1 Introduction
The introduction of non-native predatory species can impact food webs and native populations of predators and prey [1]. The most obvious effects of introduced predators are lethal or non-lethal direct effects via predation on and/or competition with native predators [2,3]. Freshwater ecosystems have little immunity to non-native fishes [4], which were introduced for many recreational and economic purposes [5]. For instance, rivers have been heavily stocked with non-native predatory fish species for recreational fisheries [6], and one of them is the pikeperch, Sander lucioperca (Linnaeus, 1758). The native distribution of the pikeperch extends from Germany in the Western Europe through the Baltic Sea drainage area to the Southeast part of Russia. Since the beginning of the 19th century, pikeperch has been introduced in several countries and nowadays, the species occurs from the Iberian Peninsula to the Aral Sea and from Scandinavia to Maghreb [7]. Specifically, pikeperch has been introduced in France since the beginning of the 20th century [5] and it has been recorded since 1960 in southwestern French rivers [8]. In its native areas, pikeperch is classified as a top-predator feeding mainly on perch Perca fluviatilis Linnaeus, 1758, smelt Osmerus eperlanus (Linnaeus, 1758) and ruffe Gymnocephalus cernuus (Linnaeus, 1758) [9]. In its introduced areas, virtually all aspects of the environmental biology of pikeperch require further studies to determine its potential effects in novel environments [5,10]. Specifically, one of the most urgent needs for basic and applied ecology is to assess the trophic ecology of pikeperch, with reference to other predatory fish species, especially native species.
During the last decade, the study of the trophic ecology and ecological effects of non-native species in freshwaters has greatly benefited from the use of stable isotope analysis (SIA) [1,11]. This method, based on the predictable relationship between the isotopic composition of consumers and their diet, has been proposed as a sensitive, powerful and cost-effective tool [12]. SIA is equally robust at discriminating trophic groups of stream fishes than conventional methods based on gut contents analyses; the difference in the information that can be obtained by both methods relies on the time scale, namely, short (gut contents) vs. mid-term (SIA) [13]. Ratios of carbon isotopes (δ13C) are used to determine sources of dietary carbon whereas δ15N ratios are powerful for estimating trophic position of organisms [14].
The aims of this study were to determine the trophic position of introduced pikeperch within the food web of river ecosystems, and to discuss the potential ecological risks associated with non-native fish introductions. To this end, predatory fish tissues were sampled in two large rivers of SW France, and carbon and nitrogen stable isotopes (δ13C and δ15N) were used to analyze the trophic position differences between co-existing species.
2 Materials and methods
The Lot River flows westward across the Massif Central Mountains for approximately 480 km with a mean discharge of 151 m3 s−1 and a total drainage area of 11,500 km2. The studied stretch in the Lot River was approximately 4 km long near the town of Puy l'Evêque (44°30′N, 1°8′E). The Tarn River is about 380 km long with a mean discharge of 144 m3 s−1 and a total drainage area of 15,700 km2. The studied stretch in the Tarn River, located between two artificial weirs near the town of Villemur-sur-Tarn (43°52′N, 1°30′E), was approximately 8 km long. Both rivers are tributaries of the Garonne River basin (Fig. 1).
Location of the sampling areas in the Lot River and in the Tarn River, southwestern France.
The predatory fish guild in both rivers was mainly composed of one native species (northern pike), and two non-native species (pikeperch and European catfish). Samples of predatory fish were collected from the two rivers by local recreational anglers from mid-April to the end of June 2007. This period corresponded to the most intensive feeding and growth periods of predatory fish [15]. Each individual was measured for total length (TL, ±1 mm) and fin-clipped for SIA. Fin clipping was chosen because stable isotope values in fin tissue correlate closely with those in muscle tissue of fish and thereby allow non-lethal sampling for SIA [16]. All fin samples were oven dried (60 °C for 48 h) and ground into a homogeneous powder using a mixer mill (Retsch MM 200). SIA were performed at the Stable Isotopes in Nature Laboratory, University of New Brunswick, Canada.
