1 Introduction
As already outlined by several authors [1–5], our knowledge of the scorpion fauna of China has seen a remarkable development since the early 2000s. This improvement concerns not only global numbers, but also the diversity of the described taxa. Although some Chinese Provinces have been extensively prospected, with the descriptions of several new species [4], others remain poorly sampled. The latter is most certainly the case for the Western Chinese Province of Xinjiang. Some contributions to the scorpion fauna of this province are available, but these mainly concern representatives of the family Buthidae C. L. Koch, 1837 [6,7].
In the present contribution, a new species belonging to the genus Scorpiops Peters, 1861 of the family Scorpiopidae Kraepelin, 1905 is described from the Taxkorgan Natural Reserve, located in the southwestern portion of the Xinjiang Province, near the borders with Afghanistan, Pakistan and Tajikistan (Fig. 1). The new species represents the first record of a scorpion from the Taxkorgan Natural Reserve and probably constitutes an endemic element of this region. The scorpions were found buried under stones at an altitude of 4500–4600 m, placing the new species among those living at high elevations (see next section).
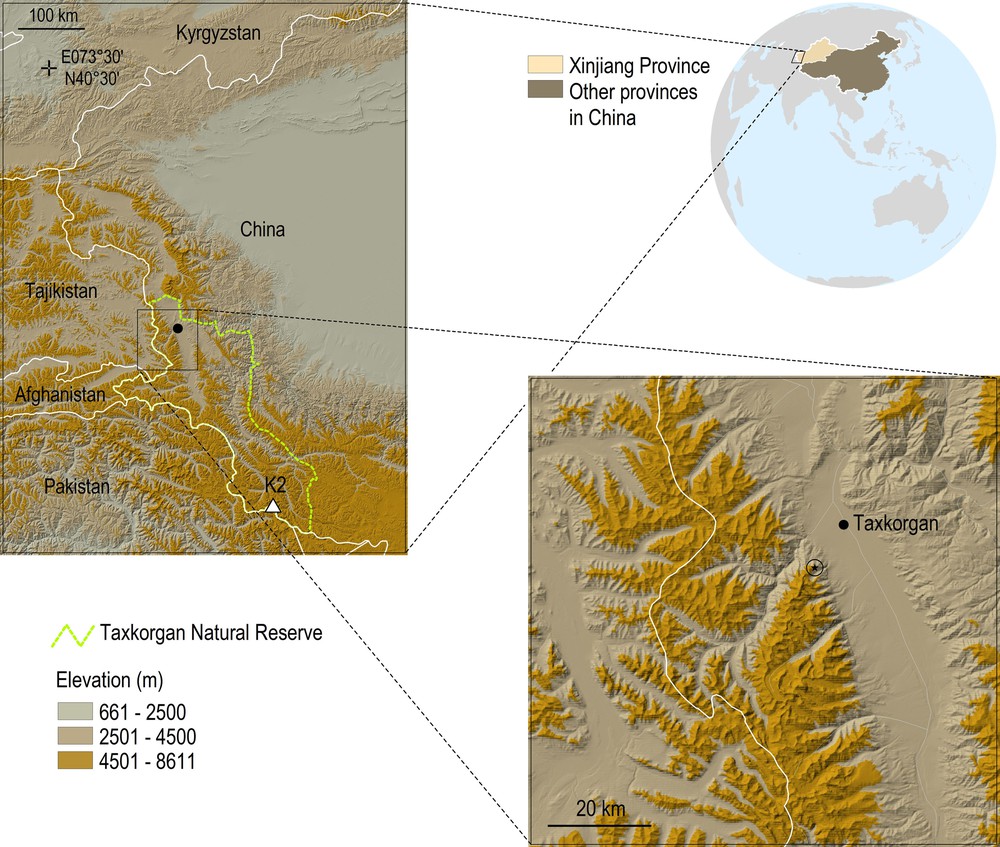
Physical map of Xinjiang Autonomous Province in China, showing the Taxkorgan Natural Reserve. Circle with black star indicates the approximate area SW of Taxkorgan where the new species was collected.
