1 Introduction
The poison gland of many myrmicine ants, including those of the genus Aphaenogaster, contain alkaloids [1,2]. This is the case for Aphaenogaster senilis (i.e. poison gland containing anabasine, anabaseine, and pyridines), but not for A. iberica [3]. As these two species are phylogenetically closely related, this difference is very surprising, but it is probably linked to the differences in their habitat, which explains why A. senilis uses alkaloids in trail pheromone [3].
Because the importance of microbiota was highlighted in the biology of organisms, including insects and among them ants [4,5], we hypothesized that alkaloid production in A. senilis may be due to a bacterial activity, something supposedly absent in A. iberica. We thus treated the workers of the two species with Rifampicin, an antibiotic frequently used in insects, including ants [6,7]. To test this hypothesis, we measured the quantities of substances in poison and Dufour glands of the two above-cited Aphaenogaster species as well as the effect of the treatment on cuticular hydrocarbons to investigate a more general effect on the ants.
2 Material and methods
We collected in Spain four colonies of A. senilis in Doñana National Park (Las Beles, 36°58′53″N, 6°29′11″W), and two colonies of A. iberica in the Sierra Nevada (37°08′67″N, 3°28′33″W, 1370 m). All colonies had a queen and abundant brood. They were maintained in artificial nests composed of a plastic box containing two to three tubes half-filled with water. Each nest was connected through a plastic tube to a large foraging arena, where food (mealworms and honey) was provided ad libitum. The room temperature was maintained at 25 °C.
For the experiments, we prepared small plastic boxes (i.e. foraging arena) with a tube provided with a watering place (i.e. the nest) and placed inside 100 workers with some brood. The ants were fed three times a week during one month with honey, whereas mealworms or maggots and pieces of orange were offered ad libitum. We made control groups (C) and groups treated with the antibiotic Rifampin (R) as a 50 μg/ml solution were mixed with honey (4 × 2 groups for A. senilis and 2 × 2 groups for A. iberica).
The ants were treated for one month, which is efficient to kill bacteria in Camponotus [7]. The workers were frozen at −20°. Cuticular hydrocarbons were extracted from the thoraxes of workers placed in vials with 0.5 ml of pentane during 1 h, and then the thoraxes were removed whereas the solvent was evaporated. The residue was re-dissolved in 50 μL of pentane, of which 2 μL were injected into a PerkinElmer GC/MS device using a DB-5 fused silica capillary column; temperature programmed from 150 °C (5 min hold) to 300 °C at 10 °C/min, and held at 300 °C for the last 10 min.
We also dissected poison glands and Dufour glands of each species and maintained them during 1 hour in 50 μL pentane, of which 2 μL were injected into the PerkinElmer GC/MS device (for the number of glands dissected, see Table 1). Separations were achieved using the same DB-5 fused silica capillary column; temperature programmed from 50 °C (2 min hold) to 300 °C at 6 °C/min, and held at 300 °C for the last 10 min [3]. In all extracts, a C20 was used as an internal standard to quantify the quantities of alkaloids and hydrocarbons .
Poison glands quantities (ng/gland) for total content and alkaloids, Dufour glands total substances in the two species A. senilis and A. iberica for controls and treated with Rifampicin.
| Control | Rifampicin | p | |||||||
| Median | Lower quartile | Upper quartile | n | Median | LQ | UQ | n | ||
| Aphaenogasrer senitis | |||||||||
| Poison gland total substances | 383.8 | 77.9 | 1264.0 | 18 | 90.4 | 55.0 | 391.0 | 20 | 0.11 |
| PG alkaloids only | 289.0 | 34.6 | 1089.8 | 17.3 | 4.6 | 217.9 | 0.008 | ||
| Dufour gland total substances | 842.5 | 394.0 | 1441.1 | 17 | 1827.7 | 102.5 | 3224.8 | 15 | 0.500 |
| DG alkaloids only | 0 | 0 | |||||||
| Aphaenogasrer iberica | |||||||||
| Poison gland total substances | 41.6 | 6.8 | 77.3 | 10 | 78.9 | 45.7 | 127.0 | 12 | 0.090 |
| Dufour gland total substances | 23.2 | 5.0 | 33.9 | 38.5 | 24.0 | 65.1 | 0.140 | ||
| U-test A. senilis/A. iberica | |||||||||
| Poison gland total substances | 0.001 | ||||||||
| Dufour gland total substances | < 0.001 |
We used Mann–Whitney's U test to compare the sample quantities and a cluster analysis (Ward method, Euclidean distances) to compare the hydrocarbon profiles (Statistica8 Software).
3 Results
3.1 Cuticule
Because there was very little worker size variation between both the colonies and species, we measured the hydrocarbon quantities per worker rather than per mg of fresh ant (Fig. 1).
