1 Introduction
This is the intriguing tale of how the previously recognised phyla Pogonophora and Vestimentifera became reduced to a family level taxon, Siboglinidae, within Annelida. The common name for the group as a whole is pogonophores (beard worms) and the two major subgroups will be referred to here as frenulates and vestimentiferans.
For those unfamiliar with the group previously referred to as frenulates (= Frenulata), they are long and extremely thin tube-living animals. The tubes range from 0.1–3 mm in diameter and can reach over a meter in length. With a few exceptions they are marine deep-water species, occurring down to near 10 000 m depth. Anteriorly the animals are provided with one or more palps that stick out from the tube.
Vestimentiferans (= Vestimentifera), or “giant tube worms” also generally live in the deep-sea, but are associated with hydrothermal vents or cold seeps. Generally, they are much stouter than frenulates, they have a tube that is attached with one end to the substratum, and they have a much larger crown of palps. They are also provided with a vestimentum which is an anterior body region provided with a pair of large flaps.
Neither frenulates nor vestimentiferans have a mouth or gut as adults and rely on symbiotic, chemoautotrophic bacteria that are situated in the trunk (Fig. 1). The blood of both groups contains hemoglobin and the palps serve for the uptake of oxygen and sulfide, thiosulfate or methane (depending on the taxa). These are transported to the bacteria and in return the host obtains nutrition from the bacteria or it digests them [1]. The more recently discovered Osedax and the unusual Sclerolinum are discussed below.
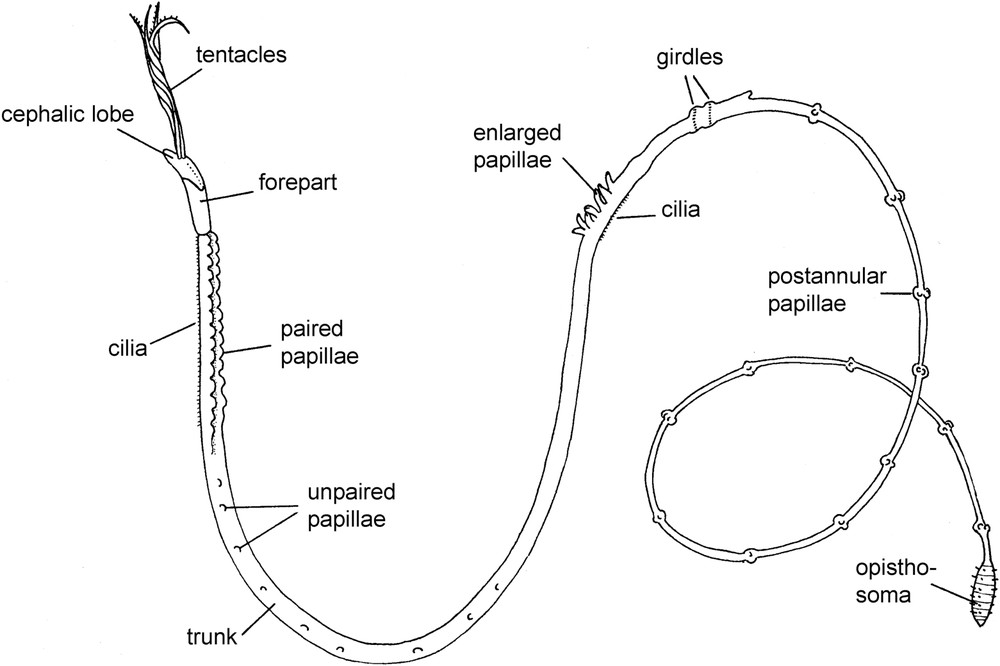
A generalized frenulate pogonophore, very much shortened. Modified from George and Southward [59].
2 The early history
We will start this story in 1914 when the French biologist Maurice Caullery described a long slender tube-living worm (Fig. 1) that had been collected in Indonesia during the Dutch Siboga expedition around 1900.