Difficulties concerning the systematics of the family Scorpiopidae and, in particular, the taxonomic positions of the genera Scorpiops and Euscorpiops Vachon, 1980 were discussed by Lourenço & Pham (and references therein) [8].
2 Scorpions at high altitudes
As noted in previous papers [9,10], although scorpions are undoubtedly most diverse in deserts, arid formations and rain forests [11,12], they also occur in most other terrestrial habitats, with the exception of tundra, high-latitude taiga and very high mountain tops [11]. Some species can be found in mountain and alpine habitats, but those living in montane sites outside tropical or subtropical areas, where climatic conditions are severe, are rare. They generally belong to a limited number of scorpion groups, families and genera. Species of the genus Euscorpius Thorell, 1876 (family Euscorpiidae) occur up to 2000 m in the Alps [11,13], and those of the genera Diplocentrus Peters, 1861 (Diplocentridae) and Vaejovis C. L. Koch, 1836 (Vaejovidae) are found up to 3000 m in North America [11,14,15]. In the Himalayas of China, India, Pakistan and Nepal, scorpions of the families Buthidae, Chaerilidae and Scorpiopidae have been found up to 4000 or 4500 m. These include species of the genera Hottentotta Birula, 1908, Chaerilus Simon, 1877 and Scorpiops Peters, 1861 [1,11,16–19].
According to Polis [11], high-altitude species are all small and feed on a diverse array of arthropods found at these heights [19]. Their small size is possibly the consequence of the short periods during which they are able to forage. According to Crawford and Riddle [20], ‘cold hardiness’ allows at least some species to survive freezing temperatures. One spectacular mechanism is the ability to ‘supercool’, a process whereby the animal's circulatory fluid can be lowered well below the freezing point without crystallization and hence without damage to the body tissues [21]. The physiological or biochemical basis for the ability of some scorpion species to ‘supercool’ is not clearly known. Increased levels of cryoprotectants, such as glycerol and sorbitol, which occur in the haemolymph of insects living in very cold environments, have not been observed for scorpions [22]. As noted by Polis [11], surprisingly, high-altitude scorpions live under rocks, in scrapes, and in relatively short burrows, rather than in deep burrows with terminal chambers below the frost line [19,20]. These characteristics seem to be in accordance with those observed for the new species described here.
3 Ecology of the Taxkorgan Reserve
As described by Schaller et al. and Schaller & Kang [23,24], the Taxkorgan Natural Reserve was established in 1984 and comprises approximately 14,000 km2 of terrain in the southwest corner of China's Xinjiang Uygur Autonomous Region, where the borders of China, Afghanistan, Pakistan, and Tajikistan meet (Fig. 1). The reserve is mountainous and about 50% of it is above 4500 m in elevation, including the northern flanks of the Karakoram, in the western edge of the Kunlun, and the eastern rim of the Pamir mountains. The southern boundary follows the Pakistan border eastward until just past K2 (Chogali), which, at 8611 m, is the world's second highest peak. For part of this border the reserve is contiguous with Pakistan's Khunjerab National Park, 2200 km2 in area, which was established in 1975 [25]. The southeast portion of the reserve is so remote that few foreign expeditions have visited it [26,27], whereas the West has been an international travel route for centuries. Flat and more than 5 km wide in places, the Taxkorgan Valley (Taghdumbash Pamir on old maps) was part of the ancient Silk Road that continued into the Chalachigu Valley and over the Mintaka Pass into Pakistan. Since the late 1960s, a highway has connected China and Pakistan via the Khunjerab Pass, a route opened to a large public in 1986. The northern and northeastern reserve boundary traces various tributaries of the Yarkand (Yeeheng) River.
