cooperated within the collaborative research projects. I offer my warmest thanks to the staff of BIOGECO and of the Experimental Units of INRA (UE Forêts Pierroton, UE Génétique et Biomasse forestière), to doctorate students, and to post-doctorate fellows for their constant help and contribution to this research.


