1 Introduction
Vasoactive intestinal peptide (VIP) is one of the earliest neuropeptides of the glucagon/secretin superfamily to be discovered. It is a 28 amino acid peptide [1], firstly isolated from porcine ileum [2] and characterized by high-sequence conservation [3,4]. VIP is produced as 170 amino acid polypetide, the pre-pro-VIP, which encompasses VIP, and VIP-related peptide, the peptide histidine-methionine amide in human or peptide histidine−isoleucine amide (PHI) in other vertebrates [4]. VIP shows 68% sequence identity with the pituitary adenylate cyclase activating polypeptide (PACAP). VIP and PACAP share common G-protein-coupled receptors, VPAC1 and VPAC2, with consequent overlapping functions [5–7] including vaso- and immune-regulation [8], neuromodulation [9–12] and reproduction control too [3,6,7]. Indeed, VIP has been widely reported in the nerve fibers present in rat testis [13], VPAC1 in human spermatozoa [14] and in vasa deferentia [15], whereas the presence of VPAC2 has been highlighted in spermatocytes [16] and Leydig cells [17,18]. No evidence of VIP was reported in mammalian seminiferous tubules [13]. The presence of the VIP/VPACR system in mammalian testis strongly suggested that VIP is involved in testis functions, including protein synthesis [19] and testosterone production [20–22], as VIP-deficient males displayed a dramatic decrease in testosterone serum concentration and testicular degeneration [23]. By contrast, in the testis of Podarcis sicula, the Italian wall lizard, the VIP/VPACR system is present in all phases of the spermatogenic cycle; indeed, VIP and VPAC2 are expressed both in germ and in somatic cells, whereas VPAC1 is expressed in Leydig cells and spermatids. Moreover, an in vitro investigation highlighted that, in Podarcis, testis VIP, as well as PACAP, is involved in 17 β-estradiol and testosterone secretion [24,25].
The goal of present study is to investigate the presence of the VIP/VPACR system in the epididymis of two vertebrates with tubular testis organization: Podarcis sicula, a seasonal reproducer, and Rattus rattus, a continuous reproducer. In Podarcis, the epididymis organization changes according to the spermatogenic cycle. During the reproductive period, the epididymis epithelium is constituted by basal and columnar cells: the former ones are stem cells, the latter are quite active cells in the synthesis of large dense vacuoles that, once discharged in the epididymal lumen, have part in epididymal fluid organization; furthermore, columnar cells highlight the presence of two nuclei testifying to their synthesis activity [26–28]. By contrast, during the non-reproductive period, basal and columnar cells are always present, but now the columnar ones highlight a single nucleus and are lacking in large dense vacuoles; on the other hand, during the non-reproductive period, spermatozoa are lacking in the epididymis lumen, whereas they are well represented during the reproductive one [27].
In the epididymis of mammals, including rats, four cellular types have been reported: main, apical, tight and basal cells [29–35]. The main cells highlight the presence of a large basal nucleus centrally located, as well as of a well-developed secretion apparatus, which synthesizes proteins in the epididymal fluid; the same apparatus is recognizable in apical cells, which, differently from main cells, show no contact with the basement membrane [34]. Tight cells are characterized by the presence of a thin cytoplasm surrounding the nucleus and typical cytoplasm extensions projecting to ward epididymal lumen as well [34]. Finally, the epididymis epithelium evidences the presence of basal cells, which are localized on the basement membrane, and have no contact with the epididymal lumen; basal cells produce control factors regulating main and tight cell activity, as well as electrolytic secretion and immune responsivity [35].
By an immunohistochemical investigation, we demonstrate that the VIP/VPACR system is widely represented in the epididymis of both experimental models, suggesting that such a system could play an active role in the reproduction of vertebrates, in particular, sperm maturation and fertilization.
2 Materials and methods
Sexually mature males of P. sicula were collected in Campania (southern Italy; latitude: 41°19′54′′N; longitude: 13°59′29′′E) during the reproductive (May 2013) and non-reproductive periods (July 2013). Once collected, the animals were maintained in a soil-filled terrarium and fed ad libitum with Tenebrio molitor larvae, for approximately 15 days, the time required to recover from the capture stress. The experiments were approved by institutional committees (Italian Ministry of Health) and organized so as to minimize the number of animals utilized in the experiment. The animals were killed by decapitation after deep anesthesia with ketamine hydrochloride (325 pg·g−1 body mass; Parke-Davis, Berlin, Germany). The sexual maturity of each animal was determined by morphological parameters and histological analysis [36–41]. We utilized six animals for our experiments.