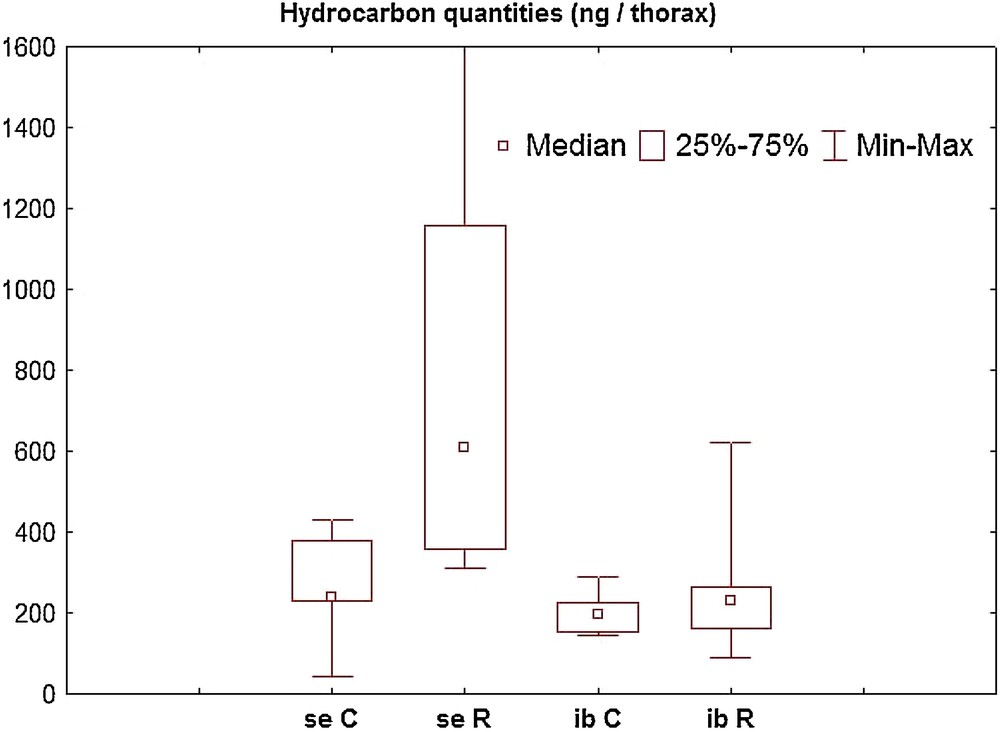
Hydrocarbon quantities (ng/thorax) in Aphaenogaster ants. se: Aphaenogaster senilis, ib: Aphaenogaster iberica, C: control, R: Rifampicin treatment. se R is significantly different from the other groups.
Concerning A. senilis, the quantity of hydrocarbons per thorax of control workers was significantly lower than that of workers treated with the antibiotic (n = 10 in both cases; median, lower and upper quartiles; 237 ng; 231.6 ng, 376.9 ng versus 610.7 ng; 356.9 ng; 1159.9 ng; U = 9; p = 0.001). Comparatively, we did not record significant differences for A. iberica workers (n = 5 in both cases; 196 ng; 152 ng; 225 ng versus 230 ng; 160 ng; 263 ng for the controls and the treated ants, respectively; U = 10; p = 0.69). Furthermore, no difference appeared between the controls of the two species (U = 13; p = 0.16).
A cluster analysis of the hydrocarbon profiles did not indicate a difference between control and treated ants in both species (figures not presented). It also showed that the antibiotic treatment increases the production of hydrocarbons in A. senilis without changing the profile, and had no effect on A. iberica.
3.2 Glands
The A. senilis poison glands contain alkanes (mean: 3%), alkenes (6.6%), aldehydes (2.6%), and a great quantity of alkaloids (87.5%) as previously presented in [3], whereas the Dufour glands, devoid of alkaloid (0%), contain alkanes (51%), alkenes (47%), and 1% of aldehydes (Table 1).
The A. iberica poison glands contain alkaloids (6.1%), alkanes (24.5%), alkenes (62.1%), and small quantities of aldehydes (3%), the composition of the Dufour gland being quite similar, containing alkaloids (1.8%), alkanes (38.4%), alkenes (54.6%), and aldehydes (3.1%).
The quantities of substances in the glands are given in Table 1. The poison glands of A. senilis are significantly bigger than those of A. iberica and contain more substances (n = 18 and n = 10 respectively; median, lower and upper quartiles; 383.8 ng versus 41.6 ng; for A. senilis and A. iberica, respectively; U = 25; p = 0.001). The same is true for the Dufour glands (n = 17 and n = 10, respectively; 842.5 ng versus 23.2 ng for A. senilis and A. iberica, respectively; U = 0; p < 0.001).
The total content of the A. senilis poison gland did not change after the antibiotic treatment (n = 18 and n = 20, respectively; 383.8 ng versus 90.4 ng, U = 125; p = 0.11; Table 1). If we consider the classes of substances, there is no difference for alkanes (U = 163; p = 0.63), alkenes (U = 168; p = 0.74), and aldehydes (U = 177; p = 0.94), but the alkaloid quantities were significantly higher in the control compared to workers treated with the antibiotic (289 ng versus 17.3 ng for controls and treated ants, respectively; U = 90; p = 0.008; Fig. 2), representing a decrease of 94%.