The westernmost 2600 km2 of the reserve represents mainly pamirs – (broad valleys) and steep hills above an elevation of 3500 m, flanked by rugged ranges. Between the eastern rim of the Taxkorgan Valley and the Yarkant River is a chaotic jumble of mountains, broken cliffs and sharp ridges cut by desolate gorges down which torrents rush. Near the junction with the Yarkand River, the Raskam, Mariang and other rivers lie below 3000 m in elevation, the lowest part of the reserve bank of the Yarkand being the Taxkuzuke Mountains, a discrete, rough range covering about 3200 km2. The southeastern section of the reserve, about 5200 km2 in extent, lies mostly above 4500 m and includes the Karakoram with its extensive glaciers, the Aghil Range, and the Oprang (Shaksgam) Valley, a region well described by Shipton [27]. The climate is cool and dry. The average monthly minimum at Taxkorgan town (3090 m) observed in 1984 was–16 °C to–17 °C during the coldest months of December and January, and the average daily maximum reached 22–23 °C during the warmest months, from June to August [23]. About 75.4 mm of precipitation was observed in 1984, 81% of it between May and September [23]. Most terrain is too high or too arid to support much vegetation. Below an elevation of about 3000 to 3200 m are usually cliffs, scree, sand and silt, a desert so dry that few plants thrive, except along streams. The only native trees in the reserve, willow (Salix) and tamarisk, are found in low-lying valleys. Tamarix occur below 3400 m, and cottonwood (Populus) and birch (Betula) below 3300 m. A few trees grow as tall as 10 m. At 4400 m, near the upper limit of the vegetation, plants grow mainly along seepages and rivulets, and at 4500 m, soil has usually given way to rock, although hardy Rhodiola, Saussurea, Tanacetum and Saxifraga may be found as high as 4600–4700 m, above which most wildlife cannot find sustenance [23]. Two main habitats can be observed between 3000 and 4500 m:
- • long streams, rivulets, moist depressions and other sites with sufficient water grow sedge meadows, dominated by Carex and Kohresia and others, and also grasses and forbs (Primula, Pnientilla, Pedicularis, Polygonum, Leontopodhun);
- • flats and slopes with alpine steppe vegetation, the ground bare except for scattered low shrubs (Artemisia, Acantholimon, Caragana, Astragalus), grass tufts, and forbs such as Oxytropis.
The vegetation has been greatly modified by human and livestock use [23,24].
4 Methods
Illustrations and measurements were made using a Wild M5 stereo-microscope with a drawing tube and an ocular micrometer. Measurements follow Stahnke [28] and are given in mm. Trichobothrial notations follow Vachon [29] and morphological terminology mostly follows Vachon [30] and Hjelle [31].
5 Taxonomic treatment
Family Scorpiopidae Kraepelin, 1905
Genus Scorpiops Peters, 1861
Scorpiops taxkorgan sp. n. (Figs. 2–6)

Habitus of Scorpiops taxkorgan sp. n., male holotype. A–B. Dorsal and ventral aspects.

Habitus of juveniles. A. Scorpiops taxkorgan sp. n., paratype female. B. Scorpiops lindbergi Vachon, 1980, female, showing distinct patterns of pigmentation.