He named it Siboglinum and placed it in the likewise new family Siboglinidae [2]. A species name was not given at that time and it was not until 1944 that he named it S. weberi [3]. One unusual feature for a non-parasitic animal was the complete lack of a digestive tract. Caullery did not refer Siboglinidae to any higher ranked taxon, but compared it to deuterostomes such as pterobranchs and enteropneusts.
Twenty years after Caullery's first paper the Russian polychaete taxonomist Uschakov [4] described another gutless animal, Lamellisabella zachsi from the Sea of Okhotsk and placed it in a new subfamily Lamellisabellinae within sabellid polychaetes (feather-duster worms). Uschakov made no references to Caullery's earlier publication. Johansson [5,6], a Swedish specialist on sabellid polychaetes, disagreed with Uschakov regarding the polychaete affinity of Lamellisabella. Based on anatomical studies he concluded that Uschakov had misinterpreted the dorsal side for the ventral and referred the animal to a new class that he named Pogonophora. In 1944 Beklemishev in a text-book [7] then raised Pogonophora to the rank of phylum among Deuterostomia, listed next to Hemichordata.
A few years later Ivanov [8] described a second species of Lamellisabella. Still no connection had been made at this time between Caullery's Siboglinum and Uschakov's Lamellisabella, and the disagreements regarding the relationships of Pogonophora were based solely on latter taxon. However, in 1951 Ivanov [9] compared Lamellisabella with Siboglinum and concluded that the two taxa were close relatives and referred also the latter genus to Pogonophora. In the coming decade a large number of pogonophores were described by Ivanov and in 1963 he published the extensive monograph “Pogonophora” [10]. By then the group had come to encompass 70 species in 14 genera (all of which, except for Sclerolinum, we now view as frenulates), and were by most authors treated as a group of deuterostomes with a dorsal nerve cord, radial cleavage and a tripartite coelom. Although most contemporary authors agreed with Johansson's and Ivanov's treatments, there were some early opponents. Hartman in the early 1950s [11,12] argued that they were polychaetes, although not necessarily monophyletic, and Livanov and Porfireva [13,14] that they were closely related to the polychaete Owenia fusiformis. Ivanov's [15], together with Jägersten's [16], rejection of Hartman's anatomical arguments (now largely considered correct; her conclusion regarding frenulate polyphyly notwithstanding) make interesting reading.
In 1964 Webb published a series of studies [17–20] with the introduction of new pogonophore taxa from Norway, including the description of the larval development and, notably, the posterior end of the animals. Whereas previous studies had described the posterior part as ending in a blunt point, Webb showed that there actually was an additional and hitherto unknown part which was annulled and provided with segmentally arranged chaetae (Fig. 2); a body region now referred to as the opisthosoma. Since the animals are fragile and this part serves as an anchoring device, it is easily detached and remains in the tube when the animals are removed, which explains why all previous authors had overlooked it.
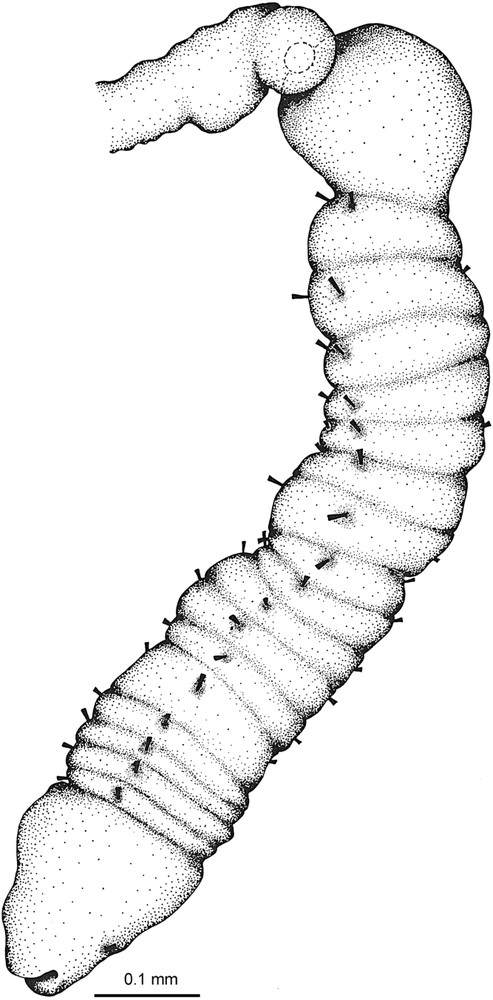
The missing piece. Lateral view of the posterior end of Siboglinum fiordicum. Modified from Webb [20].