Rattus epididymis was kindly gifted by Prof. M.P. Mollica, Department of Biology, Federico II Naples University, Italy.
3 Immunohistochemistry
For immunohistochemistry, five micrometer-thick sections of Bouin fixed testis on poly-l-lysine slides have been treated as previously reported [42–49]. Briefly, the sections were treated with 10 mM citrate buffer at pH 6.0, and then incubated in 2.5% H2O2 in methanol for endogenous peroxidase blocking. Non-specific background was reduced with the incubation in normal goat serum (Pierce, Rockford, IL) for 1 at room temperature. Sections were then treated overnight at 4 °C with the primary rabbit antibodies diluted in normal goat serum:
- • anti-human VIP (1:500) (Phoenix pharmaceuticals);
- • anti-human VPAC1 (1:300) (Santa Cruz Biotechnology);
- • anti-human VPAC2 (1:300) (Santa Cruz Biotechnology).
The reaction was revealed with a biotin-conjugated goat anti-rabbit secondary antibody and an avidin−biotin−peroxidase complex (ABC immune peroxidise kit, Pierce), using DAB (Sigma-Aldrich) as chromogen. Negative controls were carried out by omitting primary antibodies. The immunohistochemical signal was observed using a Zeiss Axioskop microscope; the images were acquired by using AxioVision 4.7 software (Zeiss).
4 Results
4.1 Podarcis sicula
4.1.1 Reproductive period
Immunohistochemistry performed with anti-VIP, -VPAC1 and -VPAC2 antibodies demonstrated that these molecules occurred within Podarcis epididymis. The immunohistochemical signal was evident in all epididymal cells, including the spermatozoa present in the lumen (Fig. 1A, B, B, inset); in details, immunolabelling for VIP was evident in both columnar and cubic cells (Figs. 1A, B). Columnar cells highlighted a VIP labelling within cytoplasm, as well as in large dense vacuoles present both in the cytoplasm of cells and in the epididymal lumen, where they were intermingled with the spermatozoa (Fig. 1B, B, inset). Furthermore, myoid and vascular endothelial cells showed immunolabelling for VIP (Fig. 1A, B). VPAC1 (Fig. 1C,C, inset, D) and VPAC2 (Fig. 1E, F) showed the same VIP distribution; indeed all epididymis cells were immunolabelled, as well as the spermatozoa, myoid, and vascular endothelial cells and the dense large vacuoles. Controls obtained by omitting the primary antibody were not immunolabelled (Fig. 1F, inset).
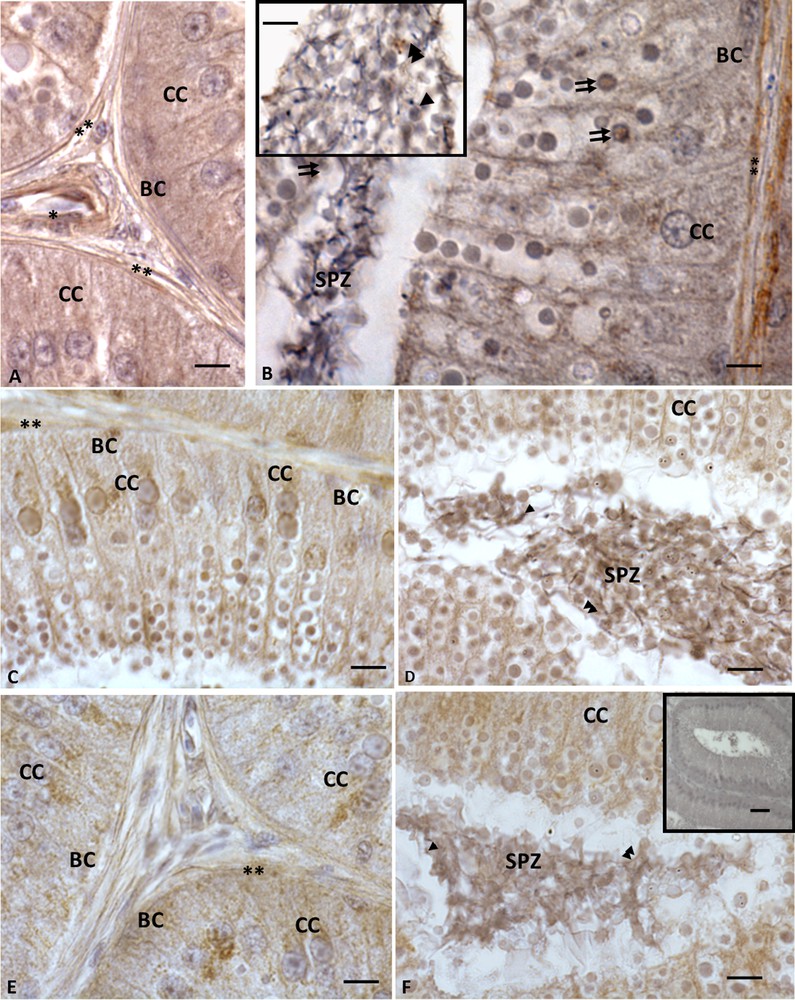