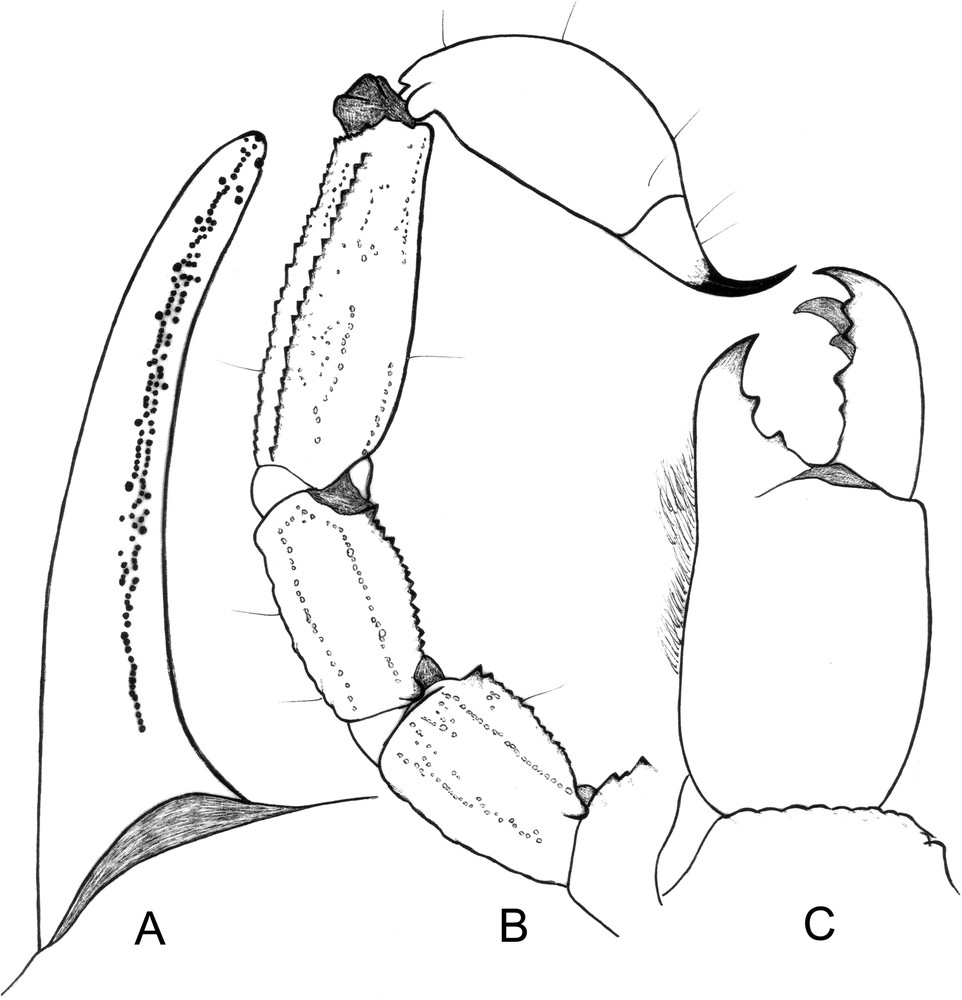
Scorpiops taxkorgan sp. n., male holotype. A. Cutting edge of chelal movable finger. B. Metasomal segments III–V and telson, lateral aspect. C. Chelicera, dorsal aspect.

Scorpiops taxkorgan sp. n., male holotype, trichobothrial pattern of chela. A–D. Dorso-external (A), external (B), internal (C) and ventral (D) aspects.
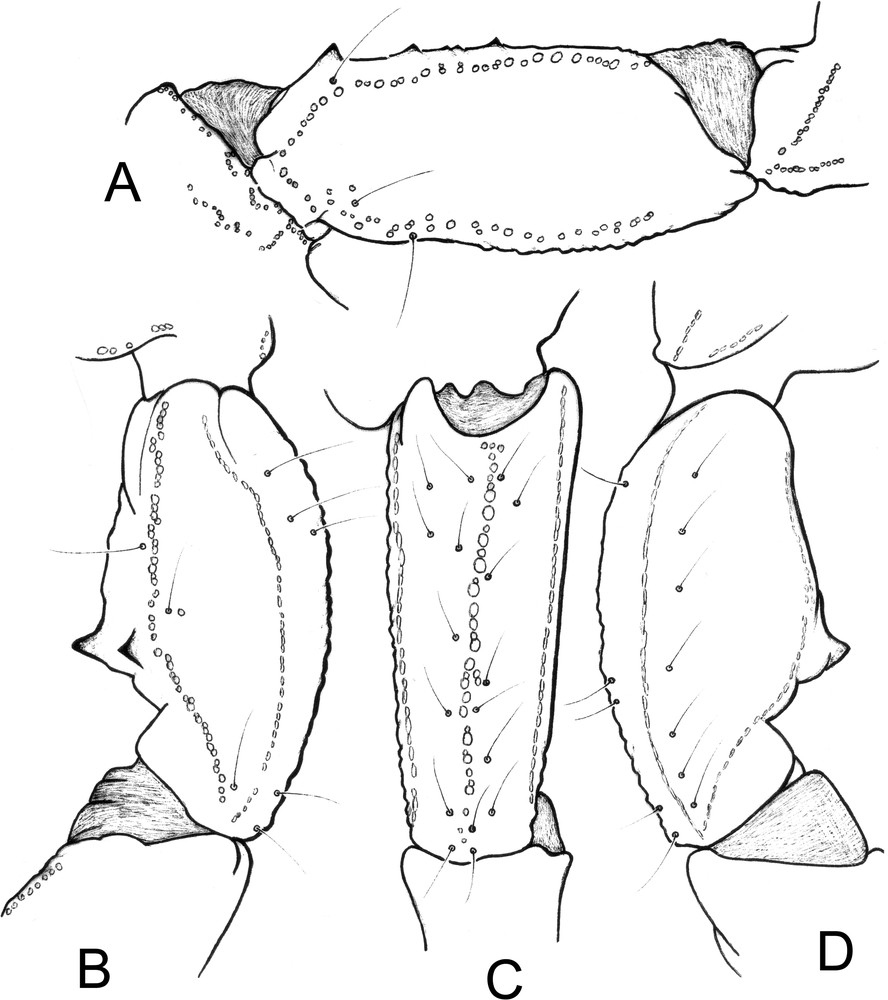
Scorpiops taxkorgan sp. n., male holotype, trichobothrial pattern. A. Femur, dorsal aspect. B–D. Patella, dorsal (B), external (C) and ventral (D) aspects.
Diagnosis: The new species presents most of the characteristics already defined for those of the genus Scorpiops [32,33]. It can, however, be separated from the other species by the following features. Medium to small size; adult male 35.2 mm in total length. General pattern of coloration yellow to reddish/yellow with infuscations in adult male; female juvenile reddish/brown to dark brown. This distinct coloration could be a neotenic character. Three lateral eyes; third pair reduced. Pectines small, with 7–8 teeth in the male and 6–6 teeth in the female. Chela fingers with two series of granules almost fused; accessory granules inconspicuous. Trichobothrium Dt of chela situated in a distal position relative to trichobothrium Eb3. Patella with 17 external and 7 ventral trichobothria in both sexes. Telson with annular ring moderately marked.