During the years that followed a number of authors started to question the deuterostome affinity of pogonophores and Ivanov's interpretations, and here the embryology, origin of the coelom, position of the nerve cord and the structure of the chaetae had a central place. Following Ivanov, the worms were said to have a dorsal brain and nerve cord, which is the classic deuterostome arrangement, whereas in protostomes (such as annelids) the brain is dorsal and joins the nerve cord by circumpharyngeal connectives which, as the name implies, form a ring around the pharynx. In the study of sections the position of the brain and the nerve cord is typically assessed with the pharynx and gut as reference, and this, of course, is complicated in pogonophores by the fact that the animals lack gut. Based on embryological studies and the development of the early larvae, Nørrevang [21,22] suggested a ventral nerve cord and that Uschakov's original dorso-ventral orientation of the animals was incorrect. Nørrevang further showed that the animals are segmented with new segments added from the posterior end, and that the coelomic sacs arise by schizocoely, not enterocoely. He also suggested that Ivanov's interpretations of the larval development in fact had them back to front, all of which Ivanov strongly rejected [23]. Later embryological studies by, e.g., Callsen-Cencic and Flügel [24] have corroborated Nørrevang's observations. More details on the other anatomical arguments are reviewed by Rouse and Fauchald [25] and Rouse [26].
Following this the consensus gradually shifted such that pogonophores changed from being viewed as deuterostomes with a dorsal nerve cord with a tripartite coelom, to protostomes with a ventral nerve cord and a segmented and chaetigerous posterior end. Not all authors, however, agreed on this interpretation, including Ivanov [23,27] and Malakhov et al. [28].
3 The vestimentiferans
In 1969 Webb [29] described an animal from deep water off southern California that shared a number of features with previously described pogonophores, but also differed in many respects. The tube was exceptionally thick with a diameter approaching 1 cm, the animal had a complex crown and an operculum to close the tube and the anterior part was provided with two prominent lateral flaps. Webb named the new animal Lamellibrachia barhami and referred it to the new taxon Vestimentifera, nested within Pogonophora.
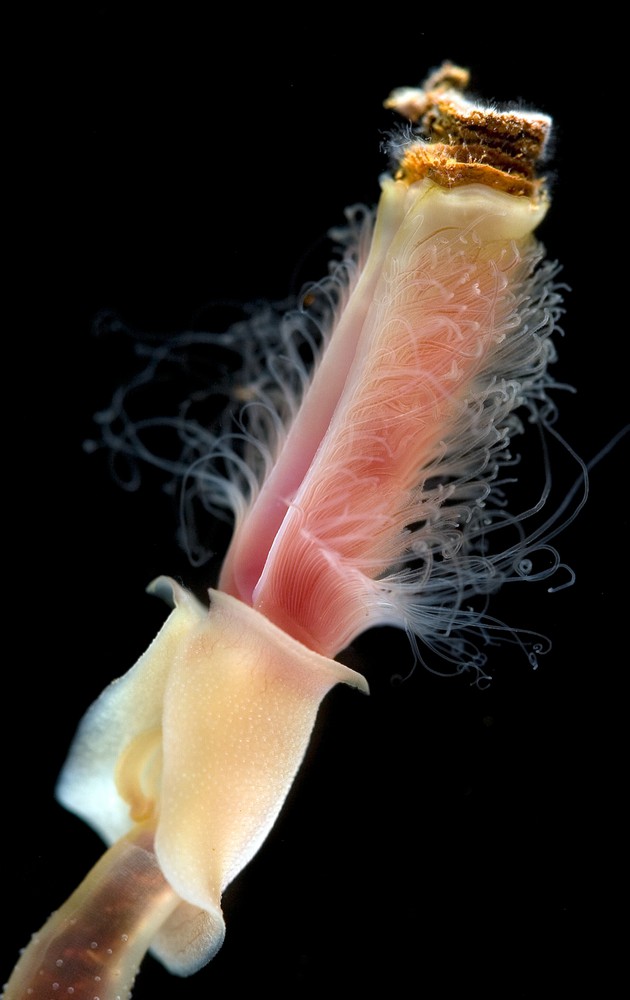
Following the amazing discovery and exploration of hydrothermal vents in the Galapagos Rift with submersibles in 1977, a number of vestimentiferans or “giant tube worms” were discovered and photographed, forming veritable gardens surrounding the smokers where Riftia pachyptila (Fig. 3), the first described species [30], can reach the impressive length of more than 1.5 m. A number of additional species were subsequently described by Jones [30,31]. Jones argued that frenulates and vestimentiferans belonged to different groups, and that vestimentiferans were closer to, but not part of, annelids. Accordingly, he raised Vestimentifera to the rank of phylum. He also provided a full classification of Vestimentifera [31] and managed to apply all basic supraspecific Linnean ranks, i.e. phylum, class, order, family and genus, for the classification of the nine known vestimentiferan species.