Immunohistochemistry for VIP and its receptors in Podarcis sicula epididymis in the reproductive period. The immunolocalization signal appears as brown areas. A−B. A strong signal for VIP is evident in basal (BC) and columnar (CC) cells, as well as in vascular endothelial (asterisk) and myoid cells (double asterisk); the signal is evident also in the large dense vacuoles (double arrows). Spermatozoa (SPZ) present in the lumen were also immunolabelled: the signal occurs in acrosome (insert, arrowhead) and tail (insert, double arrowheads). C−D. VPAC1 labelling. Staining is evident in basal (BC), columnar (CC) and in myoid cells (double asterisk). Spermatozoa (SPZ) present in the lumen were also immunolabelled: the signal occurs in the acrosome (arrowhead) and the tail (double arrowheads). The signal is also evident in the large dense vacuoles present both in columnar cells and in epididymal lumen intermingled with spermatozoa (double arrows). E−F. VPAC2 immunolocalization. The signal occurs in basal (BC), columnar (CC) and myoid cells (double asterisk), as well as in spermatozoa (SPZ), where signal occurs in acrosome (arrowhead) and tail (double arrowheads). The large dense vacuoles are also immunolabelled. No signal is evident in the negative control sections (F, inset). Scale bars correspond to 20 μm in figure F (inset), and 5 μm in A, A (inset), B, C, D, E, F.
4.1.2 Non-reproductive period
Immunohistochemistry performed with anti-VIP, -VPAC1 and -VPAC2 antibodies showed a more limited distribution. The VIP signal was found only in vascular endothelial and myoid cells (Fig. 2A, A, inset). No signal occurred in basal and columnar cells (Fig. 2A). Immunolabelling for VIP receptors was present within myoid cells, while it was lacking in epithelial cells (Figs. 2B, C). Controls obtained by omitting the primary antibody showed no positive reaction (Fig. 2C, inset).
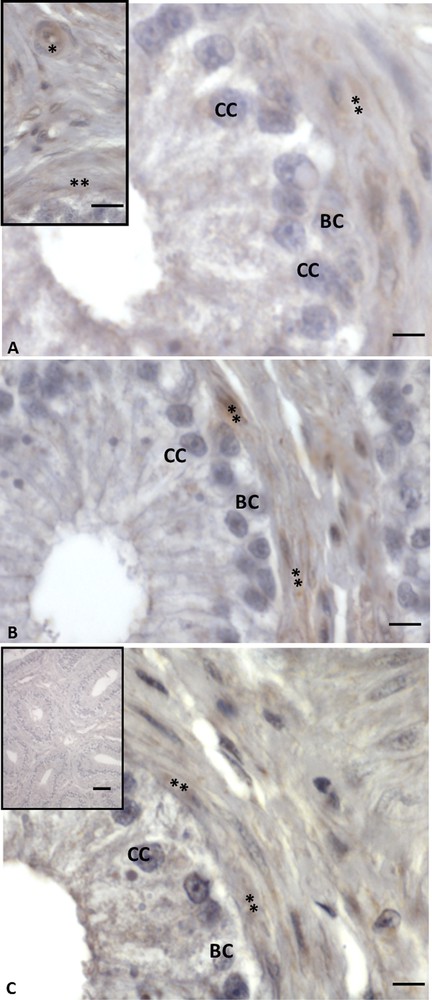
Immunohistochemistry for VIP and its receptors in Podarcis sicula epididymis in the non-reproductive period. The immunolocalization signal appears as brown areas. A. Positivity for VIP is evident only in vascular endothelial (asterisk) and myoid cells (double asterisk). No signal is evident in basal (BC) and columnar (CC) cells. B. VPAC1 labelling. The signal is evident only in vascular endothelial (asterisk) and myoid cells (double asterisk). Basal (BC), columnar (CC) cells and spermatozoa (SPZ) are unlabelled. C. VPAC2 immunostaining. It is present only in vascular endothelial (asterisk) and myoid cells (double asterisk). No signal is evident in basal (BC) and columnar (CC) cells. Negative control sections (C, inset) show no signal. The scale bars correspond to 20 μm in figure C (inset), and 5 μm in A, A (inset), B, C.