Mann–Whitney U-test was used to test for differences between fish total length between the two rivers. After inspection of normality and homoscedasticity, the level of overlapping between the trophic niche of pikeperch, pike and European catfish was determined by comparing the differences in mean δ13C and δ15N values using a one-way ANOVA followed by post-hoc tests (Tukey's multiple comparisons). Since the δ15N values cannot directly be used to compare trophic positions of consumers between ecosystems due to potential differences in baseline δ15N signatures [17], the δ15N signatures of the exotic and invasive Asian clam Corbicula fluminea (Müller, 1774) and zebra mussel Dreissena polymorpha (Pallas, 1771) were used to correct the δ15N values of fish to allow comparisons between the two rivers [14,18]. Mean δ15N values of clams were measured from both rivers and the trophic positions (TP) were calculated for each individual using the formula:
3 Results
A total of 15 and 5 pikeperch were sampled for SIA in the Lot River and in the Tarn River, respectively (Table 1). The mean (±SE) TL of pikeperch was 676 mm (±26, ) and 656 mm (±24, ), respectively, and did not differ between rivers (Mann–Whitney ; ). A total of 6 and 5 pike were sampled for SIA in the Lot River and in the Tarn River, respectively (Table 1). The mean (±SE) TL of pike was 627 mm (±20, ) and 658 mm (±57, ), respectively, and did not differ between rivers (Mann–Whitney ; ). A total of 24 catfish were sampled in both rivers. The mean (±SE) TL of European catfish was 759 mm (±40, ) and 626 mm (±42, ) in the Lot and Tarn Rivers, respectively, indicating a slight difference between the two rivers (Mann–Whitney ; ).
Number (n) of samples analyzed for stable isotopes (δ15N and δ13C) and total length (TL, minimum, maximum and mean ± SE, in mm) of pikeperch, pike and European catfish in the Lot and Tarn Rivers.
| Species | Lot River | Tarn River | ||||||
| n | Total length | n | Total length | |||||
| Min | Max | Min | Max | |||||
| Pikeperch | 15 | 580 | 890 | 676 ± 26 | 5 | 620 | 750 | 656 ± 24 |
| European catfish | 14 | 510 | 1000 | 759 ± 40 | 10 | 490 | 960 | 626 ± 42 |
| Pike | 6 | 530 | 890 | 627 ± 20 | 5 | 500 | 770 | 658 ± 57 |
In the Lot River, the mean δ13C value of pikeperch was ranging from −24.5 to , whereas the mean δ15N value was ranging from 15.48 to 17.8‰ (Fig. 2). In the Tarn River, the stable isotope signatures of pikeperch were ranging from −24.9 to for δ13C and ranging from 18.0 to 19.2‰ for δ15N.
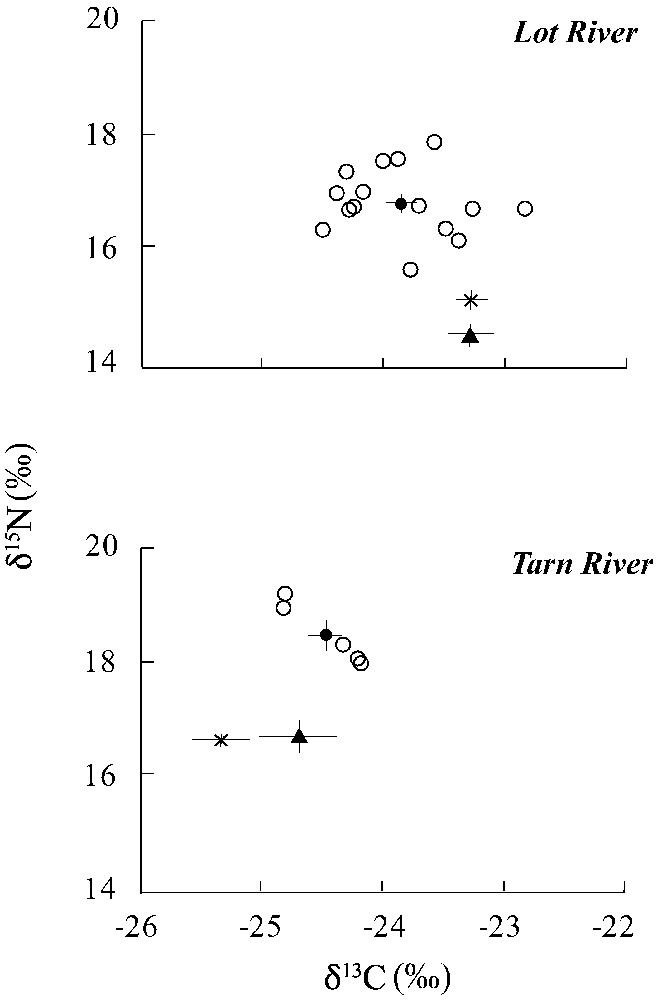
Mean stable isotope values (δ15N and δ13C) of pikeperch (●), pike (▴) and European catfish (×) from the Lot (upper panel) and Tarn (lower panel) Rivers. Individual values are given for pikeperch (○). Error-bars represent standard error (SE).