Specimens of the vestimentiferan Riftia pachyptila in situ at the East Pacific Ridge, 13°N. Photo copyright Ifremer.
Some time later, Southward [32] and Jones and Gardiner [33], in the same year, described the early development of vestimentiferans, showing that early larvae have both a mouth and gut. This allowed for an unequivocal designation of the dorso-ventral orientation of the animal and showed that the nerve cord is ventral. Further vestimentiferans have been described and today the group includes fifteen species in nine genera [34,35]. It appears to be confined to hydrothermal vents and cold seep areas (the first described one, Lamellibrachia barhami, is now believed to have been collected in a cold seep area).
4 Towards more explicit phylogenies
In 1993 Kojima et al. [36] published the first molecular phylogeny including a vestimentiferan (Lamellibrachia), in these early days necessarily with only a small selection of taxa and a single gene (elongation factor-1•), but nevertheless indicating that the vestimentiferans indeed were nested among the annelids.
In 1995 Bartolomaeus [37] suggested that the chaetal structure of both frenulates and vestimentiferans were so similar to those of polychaetes such as terebellids and sabellids that they must be closely related and so suggested bringing pogonophores inside Annelida. In the same year Rouse and Fauchald [25] carried out a morphology-based phylogenetic analysis in order to assess the position and monophyly of annelids. The results indicated that pogonophorans and vestimentiferans were a clade supported by eight morphological synapomorphies and nested within the annelids. Rouse and Fauchald also suggested that the two groups should possibly be united as a single, family level taxon within polychaetes (suggesting erroneously the family group name should be Lamellisabellidae, but later [38] correcting this suggestion to Siboglinidae). In a subsequent study [38], also morphology-based and using polychaete families as terminals, they demonstrated that frenulates and vestimentiferans are nested in a group including sabellids (in agreement with Uschakov's original allocation of Lamellisabella as mentioned above and to that of Bartolomaeus [37] and oweniids). For this taxon they applied Caullery's original family name Siboglinidae. This measure in one stroke created junior synonyms of the 12 previously used family names among the frenulates and vestimentiferans, which likely approaches a taxonomic record.
McHugh in the same year [39] published a molecular analysis based on elongation factor-1• with several polychaetes and clitellates together with other terminals. The two vestimentiferans Ridgeia (Fig. 4) and Lamellibrachia also came out as nested among polychaetes and McHugh likewise suggested that Caullery's family name should be applied. Yet another study from the same year by Black et al. [40], based on COI, showed vestimentiferans as nested within frenulates (rather than being sister taxa), and with the single included polychaete as sister taxon to these. Halanych et al. [41] in the following year carried out an 18S rDNA based analysis including four frenulates and seven vestimentiferans indicating that these groups are sister taxa, and also that they are nested within annelids.