4.2 Rattus rattus
A positive signal highlighted the presence of VIP, VPAC1, and VPAC2, at the level of all epididymis cells, including the spermatozoa present within the epididymal lumen (Fig. 3A, B). In details, VIP immunolabelling was evident in basal, tight, main and apical cells (Fig. 3A, B); in the spermatozoa, the signal was recognizable in both the acrosome and the tail (Fig. 3B). Immunolabelling for VIP was also observed in the connective tissue: indeed a strong signal occurred in myoid and vascular endothelial cells (Fig. 3A, B). VPAC1 (Fig. 3C, D) and VPAC2 (Fig. 3E, F, G) showed the same distribution of VIP: the immunohistochemical signal was observed in epididymal, and myoid cells, and finally in the acrosome and tail of the spermatozoa. Controls obtained by omitting the primary antibody showed no positive reaction (Fig. 3H).
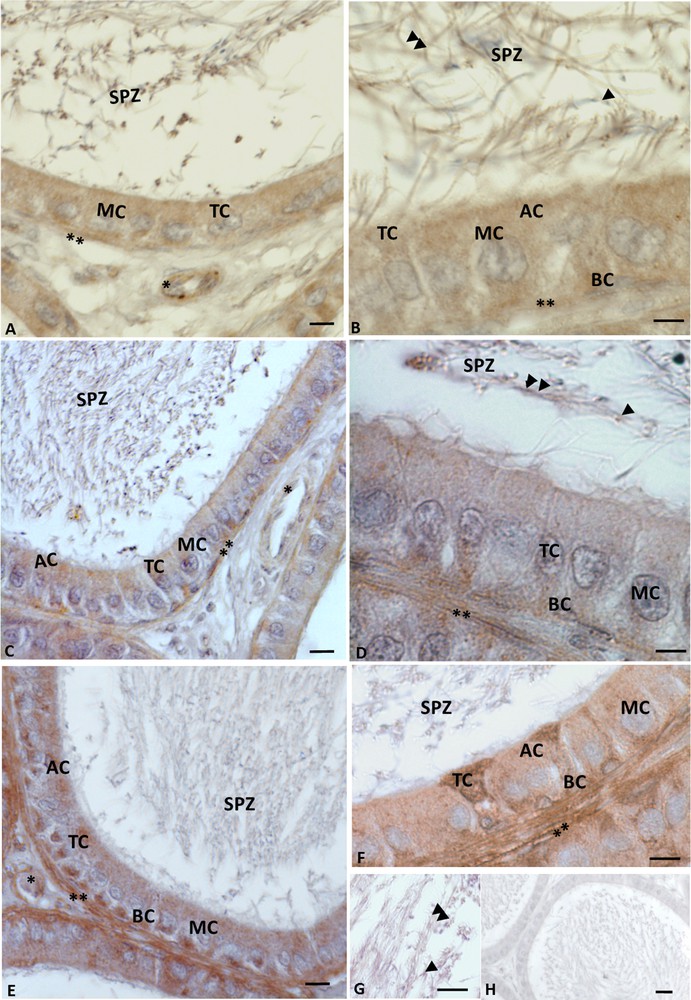
Rattus rattus epididymis: VIP and its receptor immunolabelling. Immunolabelling appears as brown areas. A−B. Anti-VIP immunolabelling. Basal (BC), tight (TC), main (MC) and apical (AP) cells are immunolabelled, as well as vascular endothelial (asterisk) and myoid cells (double asterisk). The spermatozoa (SPZ) present in the lumen were also immunolabelled: the signal occurs in acrosome (arrowhead) and tail (double arrowheads). C−D. Anti-VPAC1 immunolabelling: the signal is evident in myoid cells (double asterisk). The signal is also evident at the level of the acrosome (arrowhead) and of the tail (double arrowhead) of spermatozoa (SPZ). E−FG. VPAC2 immunolabelling is evident in basal (BC), tight (TC), main (MC) and apical (AP) cells, as well as in myoid cells (double asterisk). The signal is evident also in the spermatozoa (SPZ) at the level of the acrosome (arrowhead) and of the tail (double arrowheads). No signal is present in negative control sections (H). The scale bars correspond to 20 μm in H, 10 μm in A, C, E and 5 μm in B, D, F, G.