Relationships: the new species shows affinities with Scorpiops lindbergi Vachon, 1980, described from the region of Kabul in Afghanistan [33]. These species can, however be distinguished by a number of characters:
- • overall size and distinct morphometric values, the new species being smaller;
- • distinct pattern of coloration, the new species being generally paler in adults, whereas juveniles appear to be darker. Vachon [33] referred to juveniles of S. lindbergi, but did not include these in the type series; a re-examination of these juveniles shows that they have a yellow coloration, contrasting with the juvenile of the new species);
- • pectines smaller, with a lower number of teeth;
- • internal apophysis of pedipalp patella less marked in the new species;
- • pedipalp patella with 17 external and 7 ventral trichobothria in both sexes, versus 19 and 12–13 in S. lindbergi [33,34].
Moreover, the two species occur in quite distinct habitats at different altitudes, 1800 m for S. lindbergi versus 4500–4600 m for the new species.
Material. China, Xinjiang Autonomous Province, Taxkorgan Natural Reserve, SW of Taxkorgan, 4500–4600 m, under stones, July 1986 (collected by local people for C. M. Naumann). Male holotype and juvenile female paratype deposited in the “Muséum national d’histoire naturelle”, Paris, France.
Etymology: the specific is placed in apposition to the generic name and refers to the Taxkorgan Natural Reserve, where the new species was found.