Anterior end of the vestimentiferan Ridgeia from Juan de Fuca Ridge off the U.S. west-coast. Photo F. Pleijel.
Rouse [26] carried out a morphology-based phylogenetic analysis of siboglinids, and revised the taxonomy of the group. He identified three main groups, Frenulata and Vestimentifera, as illustrated in Fig. 5, and Monilifera. Sclerolinum had previously been treated as a frenulate, but was removed from this group by Ivanov [42] and placed in its own taxon, Monilifera, equal in rank to Frenulata and Vestimentifera. Rouse [26] applied the name Monilifera in a broader sense to group Vestimentifera with Sclerolinum. The position of Sclerolinum as sister to the vestimentiferans was also demonstrated with 18S rDNA data by Halanych et al. [43], though Schulze [44] instead suggested that Sclerolinum was sister group to Frenulata. A more recent molecular study by Rouse et al. [45] support the placement of Sclerolinum with Vestimentifera.
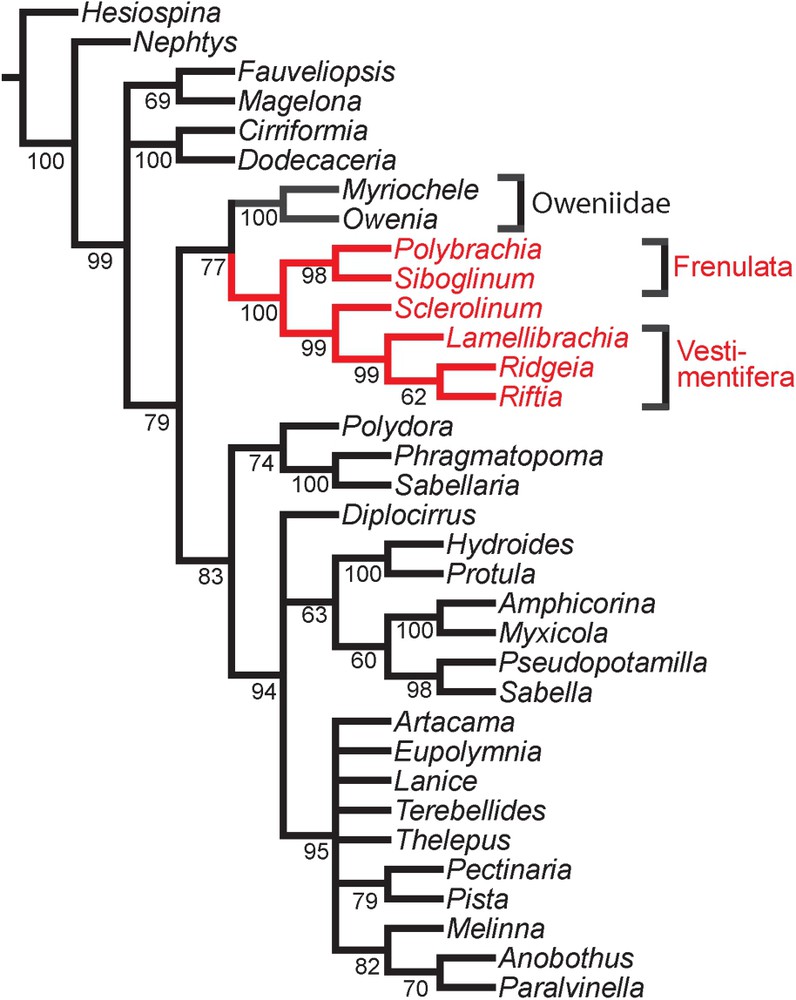
The phylogenetic position of siboglinids (red (lighter coloured) branches) among polychaetes following Rousset et al. [46]. The tree is based on a parsimony analysis of 18S rDNA, 28S rDNA and morphological data, and the provided values are jackknife support.