5 Discussion
VIP is a pleiotropic neuropeptide, acting as a hypophysiotropic and immunomodulator hormone, neuromodulator, vasoregulator and as a factor of reproduction control as well [3,6]. Indeed, the VIP/VPACR system has been localized both in mammalian and in non-mammalian vertebrate testis; in mammals, VIP has been localized only in interstitial cells [13,14]; by contrast, in non-mammalian vertebrates, the presence of the VIP/VPACR system has been reported not only in interstitial cells, but also in germ and Sertoli cells as well [36–38]. No data are available so far about the presence of the VIP/VPACR system in epididymis, whereas previous studies performed in rats highlighted the presence of PACAP, the neuropeptide closely related to VIP, and involved in the control of chlorine ion efflux in epididymis [50]. Now, we demonstrated for the first time that VIP and its receptors are well represented in the epididymis of two vertebrates: P. sicula and R. rattus. Our results showed that VIP and its receptors have a wide distribution in epididymis of both experimental models. A strong immunostaining for VIP and its receptors was found within epithelial and connective tissue as well as in the acrosome and the tail of spermatozoa present in the epididymal lumen. It strongly suggests that VIP in epididymis could be involved in sperm maturation, as well as in fertilization, as recently reported in mice for PACAP [51,52].
The involvement of VIP in the sperm maturation clearly emerges from the observations performed in Podarcis epididymis. Indeed, a strong positivity for VIP was reported in the epithelial cells of epididymis only during the productive period; during the non-reproductive period, the same positivity occurred only in connective tissue. In particular, during the reproductive period, VIP labelling in the epithelial cells occurs within large dense vacuoles, whose content is discharged in the lumen of epididymis where numerous spermatozoa are present. So, one can hypothesize that VIP may contribute to sperm maturation, as VIP occurs both in cytoplasm and in large dense vacuoles that are present both in the columnar cells and in the lumen intermingled with spermatozoa; in particular, VIP in epididymis, as PACAP [52], could stimulate an increase in intracellular cAMP, leading firstly to the activation of apical cAMP-dependent Cl− channels and then to a Cl ion efflux in the epididymal fluid, an event that promotes sperm maturation [53]. Moreover, the presence of VIP and its receptors in spermatozoa, as previously reported in seminiferous tubules [36], strongly suggests that VIP could be involved in fertilization, as reported for PACAP in mammals [51]. On the other hand, the VIP positivity evident in myoid cells could promote the release of cell content in the lumen of epididymis.
Finally, it is interesting to note that in Podarcis and Rattus testis vascular endothelial cells are immunolabelled for VIP. Lacking in situ hybridization, we can only postulate that vascular endothelial cells in the epididymis represent possible sites of synthesis for VIP, as demonstrated for PACAP in mammalian testis [14], without excluding a possible local origin, as previously reported in Podarcis testis [36–38].
In conclusion, the presence of the VIP/VPACR system in the epididymis of P. sicula and R. rattus strongly suggests that VIP interacting with VPAC1 and VPAC2, in a paracrine/autocrine manner, could be involved in the control of reproduction, particularly in sperm maturation and fertilization.
Funding
This work was supported by the Federico II Naples University.
Disclosure of interest
The authors declare that they have no competing interest.