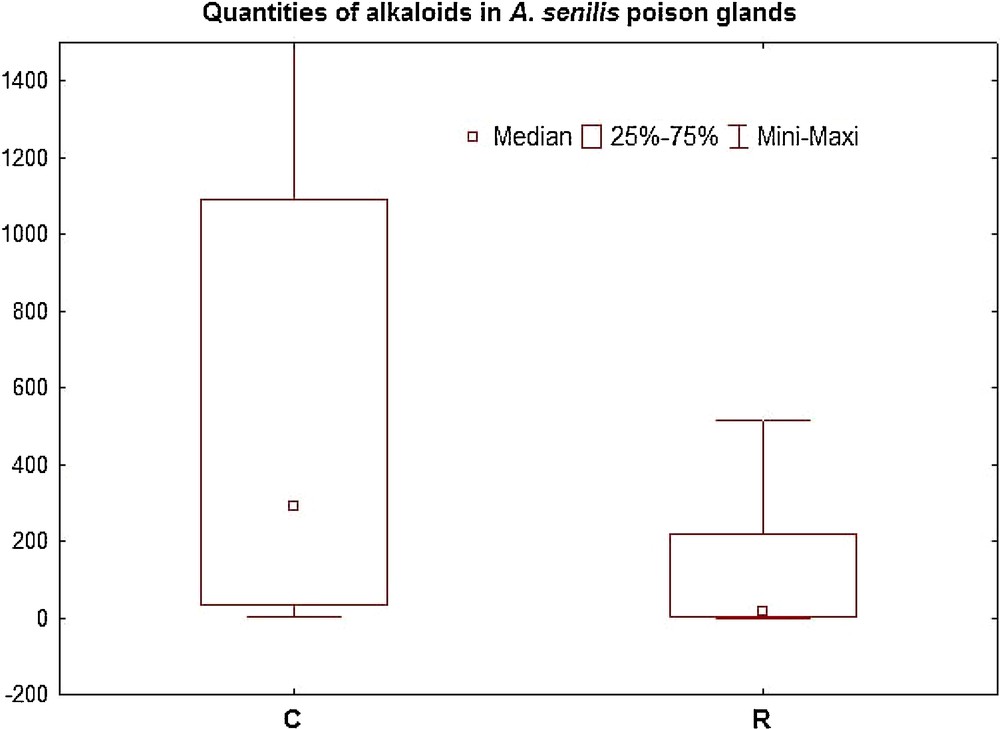
Alkaloid quantities (ng/gland) in poison glands of Aphaenogaster senilis. C: control, R: Rifampicin treatment. The difference is highly significant (p= 0.008).
Still concerning A. senilis, the total content of the Dufour gland increased after the Rifampicin treatment (842.5 ng versus 1827.7 ng for the control and the treated ants, respectively; U = 109; p = 0.50; Table 1).
The content of the A. iberica glands did not change with the treatment (Table 1; poison gland: 41.6 ng versus 78.9 ng; U = 34; p = 0.09; Dufour glands: 23.2 ng versus 24.0 ng; U = 37; p = 0.14).
4 Discussion
We observed a general effect of the antibiotic in A. senilis with an increase in the cuticular hydrocarbon quantities likely corresponding to a reaction of the ants to the stress induced by the decrease in bacteria quantities. This is reminiscent of the case of Camponotus ants that have mutualistic endosymbiotic bacteria with alimentary and immune functions [7]. Here too, the antibiotic treatment results in higher production of cuticular hydrocarbons compared to controls, plus a melanization of the cuticle [8]. In Camponotus spp. as well as A. senilis, the cuticular hydrocarbon profiles are not modified by the antibiotic treatment, but by a larger quantity of hydrocarbons produced, likely in response to a decrease in the number of symbiotic bacteria.
We also observed a spectacular effect of the antibiotic on the production of alkaloids by the poison gland of A. senilis with a decrease of 94%. This can be due to a direct production by bacteria which may be endosymbiotic in the membrane of the poison gland or by other bacteria in the ant body acting on the synthesis pathway of the alkaloids. In their review on toxins, Touchard et al. [9] said that “It is also possible that a microbial symbiont may have been involved in the production of intermediary compounds, as was suggested for some alkaloids found in sponges and briefly hinted at in ants by Saporito et al. [10].” Also, the production of pyrazines depending on bacteria was noted in Attine ants [11], and it was suggested by different researches in ant-fungus species [12,13]. Nevertheless, the production of alkaloids by free bacteria was noted only in a few marine species [14].
Aphaenogaster iberica differs from A. senilis in its geographical distribution and its mode of recruitment of nestmates [15]. We saw here that these two ant species also differ in their cuticular hydrocarbons and glandular contents, A. iberica lacking alkaloids, or they are present only as traces [3]. The fact that A. iberica did not react to the antibiotic treatment is likely due to the absence of symbiotic bacteria in this species. More research is necessary to know in detail the microbiome of these ant species.
Acknowledgments
This work was supported by the PRES Centre Val-de-Loire Université (APR–IA 2012). We are grateful to Andrea Yockey-Dejean for proofreading the manuscript.