Rousset et al. [46] published phylogenetic analyses based on combined morphological data with 18S rRNA and 28S rRNA in order to assess the sister group relationship of Siboglinidae within the annelids. Among a selection of 16 canalipalpate polychaetes and outgroups, the Siboglinidae came out as sisters to oweniids (Fig. 5), in contrast to earlier studies [37,38] which had advocated sabellid or terebelliform relationships for them (with the early aforementioned exception of Livanov and Porfireva [13,14]). A less serious nomenclatural issue in this story is Brusca and Brusca's [47] introduction in their text book “Invertebrates” of the name Pogonophoridae for frenulates and vestimentiferans. Apart from being a junior synonym of a large number of family names, it is also a nomen nudum in not being based on any existing generic name.
5 Osedax
In 2004 Rouse et al. [45] describes two new species of a remarkably odd annelid group, found on the bones of a dead gray whale in nearly 3000 m depth off the Californian coast [48] (Fig. 6A). The community that forms around a whale-fall is complex in spatial and temporal structure. Similar to vent habitats, sampling at whale fall sites requires a direct manned submarine or a video-controlled remotely operated vehicle [49]. The experience of seeing the red plumes characteristic of some of these new forms later named Osedax, covering the whale-bones, may have been just as thrilling and confusing as when the first tubeworms were found at the vents. The two first described species, Osedax rubiplumus and O. frankpressi, were very abundant and each had four pinnule-bearing palps and a long oviduct on a trunk enclosed in a transparent mucous tube and extending out of the bone tissue [45]. Reaching into the bone were “roots” extending from an ovisac that was filled with oocytes. The “roots” were packed with symbiotic bacteria. Associated with the trunk but attached to mucous tube of these 2–7 centimetre-sized females of Osedax were dwarf males that were several orders of magnitude smaller [45,50]. There was little in terms of morphology that indicated a polychaete affinity of these animals, although the microscopic males actually are provided with a few and very tiny chaetae on the posterior part of the body (Fig. 6B). Molecular evidence, however, was unequivocal, and Osedax is sister to Monilifera (Sclerolinum plus vestimentiferans) (Fig. 7).

Osedax roseus. A. Females on a bone from a blue whale carcass at near 1018 m depth off California. Photo F. Pleijel. B. Dwarf male of the same species (150 μm long) with posterior chaetae, an anterior ciliary ring (prototroch), and developing sperm in the midbody. Photo G. Rouse.
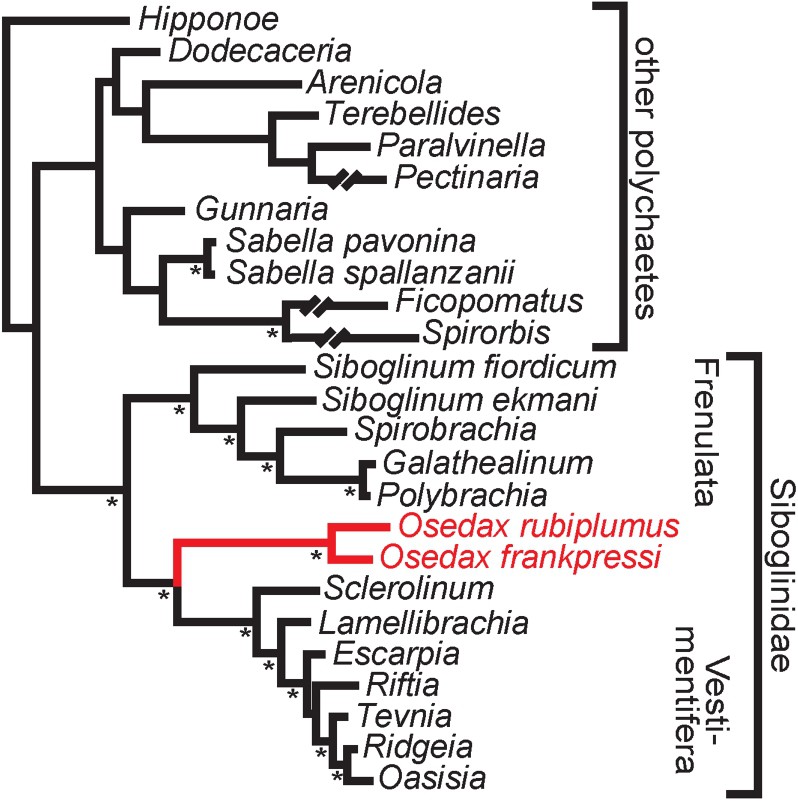
Bayesian tree showing the phylogenetic position of Osedax (red (lighter coloured) branches) following Rouse et al. [45], based on 16S rDNA and 18S rDNA. Nodes with asterisks represent posterior probabilities of 100%.