Description: Total body length 35.2 mm for the male. Body and pedipalps moderately slender. Coloration yellow to reddish/yellow with infuscations in the adult male; reddish/brown to brown in the female juvenile. In the adult male, carapace reddish-yellow to reddish/brown with paler zones on the posterior and lateral edges. Tergites yellow to reddish/yellow, paler than the carapace. Metasomal segments reddish/yellow with infuscations; telson brownish/yellow; base of aculeus yellow and tip reddish. Chelicerae yellow, with diffuse, variegated spots at the base of the fingers; fingers yellow with dark, variegated spots. Pedipalps reddish/brown; chela manus darker at the base of the fingers. Legs pale yellow. Venter yellow, with a few infuscations on sternite VII.
Morphology. Carapace weakly granular with punctations; furrows moderately to weakly deep. Anterior margin with a strong concavity. Median eyes small and anterior to the middle of the carapace; three pairs of lateral eyes, third pair reduced. Sternum pentagonal, slightly wider than long. Tergites weakly granular; VII with five carinae, moderately to weakly marked. Pectines narrow; pectinal tooth count 7–8 in the male and 6–6 in the female; fulcra vestigial. Sternites smooth and punctate; spiracles oval; sternite VII with four weakly marked carinae and vestigial granulation. Metasomal segment I wider than long; segments II to V longer than wide; 10–8–8–8–7 carinae present on segments I–V; segment II without intermediate carina; dorsal carinae on segments II–IV with one small posterior spinoid granule; metasomal tegument moderately granular; ventral carina on segment V with strongly marked spinoid granules. Telson vesicle smooth, without granulation. Setation weak on the metasomal segments and the telson. Pedipalps: femur with dorsal internal, dorsal external, ventral internal and ventral external carinae moderately marked; tegument moderately granular. Patella with dorsal internal, dorsal external, ventral internal, ventral external and external carinae moderately to strongly marked; two moderately marked spinoid granules of distinct size present on the internal aspect; tegument weakly granular to smooth. Chela with dorsal marginal, external secondary, ventral internal and ventral carinae moderately to strongly marked; other carinae moderately marked; tegument moderately granulated dorsally and ventrally, and strongly granulated internally. Chelal fingers with two longitudinal series of granules almost fused; inner and outer accessory granules present, but inconspicuous. Cheliceral dentition, as defined by Vachon for the Scorpiopidae [33,35]: movable finger with reduced teeth and 4–5 teeth on the ventro-internal face. Trichobothriotaxy of type C [29,33], as shown in Figs. 5–6. Trichobothrial pattern with three trichobothria on the femur: dorsal, internal and external. Patella with 2 dorsal, 1 internal, 7 ventral and 17 external trichobothria. Chelal manus with 4 ventral, 2 dorsal (Dt, Db), 2 internal (ib, it), 1 Est, 5 Et, 1 Esb and 3 trichobothria in Eb series. Trichobothrium Dt of chela situated in a distal position relative to Eb3 [29,33].
Morphometric values (in mm) of the male holotype. Total length (including telson) 35.2. Carapace: length 5.4; anterior width 3.5; posterior width 5.2. Mesosoma length 12.7. Metasomal segment I: length 1.8, width 2.0; II: length 2.0, width 1.7; III: length 2.2, width 1.6; IV: length 2.4, width 1.5; V: length 4.1, width 1.4, depth 1.2. Telson length 4.6. Vesicle: width 1.4, depth 1.5. Pedipalp: femur length 5.1, width 2.1; patella length 4.6, width 2.2; chela length 9.5, width 3.0, depth 2.2; movable finger length 4.6.
Disclosure of interest
The author declares that he has no competing interest.
Acknowledgements
I am most grateful to Lucienne Wilmé (Missouri Botanical Garden) for preparing Fig. 1 (map) and to Élise-Anne Leguin (MNHN, Paris) for her help with the preparation of the photographs and the plates. My thanks also go to Mark Judson (MNHN, Paris) for revising the English text.