In the Lot River, mean δ13C values differed significantly between pikeperch and the two other predatory fish (ANOVA, , , Fig. 2), with pikeperch being significantly 13C-depleted compared to pike (Tukey's test, ) and European catfish (Tukey's test, ). No significant differences in δ13C were found between pikeperch and the two other predatory fish in the Tarn River.
The trophic positions of pikeperch, pike and European catfish differed significantly (, ) in the Lot River (Fig. 3). Specifically, pikeperch had a higher trophic position than pike (Tukey's test, ) and European catfish (Tukey's test, ). The same pattern was observed in the Tarn river (Fig. 3) where the three predatory species occupy significantly different trophic positions (, ). Specifically, pikeperch had a higher trophic position than the two other predatory species (Tukey's test, for both pike and European catfish). Trophic positions of pike and European catfish were not significantly different neither in the Lot (Tukey's test, ) nor in the Tarn (Tukey's test, ).
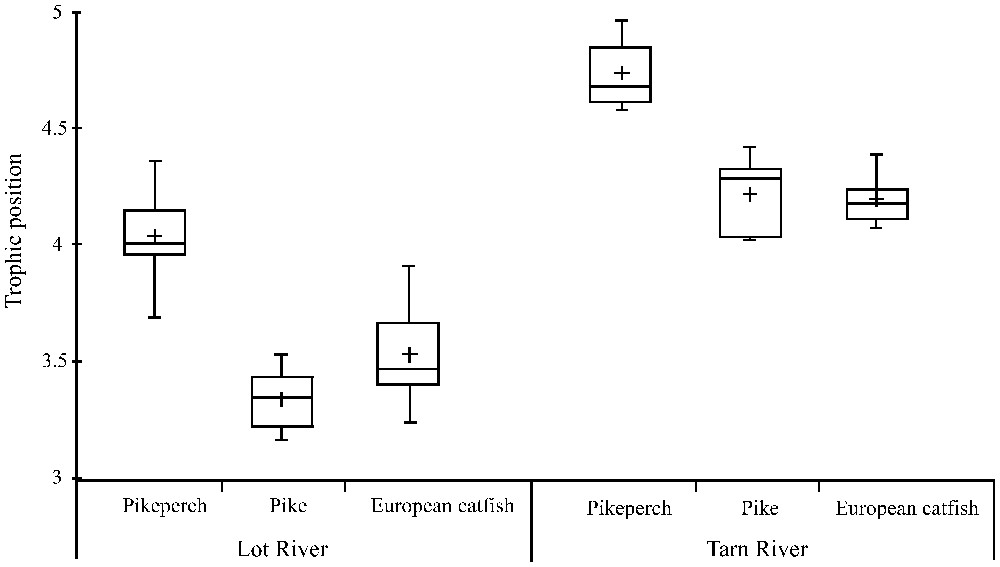
Trophic position of pikeperch, pike and European catfish in the Lot and Tarn Rivers. The boxes represent the interquartile range, the line across is the median value and the cross is the mean value. The whiskers show the lowest and the highest values.