In 2005 Glover et al. [51] described a third species from an experimentally sunk minke whale at shelf depth of 125 m in Skagerrak in the eastern North Atlantic. Named O. mucofloris, the new species was later found also at a pilot whale carcass implanted near the type locality, but as shallow as 34 m depth [52]. The known geographical range for Osedax was extended in 2006 to the western Pacific when a fourth new species was described from sperm whales sunk at 200 m depth off southern Japan [53,54]. A fifth species, the third from Monterey Bay, has now also been described [50]. In addition to these five described species, five additional ones are known and awaiting formal description from a handful of experimentally sunk whale and bone implantations in the Monterey Bay area [48,50,55,56]. An eleventh species is under description from material collected at whale-falls off southern California (Glover et al., in prep). Although the known range of Osedax species now include both of the major ocean basins, the entire known morphological diversity of the group can be found at a few whale-falls in a small area at the continental shelf and slope in Monterey Bay. Morphological characters that have been used to distinguish females of the different species are related to the palps, oviduct, trunk and ovisac, as mentioned above and summarized in some detail by Fujikura [53] and Braby [55]. They include colour of palps, placement and type of pinnules on the palps, length of and place from where the oviduct extend on the trunk, length of trunk, shape of and the way on which the root structure extend within the substrate. One species has only been found in the sediments near whale falls living out of buried remnants from the whale carcass [55]. The substrate used by the females of some species has also been found to include cow bones suggesting that also carcasses from other vertebrates could sustain populations of Osedax [56]. In contrast to other siboglinids, Osedax species lack a trophosome, the organ that holds the symbiotic bacteria in frenulates and vestimentiferans [42]. Instead the symbiotic bacteria are housed in the female root structure together with the ovaries. The organization of the root structure with an ovisac covered with tissue packed with bacteria and forming a sheet as seen in O. frankpressi [42], is less obvious in other species where bacteria-containing tissue appear to be more integrated with ovaries (e.g. O. mucofloris). Research on the nature of Osedax-bacteria symbiosis [45,48,57] have revealed several fascinating features contrasting those found in most other symbiotic relationships between bacteria and marine invertebrates. While chemoautotrophic sulphur-oxidizing bacteria are found in almost all other cases, the most common endosymbionts in known Osedax taxa belong to Oceanospirales, a group of heterotrophic bacteria often associated with degradation of complex organic compounds [45,48,57]. Analyses of the genetic diversity of bacteria assemblages associated to Osedax worm have further shown high levels of heterogeneity, similar to that found in shipworm symbionts [58] but unprecedented in taxa such as other siboglinids, gutless oligochaetes and mussels [48]. The remarkable diversity of Osedax species identified from a very restricted sample suggests that more research of large organic falls and other ephemeral habitats is required for us to understand the extent and evolution of siboglinid annelids.
The lesson of this intriguing tale is one of humbleness and the difficulties in tracing evolution from ancient signs that are difficult to interpret. Through the course of their taxonomic history these animals have been turned upside down and back to front, moved from deuterostomes to protostomes, discovered to be incomplete, raised to several phyla and then sunk into a family of polychaetes. It serves a reminder that we all are likely to make mistakes, and that strength is shown in recognizing and admitting error when evidence point us in new directions. Whatever the future, we certainly have not seen the end of this story.
Acknowledgements
We wish to thank Hervé Le Guyader for an invitation to contribute to this volume, and Daniel Desbruyères for providing the photograph of Riftia.