4 Discussion
Information on the trophic ecology of co-existing native and non-native predators is of importance to virtually all aspects of risk assessment in relation to biological invasions. Particularly, understanding the potential impacts of introduced predators on co-occurring predators and prey can improve our ability to forecast the effects of changes in the composition of the predator guild on the overall community. Basically, such an understanding can be gained by assessing the trophic positions of introduced predators, and potential diet overlap with other species of the predatory guild. Fish species are often studied as model organisms under this topic due to strong fisheries and/or economical concerns [6]. Moreover, many non-native fish species may become rapidly established through human-assisted dispersal (e.g. sport and bait species). Although several studies have described the effects of introduced predatory fish on native prey [19,20], studies focusing on the non-native and native piscivorous fish in rivers are still scarce, but some exists in lakes [1,21]. One of the most successful introduced piscivorous fish, pikeperch, has been widely introduced outside its native area throughout Europe for recreational fishing and commercial value; specifically in areas where its abundance is high enough to support small scale commercial fisheries [22]. This species is also used as a biomanipulation tool to control planktivorous fish abundance in many lake restoration attempts [23].
Here, we assessed the trophic position of the pikeperch within the predatory fish guild in two large rivers using SIA, while other existing studies rather relied on stomach content analyses (but see [24]). SIA is relevant for studying the diet of pikeperch, notably because of the frequency of empty stomachs in predatory fish. Moreover, even if not empty, typically only one or two prey species are found per stomach, and this may lead to highly biased estimates of temporally variable diets [25]. Conversely, SIA integrates diet information over longer time periods [14]. Last, predatory fish are not very abundant in the two studied rivers and they are popular for recreational fishing; hence non-lethal sampling is needed in this context. By the way, we implicitly show that non-specialists (here local anglers) can contribute efficiently to scientific studies on fish diet, by providing researchers with fresh tissue samples. Such a contribution would have probably been complicated, if not impossible, if stomach tubes or pulsed gastric lavage were required for gut contents.
Our results suggest that pikeperch can be categorized as a top-predator in the two studied rivers, but that it occupied a higher trophic position compared to other predatory fish species. Such high trophic levels of pikeperch have been reported in lagoons [24], lakes [19,26,27], as well as in marine embayments [28]. However, little was known to date in rivers, although the species is widely introduced in all European rivers [29]. The high trophic position of pikeperch in lakes and reservoirs, assessed from gut content analyses, was often attributed to their feeding preferences on omnivorous fishes like roach (Rutilus rutilus), bleak (Alburnus alburnus) or smelt as already observed in some French river [25] but also on piscivorous fishes like perch [26,27] when they reach larger sizes. Furthermore, cannibalism seems to occur throughout the life of pikeperch. Although its importance is modest for individuals smaller than 250 mm [19], cannibalism becomes more important with increasing body size [9,19,30] until pikeperch congeners can become the major part of the diet at the adult stage [19] leading to a higher trophic position.
A crucial question about the establishment of non-native species concerns their potential impact on the predator guild. Indeed, most studies concerning resource use frequently treat predatory fish as ecologically equivalent. This simplification would imply that inter-species variation is so weak that it has a limited influence upon interspecific relationships within the predatory guild, and upon the functional organization of biological communities. Our results indicate that such simplification is not justified and that some predators can be highly specialized compared to other species within the same trophic guild, occupying a higher trophic niche. This study therefore highlights the apparent specialization of a top-predator, which can have important community level implications through intraguild competition and/or predation in the context of species introductions.
Acknowledgements
We are grateful to the numerous anglers that provided us samples for SIA. The study was funded by the ‘Fédération de pêche du Lot, Agence de l'eau Adour Garonne, Conseil Régional Midi-Pyrénées, Conseil Général du Lot, Fédération nationale pour la pêche en France, EDF’ (contract: No 08005117). We thank N. Poulet for his comments on an earlier version of the manuscript. We wish to thank an anonymous Reviewer who provided valuable comments to improve the manuscript.


